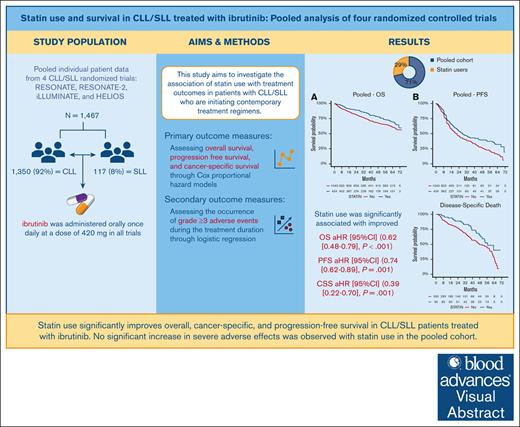
Key Points
Statin use is significantly associated with overall, cancer-specific, and progression-free survival in CLL/SLL patients treated with contemporary regimens, including ibrutinib.
No significant increase in severe adverse effects was observed with statin use in the study cohort.
Visual Abstract
Patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) have seen significant treatment advancements with the emergence of Bruton tyrosine kinase inhibitors like ibrutinib. Statin use has been linked to reduced mortality in several cancers, including CLL. However, their concomitant use with targeted therapies such as ibrutinib remains unexplored. This study investigates the association of statin use with survival and adverse event outcomes in patients with CLL/SLL initiating contemporary treatment regimens, including ibrutinib. Individual participant data from 4 randomized trials—RESONATE, RESONATE-2, iLLUMINATE, and HELIOS—were used. Associations between baseline statin use and treatment outcomes were examined using Cox proportional hazards models for overall survival (OS), progression-free survival (PFS), and cancer-specific survival (CCS), and logistic regression models for grade ≥3 adverse effects. Analyses were adjusted for age, sex, weight, Eastern Cooperative Oncology Group performance status, disease diagnosis, bulky disease (≥5 cm), time since diagnosis, comorbidity count, and the use of beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and diuretics. Of 1467 patients, 424 (29%) were using statins. Statin use was significantly associated with improved OS (adjusted hazard ratio [aHR] 0.62 [95% CI, 0.48‑0.79], P < 0.001), PFS (aHR 0.74 [95% CI, 0.62-0.89], P = 0.001), and CCS (aHR 0.39 [95% CI, 0.22–0.70], P = 0.001). Findings were consistent across ibrutinib vs nonibrutinib treatment arms and CLL vs SLL diagnosis. No significant association with grade ≥3 adverse effects was observed. Statin use was identified as an independent positive prognostic factor in patients with CLL/SLL, irrespective of the treatment employed. Further research is needed to validate these results and explore the underlying impacts of statins in CLL/SLL. These trials were registered at www.ClinicalTrials.gov as #NCT01578707, #NCT01722487, #NCT02264574, and #NCT01611090.
Introduction
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are B-cell lymphoid malignancies characterized by the clonal proliferation and accumulation of mature lymphocytes.1 Recent advancements in treatment strategies, including the emergence of novel Bruton tyrosine kinase (BTK)–targeted therapies, such as ibrutinib, the first-in-class BTK covalent inhibitor, have revolutionized the management of CLL/SLL.1,2 However, substantial heterogeneity persists in both survival and treatment-related adverse outcomes. This highlights the need for continued exploration of prognostic factors that can predict likely outcomes and aid in the selection of treatment.3
In the clinical setting, concomitant medications are often prescribed to manage either comorbidities or treatment-emergent adverse effects.4 Among these, statins, which are commonly prescribed for their lipid-lowering properties, have garnered considerable attention for their potential effects beyond cholesterol reduction. These include antineoplastic, anti-inflammatory, and immunomodulatory properties.5-8 Decreased cholesterol availability may halt cancer-cell proliferation and migration.5 In addition, the use of statins reduces the downstream products in the mevalonate pathway, leading to various potential anticancer effects as well as reducing cancer recurrence.6 Statins have also demonstrated the ability to slow down cell-cycle progression and enhance radiosensitization.7 In CLL, they may also reduce inflammatory cytokine synthesis and cause apoptosis of CLL cells. Preclinical studies suggest that statins may exert antitumor effects in CLL and SLL through various mechanisms, including the disruption of cell signaling pathways crucial for leukemic cell survival and proliferation.8
Although the association between statin use and clinical outcomes has been investigated across various cancer types,9-11 its impact on CLL/SLL remains largely unexplored, particularly in the context of novel targeted therapies, such as ibrutinib. This knowledge gap is especially relevant given the increasing use of BTK inhibitors as a frontline therapy for CLL/SLL. Therefore, this study aims to investigate the association of statin use with overall survival (OS) and progression-free survival (PFS), as well as the occurrence of grade ≥3 adverse effects in patients with CLL/SLL, who are initiating contemporary treatment regimens.
Methods
Patient population
Individual patient data was pooled from 4 randomized clinical trials: RESONATE (NCT01578707, data cutoff: 27 November, 2018),12 RESONATE-2 (NCT01722487, data cutoff: 4 May 2015),13 iLLUMINATE (NCT02264574, data cutoff: 26 March 2018),14 and HELIOS (NCT01611090, data cutoff: 23 January 2019).15 All participants were adults aged 18 years or older with CLL or SLL.
RESONATE assessed the efficacy of ibrutinib monotherapy over ofatumumab in improving survival outcomes for patients with relapsed or refractory disease; RESONATE-2 evaluated the safety and efficacy of ibrutinib in comparison with chlorambucil for treatment-naïve patients aged 65 years and older. The iLLUMINATE trial investigated ibrutinib efficacy combined with obinutuzumab relative to chlorambucil and obinutuzumab combination in treatment-naïve patients. Finally, the HELIOS trial assessed the safety and efficacy of ibrutinib in combination with bendamustine and rituximab in patients with relapsed or refractory disease.
In all trials, ibrutinib was administered orally at a daily dose of 420 mg, although specific regimens varied across trials. In RESONATE, ibrutinib was compared to ofatumumab, which was administered intravenously over up to 24 weeks, with an initial dose of 300 mg in week 1, followed by 2000 mg weekly for 7 weeks, and every 4 weeks for the next 16 weeks.12
In RESONATE-2, ibrutinib was compared to chlorambucil, which was given on days 1 and 15 of each 28-day cycle, starting at 0.5 mg/kg and increased up to 0.8 mg/kg based on tolerability.13
In iLLUMINATE, ibrutinib was given with obinutuzumab (100 mg on day 1, 900 mg on day 2, then 1000 mg on day 8 and day 15 of cycle 1, and 1000 mg on day 1 of each subsequent 28-day cycle for up to 6 cycles). This was compared to obinutuzumab with chlorambucil (0.5 mg/kg) on days 1 and 15 of 28-days cycle each for 6 cycles.14
In HELIOS, ibrutinib was used in combination with bendamustine (70 mg/m2 intravenously on days 2-3 of the first cycle and days 1-2 of the subsequent cycles for up to 6 cycles) and rituximab (375 mg/m2 on day 1 of cycle 1, then 500 mg/m2 on day 1 of cycles 2-6). This was compared to placebo plus bendamustine and rituximab.15
All trials conformed to the guidelines outlined by the International Conference on Harmonization for Good Clinical Practice and the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. The secondary analysis of deidentified data presented in this study was approved by the University of Sharjah Ethics Committee (approval reference number: REC-23-11-07-01-F) and was classified as a research activity with negligible risk. Data access followed Pharmacyclics policy through The Yale University Open Data Access Project (https://yoda.yale.edu/).
Outcome and predictor data
The primary outcomes in this study were OS, PFS, and cancer-specific survival. The incidence of grade ≥3 adverse events during the treatment period were assessed as a secondary outcome.
In each of the RESONATE, RESONATE-2, iLLUMINATE, and HELIOS trials, PFS was defined as the time from patient randomization to either disease progression (as determined by the Independent Review Committee based on the International Workshop on CLL criteria, with clarification for treatment-related lymphocytosis) or death, whichever occurred first. OS was defined as the time from the date of randomization to the date of death.
Cancer-specific survival was determined based on the cause of death classification, distinguishing between CLL/SLL-specific mortality (“progressive disease”) and non-CLL/SLL–specific causes (“others”). Deaths attributed to CLL/SLL progression were considered events, whereas deaths from unrelated causes were censored to ensure the analysis focused specifically on cancer-specific mortality.
The primary covariate in this study was documented pretreatment statin use. Additional pretreatment variables included age, sex, weight, Eastern Cooperative Oncology Group performance status (ECOG PS) score, CLL or SLL diagnosis, bulky disease (≥5 cm), time since diagnosis, comorbidity count, and pretreatment use of beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), and diuretics.
Statistical analysis
The association of statin use with OS and PFS was investigated using Cox proportional hazard regression models. Both univariate and adjusted analyses were employed to assess statin independence from other prognostic factors, with results reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs). A P value of <.05 was considered statistically significant. Complete case analysis was performed. All analyses were stratified by study and treatment arm. Heterogeneity in the association of statins with outcomes across different studies and treatment regimens was assessed using a treatment-by-biomarker interaction term in the Cox regression model. Kaplan-Meier plots were utilized to illustrate and estimate survival probabilities based on statin use.
The association between statin use and the occurrence of any grade ≥3 adverse events within the first 12 months of treatment initiation was assessed using logistic regression analysis, with results reported as odds ratios (ORs) and their corresponding 95% CIs. All analyses were conducted using R version 4.3.
Results
Patient population
Table 1 presents a summary of the baseline characteristics of patients across different study cohorts. Out of the total 1467 patients, 424 (29%) were on statins at baseline. The distribution across the studies was as follows: 116 (30%) in RESONATE, 102 (38%) in RESONATE2, 77 (34%) in iLLUMINATE, and 129 (22%) in HELIOS. As for diagnosis, 1350 (92%) patients were diagnosed with CLL, whereas 117 (8%) patients had SLL.
Baseline patients’ characteristics in each study cohort
| . | Total N = 1467 . | HELIOS n = 578 . | iLLUMINATE n = 229 . | RESONATE n = 391 . | RESONATE2 n = 269 . | P value . |
|---|---|---|---|---|---|---|
| Arm of the clinical study, n (%) | <.001 | |||||
| Chlorambucil | 133 (9) | 0 (0) | 0 (0) | 0 (0) | 133 (49) | |
| Chlorambucil plus obinutuzumab | 116 (8) | 0 (0) | 116 (51) | 0 (0) | 0 (0) | |
| Ibrutinib | 331 (23) | 0 (0) | 0 (0) | 195 (50) | 136 (51) | |
| Ibrutinib plus bendamustine and rituximab | 289 (20) | 289 (50) | 0 (0) | 0 (0) | 0 (0) | |
| Ibrutinib plus obinutuzumab | 113 (8) | 0 (0) | 113 (49) | 0 (0) | 0 (0) | |
| Ofatumumab | 196 (13) | 0 (0) | 0 (0) | 196 (50) | 0 (0) | |
| Placebo plus bendamustine and rituximab | 289 (20) | 289 (50) | 0 (0) | 0 (0) | 0 (0) | |
| Diagnosis, n (%) | .002 | |||||
| CLL | 1350 (92) | 514 (89) | 214 (93) | 373 (95) | 249 (93) | |
| SLL | 117 (8) | 64 (11) | 15 (7) | 18 (5) | 20 (7) | |
| Age, y (IQR) | 65 (60-72) | 60 (55-70) | 70 (66-76) | 64 (60-72) | 70 (63-77) | <.001 |
| Sex, n (%) | .50 | |||||
| Male | 963 (66) | 382 (66) | 146 (64) | 266 (68) | 169 (63) | |
| Female | 504 (34) | 196 (34) | 83 (36) | 125 (32) | 100 (37) | |
| Weight, kg | .016 | |||||
| Median (IQR) | 75 (66-86) | 76 (68-87) | 74 (65-86) | 76 (66-88) | 72 (64-83) | |
| Missing, n (%) | 16 (1) | 8 (1) | 0 (0) | 8 (2) | 0 (0) | |
| ECOG PS2, n (%) | .32 | |||||
| 0 | 632 (43) | 251 (43) | 110 (48) | 159 (41) | 112 (42) | |
| ≥1 | 835 (57) | 327 (57) | 119 (52) | 232 (59) | 157 (58) | |
| Bulky disease ≥5 cm (Y/N), n (%) | <.001 | |||||
| Y | 714 (49) | 324 (56) | 74 (32) | 222 (57) | 94 (35) | |
| N | 744 (51) | 254 (44) | 155 (68) | 165 (42) | 170 (63) | |
| Missing | 9 (1) | 0 (0) | 0 (0) | 4 (1) | 5 (2) | |
| Time since initial diagnosis, mo (IQR) | 64 (31-106) | 70 (42-107) | 32 (11-61) | 91 (60-137) | 31 (9-74) | <.001 |
| Statin, pretreatment, n (%) | 424 (29) | 129 (22) | 77 (34) | 116 (30) | 102 (38) | <.001 |
| Beta-blockers, pretreatment, n (%) | 328 (22) | 113 (20) | 63 (28) | 81 (21) | 71 (26) | .026 |
| CCBs, pretreatment, n (%) | 193 (13) | 65 (11) | 41 (18) | 48 (12) | 39 (14) | .070 |
| ACEI/ARBs, pretreatment, n (%) | 414 (28) | 145 (25) | 86 (38) | 84 (21) | 99 (37) | <.001 |
| Diuretics, pretreatment, n (%) | 246 (17) | 84 (15) | 45 (20) | 58 (15) | 59 (22) | .022 |
| Comorbidity count | <.001 | |||||
| Median (IQR) | 7 (4-12) | 6 (3-10) | 8 (5-12) | 8 (5-15) | 8 (5-13) | |
| Missing, n (%) | 32 (2) | 24 (4) | 1 (<1) | 3 (1) | 4 (1) | |
| Cause of death, n (%) | <.001 | |||||
| Progressive disease | 133 (9) | 48 (8) | 5 (2) | 75 (19) | 5 (2) | |
| Other | 282 (19) | 135 (23) | 31 (14) | 101 (26) | 15 (6) | |
| . | Total N = 1467 . | HELIOS n = 578 . | iLLUMINATE n = 229 . | RESONATE n = 391 . | RESONATE2 n = 269 . | P value . |
|---|---|---|---|---|---|---|
| Arm of the clinical study, n (%) | <.001 | |||||
| Chlorambucil | 133 (9) | 0 (0) | 0 (0) | 0 (0) | 133 (49) | |
| Chlorambucil plus obinutuzumab | 116 (8) | 0 (0) | 116 (51) | 0 (0) | 0 (0) | |
| Ibrutinib | 331 (23) | 0 (0) | 0 (0) | 195 (50) | 136 (51) | |
| Ibrutinib plus bendamustine and rituximab | 289 (20) | 289 (50) | 0 (0) | 0 (0) | 0 (0) | |
| Ibrutinib plus obinutuzumab | 113 (8) | 0 (0) | 113 (49) | 0 (0) | 0 (0) | |
| Ofatumumab | 196 (13) | 0 (0) | 0 (0) | 196 (50) | 0 (0) | |
| Placebo plus bendamustine and rituximab | 289 (20) | 289 (50) | 0 (0) | 0 (0) | 0 (0) | |
| Diagnosis, n (%) | .002 | |||||
| CLL | 1350 (92) | 514 (89) | 214 (93) | 373 (95) | 249 (93) | |
| SLL | 117 (8) | 64 (11) | 15 (7) | 18 (5) | 20 (7) | |
| Age, y (IQR) | 65 (60-72) | 60 (55-70) | 70 (66-76) | 64 (60-72) | 70 (63-77) | <.001 |
| Sex, n (%) | .50 | |||||
| Male | 963 (66) | 382 (66) | 146 (64) | 266 (68) | 169 (63) | |
| Female | 504 (34) | 196 (34) | 83 (36) | 125 (32) | 100 (37) | |
| Weight, kg | .016 | |||||
| Median (IQR) | 75 (66-86) | 76 (68-87) | 74 (65-86) | 76 (66-88) | 72 (64-83) | |
| Missing, n (%) | 16 (1) | 8 (1) | 0 (0) | 8 (2) | 0 (0) | |
| ECOG PS2, n (%) | .32 | |||||
| 0 | 632 (43) | 251 (43) | 110 (48) | 159 (41) | 112 (42) | |
| ≥1 | 835 (57) | 327 (57) | 119 (52) | 232 (59) | 157 (58) | |
| Bulky disease ≥5 cm (Y/N), n (%) | <.001 | |||||
| Y | 714 (49) | 324 (56) | 74 (32) | 222 (57) | 94 (35) | |
| N | 744 (51) | 254 (44) | 155 (68) | 165 (42) | 170 (63) | |
| Missing | 9 (1) | 0 (0) | 0 (0) | 4 (1) | 5 (2) | |
| Time since initial diagnosis, mo (IQR) | 64 (31-106) | 70 (42-107) | 32 (11-61) | 91 (60-137) | 31 (9-74) | <.001 |
| Statin, pretreatment, n (%) | 424 (29) | 129 (22) | 77 (34) | 116 (30) | 102 (38) | <.001 |
| Beta-blockers, pretreatment, n (%) | 328 (22) | 113 (20) | 63 (28) | 81 (21) | 71 (26) | .026 |
| CCBs, pretreatment, n (%) | 193 (13) | 65 (11) | 41 (18) | 48 (12) | 39 (14) | .070 |
| ACEI/ARBs, pretreatment, n (%) | 414 (28) | 145 (25) | 86 (38) | 84 (21) | 99 (37) | <.001 |
| Diuretics, pretreatment, n (%) | 246 (17) | 84 (15) | 45 (20) | 58 (15) | 59 (22) | .022 |
| Comorbidity count | <.001 | |||||
| Median (IQR) | 7 (4-12) | 6 (3-10) | 8 (5-12) | 8 (5-15) | 8 (5-13) | |
| Missing, n (%) | 32 (2) | 24 (4) | 1 (<1) | 3 (1) | 4 (1) | |
| Cause of death, n (%) | <.001 | |||||
| Progressive disease | 133 (9) | 48 (8) | 5 (2) | 75 (19) | 5 (2) | |
| Other | 282 (19) | 135 (23) | 31 (14) | 101 (26) | 15 (6) | |
Data are median (IQR) or number of patients (%). P values per χ2 test for categorical data and Kruskal-Wallis test for continuous data.
CCB, calcium channel blocker; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IQR, interquartile range; N, no; Y, yes.
The median follow-up time for the pooled cohort was 60.5 months for OS and 22 months for PFS. Specifically, the median OS follow-up times were 65.4 months for RESONATE, 18.4 months for RESONATE2, 31.3 months for iLLUMINATE, and 63.7 months for HELIOS. The median PFS follow-up times were 64.9 months for RESONATE, 18.3 months for RESONATE2, 29.5 months for iLLUMINATE, and 17.7 months for HELIOS.
Supplemental Table 1 provides a summary of patients’ baseline characteristics by statin use. Patients on statins were older, had a greater weight, and a higher number of comorbidities (P < .05). These patients were also more likely to have an ECOG PS ≥1 and a larger proportion of them were female (P < .05).
The statins used across the cohort (n = 424) included simvastatin (n = 149, 35%), rosuvastatin (n = 69, 16%), atorvastatin (n = 170, 40%), fluvastatin (n = 2, <1%), pravastatin (n = 25, 6%), and lovastatin (n = 9, 2%). Other cardiovascular drugs used at baseline included beta-blockers (328 patients, 22%), calcium channel blockers (193 patients, 13%), ACEI/ARBs (414 patients, 28%), and diuretics (246 patients, 17%; Table 1).
Association of statin use with OS and PFS outcomes
In the univariable analyses of the pooled cohort, statin use was significantly associated with improved OS (HR, 0.68; 95% CI, 0.54-0.86; P = .001) and PFS (HR, 0.77; 95% CI, 0.65-0.91; P = .002; supplemental Table 2). After adjusting for variables including patient’s diagnosis, age, sex, weight, ECOG PS, bulky disease ≥5 cm, time since initial diagnosis, comorbidity count, and use of other cardiovascular drugs (beta-blockers, calcium channel blockers, ACEI/ARBs, and diuretics), statin use remained significantly associated with improved OS (HR, 0.62; 95% CI, 0.48-0.79; P < .001) and PFS (HR, 0.74; 95% CI, 0.62-0.89; P = .001; Table 2).
Adjusted pooled cohort analysis of the association of statin use with survival outcomes
| Pooled cohort . | OS . | PFS . | ||||
|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | |
| Statin use | ||||||
| No | 314/993 | 1 | 547/993 | 1 | ||
| Yes | 92/418 | 0.62 (0.48-0.79) | <.001 | 195/418 | 0.74 (0.62-0.89) | .001 |
| Beta-blockers use | ||||||
| No | 316/1089 | 1 | 583/1089 | 1 | ||
| Yes | 90/322 | 1.06 (0.82-1.36) | .670 | 159/322 | 1.06 (0.88-1.28) | .529 |
| Calcium channel blockers use | ||||||
| No | 349/1222 | 1 | 650/1222 | 1 | ||
| Yes | 57/189 | 1.04 (0.77-1.39) | .808 | 92/189 | 0.76 (0.61-0.96) | .023 |
| ACEI/ARBs use | ||||||
| No | 297/1003 | 1 | 548/1003 | 1 | ||
| Yes | 109/408 | 1.11 (0.87-1.43) | .409 | 194/408 | 0.94 (0.78-1.13) | .506 |
| Diuretics use | ||||||
| No | 341/1169 | 1 | 623/1169 | 1 | ||
| Yes | 65/242 | 0.96 (0.72-1.30) | .809 | 119/242 | 1.04 (0.83-1.31) | .713 |
| SLL diagnosis | ||||||
| No | 37/1298 | 1 | 682/1298 | 1 | ||
| Yes | 34/113 | 1.11 (0.78-1.59) | .564 | 60/113 | 1.06 (0.81-1.40) | .668 |
| Sex (female) | ||||||
| No | 281/918 | 1 | 515/918 | 1 | ||
| Yes | 125/493 | 0.65 (0.52-0.83) | <.001 | 227/493 | 0.69 (0.58-0.82) | <.001 |
| Bulky disease | ||||||
| No | 163/718 | 1 | 322/718 | 1 | ||
| Yes | 243/693 | 1.46 (1.19-1.79) | <.001 | 420/693 | 1.56 (1.34-1.82) | <.001 |
| ECOG PS | ||||||
| 0 | 144/609 | 1 | 304/609 | 1 | ||
| ≥1 | 262/802 | 1.50 (1.21-1.85) | <.001 | 438/802 | 1.19 (1.03-1.39) | .022 |
| Weight | — | 0.99 (0.98-1.00) | .005 | — | 0.99 (0.99-1.00) | .015 |
| Age | — | 1.02 (1.01-1.03) | .004 | — | 1.00 (0.99-1.01) | .853 |
| Time since initial diagnosis | — | 1.00 (1.00-1.00) | .813 | — | 1.00 (1.00-1.00) | .215 |
| Comorbidity count | — | 1.00 (0.99-1.01) | .888 | — | 1.00 (1.00-1.01) | .348 |
| Pooled cohort . | OS . | PFS . | ||||
|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | |
| Statin use | ||||||
| No | 314/993 | 1 | 547/993 | 1 | ||
| Yes | 92/418 | 0.62 (0.48-0.79) | <.001 | 195/418 | 0.74 (0.62-0.89) | .001 |
| Beta-blockers use | ||||||
| No | 316/1089 | 1 | 583/1089 | 1 | ||
| Yes | 90/322 | 1.06 (0.82-1.36) | .670 | 159/322 | 1.06 (0.88-1.28) | .529 |
| Calcium channel blockers use | ||||||
| No | 349/1222 | 1 | 650/1222 | 1 | ||
| Yes | 57/189 | 1.04 (0.77-1.39) | .808 | 92/189 | 0.76 (0.61-0.96) | .023 |
| ACEI/ARBs use | ||||||
| No | 297/1003 | 1 | 548/1003 | 1 | ||
| Yes | 109/408 | 1.11 (0.87-1.43) | .409 | 194/408 | 0.94 (0.78-1.13) | .506 |
| Diuretics use | ||||||
| No | 341/1169 | 1 | 623/1169 | 1 | ||
| Yes | 65/242 | 0.96 (0.72-1.30) | .809 | 119/242 | 1.04 (0.83-1.31) | .713 |
| SLL diagnosis | ||||||
| No | 37/1298 | 1 | 682/1298 | 1 | ||
| Yes | 34/113 | 1.11 (0.78-1.59) | .564 | 60/113 | 1.06 (0.81-1.40) | .668 |
| Sex (female) | ||||||
| No | 281/918 | 1 | 515/918 | 1 | ||
| Yes | 125/493 | 0.65 (0.52-0.83) | <.001 | 227/493 | 0.69 (0.58-0.82) | <.001 |
| Bulky disease | ||||||
| No | 163/718 | 1 | 322/718 | 1 | ||
| Yes | 243/693 | 1.46 (1.19-1.79) | <.001 | 420/693 | 1.56 (1.34-1.82) | <.001 |
| ECOG PS | ||||||
| 0 | 144/609 | 1 | 304/609 | 1 | ||
| ≥1 | 262/802 | 1.50 (1.21-1.85) | <.001 | 438/802 | 1.19 (1.03-1.39) | .022 |
| Weight | — | 0.99 (0.98-1.00) | .005 | — | 0.99 (0.99-1.00) | .015 |
| Age | — | 1.02 (1.01-1.03) | .004 | — | 1.00 (0.99-1.01) | .853 |
| Time since initial diagnosis | — | 1.00 (1.00-1.00) | .813 | — | 1.00 (1.00-1.00) | .215 |
| Comorbidity count | — | 1.00 (0.99-1.01) | .888 | — | 1.00 (1.00-1.01) | .348 |
Adjustment variables: diagnosis, age, sex, weight, ECOG PS, bulky disease ≥5 cm, time since initial diagnosis, comorbidity count, use of beta-blockers, calcium channel blockers, ACEIs/ARBs, and diuretics.
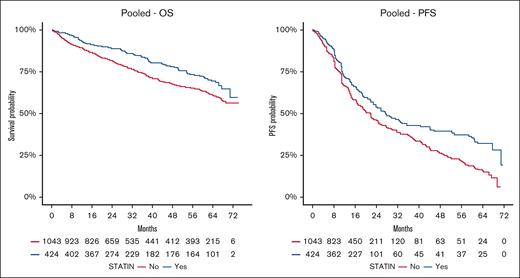
Baseline statin users exhibited a higher 2-year survival probability compared to nonusers. The 2-year OS probability was 89% (95% CI, 86-92) for statin users, vs 82% (95% CI, 79-84) for nonusers. The 2-year PFS was 54% (95% CI, 49-60) for statin users, compared to 46% (95% CI, 42-50) for nonusers.
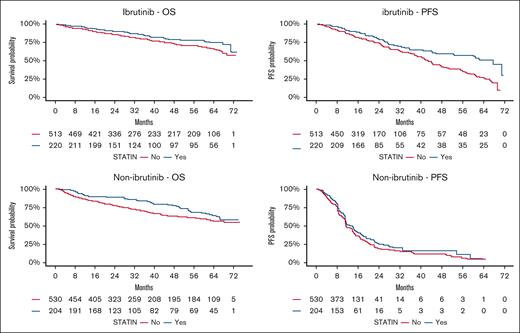
No significant heterogeneity was observed in the identified association between ibrutinib vs non-ibrutinib arms (Table 3; OS Pinteraction = .92, PFS Pinteraction = .09) and CLL vs SLL diagnosis (supplemental Table 3; OS Pinteraction = .56, PFS Pinteraction = .19). In addition, no significant heterogeneity was observed in the identified associations between study cohorts (supplemental Figure 1). This suggests a generally consistent association of statin use with improved survival outcomes across different treatment types and study cohorts.
Adjusted analysis of the association of statin use with survival outcomes by treatment type (ibrutinib vs nonibrutinib arms)
| Treatment type . | Variable . | OS . | Pinteraction . | PFS . | Pinteraction . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | ||||
| Ibrutinib arms | Statin use | .013 | .92 | <.001 | .09 | ||||
| No | 133/491 | 1 | 184/491 | 1 | |||||
| Yes | 42/218 | 0.63 (0.43-0.90) | 61/218 | 0.55 (0.40-0.76) | |||||
| Non-ibrutinib arms | Statin use | .003 | .126 | ||||||
| No | 181/502 | 1 | 363/502 | 1 | |||||
| Yes | 50/200 | 0.60 (0.43-0.84) | 134/200 | 0.84 (0.68-1.05) | |||||
| Treatment type . | Variable . | OS . | Pinteraction . | PFS . | Pinteraction . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | ||||
| Ibrutinib arms | Statin use | .013 | .92 | <.001 | .09 | ||||
| No | 133/491 | 1 | 184/491 | 1 | |||||
| Yes | 42/218 | 0.63 (0.43-0.90) | 61/218 | 0.55 (0.40-0.76) | |||||
| Non-ibrutinib arms | Statin use | .003 | .126 | ||||||
| No | 181/502 | 1 | 363/502 | 1 | |||||
| Yes | 50/200 | 0.60 (0.43-0.84) | 134/200 | 0.84 (0.68-1.05) | |||||
Adjustment variables: diagnosis, age, sex, weight, ECOG PS, bulky disease ≥5 cm, time since initial diagnosis, comorbidity count, use of beta-blockers, calcium channel blockers, ACEIs/ARBs, and diuretics.
The association of statin use with OS and PFS outcomes in the pooled cohort and by treatment type are visualized in Figures 1 and 2, respectively, using Kaplan-Meier plots.
Kaplan-Meier estimates for overall survival (OS) and progression-free survival (PFS) by statin use in the pooled cohort.
Kaplan-Meier estimates for overall survival (OS) and progression-free survival (PFS) by statin use in the pooled cohort.
Kaplan-Meier estimates for overall survival (OS) and progression-free survival (PFS) by statin use and treatment type (ibrutinib vs nonibrutinib).
Kaplan-Meier estimates for overall survival (OS) and progression-free survival (PFS) by statin use and treatment type (ibrutinib vs nonibrutinib).
Association of statin use with cancer-specific survival
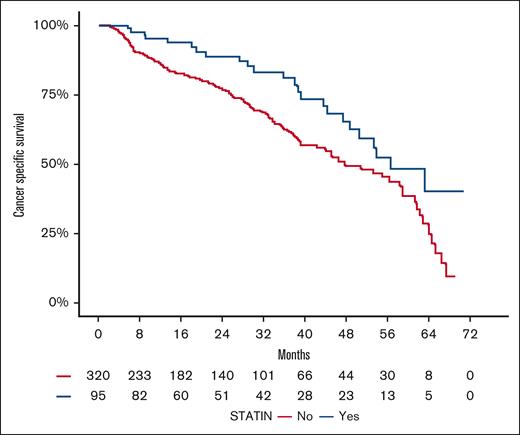
Among the 1467 patients, 133 (9%) patients experienced deaths due to progressive disease (ie, CLL/SLL–specific death), whereas 282 (19%) deaths were attributed to other causes including treatment-emergent adverse events, unknown cause, cardiovascular complications, second malignancies, and sudden death. The distribution of cancer-specific deaths varied significantly across the 4 studies (P < .001), likely reflecting differences in the studies follow-up durations (Table 1). Among the 424 statin users, 24 (6%) died from progressive disease, whereas 71 (17%) of statin users experienced deaths due to other causes (supplemental Table 1).
In the univariate analysis of the pooled cohort, statin use was significantly associated with improved cancer-specific survival (HR, 0.44; 95% CI, 0.27-0.72; P = .001). This association remained statistically significant in the adjusted analysis (HR, 0.39; 95% CI, 0.22-0.70; P = .001; Table 4), suggesting an independent association between statin use and favorable survival outcomes specific for CLL/SLL. Kaplan-Meier plot visualizing the association between statin use and cancer-specific survival in the pooled cohort is presented in Figure 3.
Univariate and adjusted analysis of the association of statin use with cancer-specific death
| Variable . | Univariate . | Adjusted . | ||||
|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | |
| Statin use | .001 | .001 | ||||
| No | 109/320 | 1 | 106/314 | 1 | ||
| Yes | 24/95 | 0.44 (0.27-0.72) | 23/92 | 0.39 (0.22-0.70) | ||
| Variable . | Univariate . | Adjusted . | ||||
|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | Events/patients . | HR (95% CI) . | P value . | |
| Statin use | .001 | .001 | ||||
| No | 109/320 | 1 | 106/314 | 1 | ||
| Yes | 24/95 | 0.44 (0.27-0.72) | 23/92 | 0.39 (0.22-0.70) | ||
Adjustment variables: diagnosis, age, sex, weight, ECOG PS, bulky disease ≥5 cm, time since initial diagnosis, comorbidity count, use of beta-blockers, calcium channel blockers, ACEIs/ARBs, and diuretics.
Kaplan-Meier estimates for cancer-specific survival (CCS) by statin use in the pooled cohort.
Kaplan-Meier estimates for cancer-specific survival (CCS) by statin use in the pooled cohort.
Association of statin use with grade ≥3 adverse effects
No significant association was identified between statin use and grade ≥3 adverse effects in both univariable (OR, 0.93; 95% CI, 0.69-1.24) and adjusted analysis (OR, 0.92; 95% CI, 0.65-1.28).
Discussion
This study provides valuable insights into the associations of baseline statin use with survival and adverse event outcomes in patients with CLL/SLL initiating contemporary treatment regimens. The findings revealed a statistically significant association between statin utilization and improved OS, PFS, and cancer-specific survival. The latter suggests a potential disease-modifying effect of statins in this patient population. No significant associations were observed between statin use and the occurrence of grade ≥3 adverse events. Notably, statin use emerged as a consistent positive prognostic factor irrespective of the specific treatment type or diagnosis, suggesting a consistent association between statin use and enhanced clinical outcomes. To our knowledge, this study represents the first comprehensive investigation of the association of statin use with clinical outcomes in patients with CLL/SLL receiving contemporary treatment regimens, including frontline ibrutinib therapy.
Several studies have investigated the effects of statins on the pathogenesis and treatment outcomes in patients with CLL.16-19 In alignment with our findings, a study assessing 2124 patients with CLL demonstrated a statistically significant improvement in OS among those receiving statins either before or after diagnosis (adjusted HR, 0.53; 95% CI, 0.46-0.60; P < .001).16 However, the previous study included patients diagnosed between 2000 and 2005, with follow-up until 2014, which was before the introduction of contemporary treatments like ibrutinib. In addition, the study excluded a significant portion of patients with CLL aged <66 years, which may limit the generalizability of its findings to younger patients. Similarly, another investigation (n = 280) revealed a 66% reduction in disease progression and a 60% decrease in the risk of death in patients with CLL with relapsed/refractory disease who received salvage FCR (fludarabine, cyclophosphamide, and rituximab) along with statins and aspirin concurrently (PFS: HR, 0.34; 95% CI, 0.18-0.65; P < .001; OS: HR, 0.40; 95% CI, 0.21-0.79; P = .008).17 Furthermore, other studies on patients with CLL have shown that statin use correlates with favorable outcomes by increasing the time to first treatment19-21 and reducing the risk of CLL development.18 Nevertheless, results remain conflicting, as evidenced by 2 observational studies that found no significant associations between statin use and clinical outcomes.21,22
As for SLL, our study fills a gap in the literature by examining the association of statin use with survival outcomes for patients, an area that, to our knowledge, has not been previously explored. Notably, our study demonstrated that statin use was a consistent positive prognostic factor for both SLL and CLL, underscoring the potential advantages of statins in these patient populations. These results are not surprising given that CLL and SLL are now considered biologically the same disease.
Statins, known for their cholesterol-lowering effects, also exhibit anti-inflammatory, antiangiogenic, antioxidant, and anticancer activities.23,24 These effects include inducing cell-cycle arrest, apoptosis, and inhibiting migration, invasion, and angiogenesis,25-27 particularly in CLL cells where statins induce apoptosis by lowering the BCL-2:Bax ratio and activating caspase 9.28 The sensitivity of CLL/SLL cells to statins may be due to the role of cholesterol in the pathogenesis of these malignancies, as CLL cells rely on lipid metabolism for proliferation.29 Furthermore, 3-hydroxy-3-methylglutaryl-coenzyme A reductase levels, targeted by statins, are elevated up to 20-fold in hematological malignancies, including CLL cells, suggesting a potential mechanism for their cytotoxic effects.30 Clinical studies have shown that CLL patients often have dyslipidemia and hypercholesterolemia, which are associated with disease progression and poor prognosis. Statins may improve outcomes by reducing low-density lipoproteins levels and intracellular cholesterol, leading to fewer circulating CLL cells and longer lymphocyte doubling times.31 However, baseline lipid levels were not available in the trial data, which limited our ability to evaluate the relationship between changes in lipid levels, statin use, and CLL outcomes.
Given these promising effects of statins on CLL cells, it is important to explore their potential in combination with other treatments, such as ibrutinib. The potential effects of statins in combination with ibrutinib have not been explored in previous retrospective or clinical studies. In our cohort, no treatment effects were established between ibrutinib and statin use. However, an in vitro study reported a synergistic effect between statins and ibrutinib, demonstrating that incubation with various statins along with ibrutinib significantly reduced CLL cell viability. Furthermore, the proliferation of CLL cells decreased after treatment with ibrutinib or statins alone, and this antiproliferative effect was significantly enhanced when statins were combined with ibrutinib.32
Statins are generally well-tolerated, but long-term adherence can be affected by adverse events such as muscle toxicity, hepatic and renal dysfunction, peripheral neuropathy, and an increased risk of diabetes mellitus.33-36 These adverse effects are significant for patients with cancer as they can impact treatment outcomes. However, in our study, statin use was not significantly associated with an increased risk of grade ≥3 adverse events. Similarly, a study on statin safety in solid tumors found no increased adverse events with statin use.37 These findings suggest that statins may be relatively safe for patients with cancer, although further research is needed to fully understand their safety profile.
Limitations of this study include the generalizability of the findings to real-world populations, constrained by the eligibility criteria applied in the clinical trials. Moreover, statin use may be correlated with other unmeasured factors associated with improved survival, such as socioeconomic status or greater access to health care. For example, the observed associations may be influenced by differences in the quality of primary care, as statin users tended to be older and may have more documented comorbidities, potentially reflecting more comprehensive medical management rather than a direct effect of statins alone. Furthermore, data limitations, including the absence of immunoglobulin heavy chain and TP53 mutation status, patient crossover data to BTK inhibitor regimens, and insufficient event counts within individual statin subgroups, restricted our ability to evaluate the associations of these variables with survival outcomes.
Finally, to our knowledge, while this study is the first to examine BTK inhibitor containing regimens in the context of statin use, ibrutinib is now less widely used given the availability of acalabrutinib and zanubrutinib, and none of the control arm regimens of these studies are in widespread clinical use anymore. Further studies utilizing data from more recent clinical trials are warranted.
In conclusion, our findings demonstrate for the first time that the concurrent use of statins in a diverse population of treatment-naïve or relapsed/refractory patients with CLL/SLL initiating contemporary treatment regimens, including ibrutinib, is significantly associated with improved OS and PFS. Further research is warranted to explore the underlying mechanisms by which statins exert their beneficial effects in patients with CLL/SLL and to investigate whether these associations vary between different statin classes and treatment regimens. Ultimately, prospective studies are needed to confirm these results and evaluate the potential application of statins as adjunctive therapeutic agents in the management of CLL/SLL.
Acknowledgments
This study, carried out under Yale University Open Data Access (YODA) Project 2022-5066, used data obtained from the YODA Project, which has an agreement with Janssen Research & Development, LLC.
This study was supported by University of Sharjah Targeted Research grant 2301110392. A.M.H. is supported by an Emerging Leader Investigator Fellowship from the National Health and Medical Research Council, Australia (grant no. APP2008119). A.M.H. is also a National Health and Medical Research Council Fellow (APP2008119), who is supported by research funding from Tour De Cure (grant no. RSP-117-FY2023).
The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the YODA Project or Janssen Research & Development, LLC.
Authorship
Contribution: A.Y.A. conceived and designed the study; A.Y.A. and A.M.H. developed the methodology; A.Y.A. acquired the data; S.A.A., J.R.B., A.M.H., Z.A., H.O.A.-S., Z.K., M.H.S., S.A., Y.B., A.A., R.A.M., M.J.S., K.H.A., and A.Y.A. analyzed and interpreted the data (eg, statistical analysis, biostatistics, and computational analysis); S.A.A., J.R.B., A.M.H., Z.A., H.O.A.-S., Z.K., M.H.S., S.A., Y.B., A.A., R.A.M., M.J.S., K.H.A., and A.Y.A. wrote, reviewed, and/or revised the manuscript; S.A.A., S.A., and A.Y.A. provided administrative, technical, or material support (ie, reporting or organizing data and constructing databases); A.M.H. and A.Y.A. supervised the study; and all authors have read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: H.O.A.-S. is on the advisory board of Roche, Merck & Co, Inc, Bristol Myers Squibb, AstraZeneca, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Ahmad Y. Abuhelwa, Department of Pharmacy Practice and Pharmacotherapeutics, University of Sharjah, Sharjah, United Arab Emirates; email: Ahmad.Abuhelwa@sharjah.ac.ae.
References
Author notes
Data were accessed according to Yale University Open Data Access policy and process for clinical study data sharing and is available for request at https://yoda.yale.edu/.
The full-text version of this article contains a data supplement.