Visual Abstract
TO THE EDITOR:
The capitalized research and development investment to bring a new drug to market is estimated at $2771.60 million per antineoplastic drug1 underscoring the significant time and financial and human resources for drug development. Targeted therapies, such as tyrosine kinase inhibitors, have significantly improved long-term outcomes of patients with chronic myeloid leukemia (CML), leading to a new standard of care.2 On the other hand, the prognosis of acute myeloid leukemia (AML) has not improved despite the recent approvals of new targeted therapies.3 Thus, identifying areas for improvement in the design of clinical trials (CTs) and in how they are reported, is critical to guide future research. This topic has not been explored much in leukemias.4 To fill this gap and to identify the regulatory approval rate of early trials and the factors associated with achieving such outcome, we evaluated abstracts reporting CTs which were presented in the annual meetings of at least one of the following organizations: the American Society of Clinical Oncology (ASCO), the American Society of Hematology (ASH), and/or the European Hematology Association (EHA).
We retrieved abstracts presented at the annual meetings of all 3 organizations between 2011 and 2015 to assess the information available and performance of CTs through the drug development process. We also tracked the progression of the trial throughout its phases, obtained regulatory history, and publication of articles. The regulatory history was retrieved from the countries of all sites included in each abstract. After screening the abstracts of ASCO, ASH, and EHA meetings and removing duplicates/triplicates, the final number of CT abstracts was 393 (supplemental Figure 1). Four multicountry trials led to the drug approval in only some of the participating countries, representing 25%, 33%, and 50% (for 2 trials) of the countries that enrolled patients.
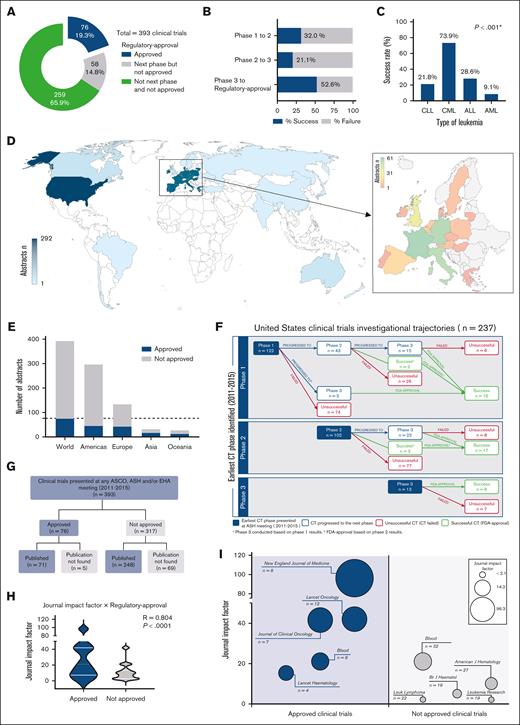
The earliest captured phase of most abstracts was phase 2 (47.8%, supplemental Table 1). Supplemental Table 2 shows CT features based on their phase and approval (ie, regulatory approval for the same indication as studied). Seventy-six of the 393 CTs reported new drugs or new indications of previously approved drugs that resulted in approval by the regulatory agency (Figure 1A). The main bottleneck for drug development in leukemia was the transition from CT phase 2 to phase 3 (78.9% did not progress any further, Figure 1B,F).
CT abstracts investigational trajectories and output (n = 393). (A) Distribution of the CT according to their highest clinical phase reached. (B) Proportion of drug approval vs not of the trials according to their earliest phase presented in the study period. (C) Approval rates of CT by type of leukemia. χ2; P < .05 indicates statistical significance. (D) Spatial distribution of abstracts based on the countries where the studies were conducted. Europe is represented as a single entity on the world map and shown in more detail in the zoomed-in view. (E) Proportion of abstracts that reached or not the regulatory approval by continent. (F) Trajectory of the trials including at least 1 site in the United States across the phases according to their earliest phase captured in the study period. (G) Distribution of the CTs according to the availability of the publication as full-length manuscripts. (H) Correlation of the journal's impact factor with drug approval resulting from the CT. Spearman correlation test; P < .05 indicates statistical significance. (I) Top 5 journals reporting the trials that led to approvals and those that did not. Bubble sizes are proportional to the impact factor of the journal. Impact factor: 2023 Journal Citation Reports (JCR) by Clarivate. ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; FDA, US Food and Drug Administration.
CT abstracts investigational trajectories and output (n = 393). (A) Distribution of the CT according to their highest clinical phase reached. (B) Proportion of drug approval vs not of the trials according to their earliest phase presented in the study period. (C) Approval rates of CT by type of leukemia. χ2; P < .05 indicates statistical significance. (D) Spatial distribution of abstracts based on the countries where the studies were conducted. Europe is represented as a single entity on the world map and shown in more detail in the zoomed-in view. (E) Proportion of abstracts that reached or not the regulatory approval by continent. (F) Trajectory of the trials including at least 1 site in the United States across the phases according to their earliest phase captured in the study period. (G) Distribution of the CTs according to the availability of the publication as full-length manuscripts. (H) Correlation of the journal's impact factor with drug approval resulting from the CT. Spearman correlation test; P < .05 indicates statistical significance. (I) Top 5 journals reporting the trials that led to approvals and those that did not. Bubble sizes are proportional to the impact factor of the journal. Impact factor: 2023 Journal Citation Reports (JCR) by Clarivate. ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; FDA, US Food and Drug Administration.
CTs that resulted in approvals had higher number of patients (supplemental Table 1). AML had the lowest approval rate, 9.1% (Figure 1C), despite accounting for 47.3% of the CTs. All continents except Africa had sites developing CTs, with higher concentrations in America and Europe (Figure 1 D-E). Multicenter and international (including >1 country) studies performed better. Although phase 1 CTs indicated that the drug was safe in 85.6% of cases, only 14% received the regulatory approval. Similarly, most phase 2 and 3 CTs (78.3%) described the study drug or regimen as promising to reach regulatory approval but only 28.2% of them were approved (supplemental Table 1).
Once AML represented nearly half of abstracts (47.3%), we analyzed each of these studies separately. Only the number of authors by abstract was associated with higher approval rates (supplemental Table 3). We also performed a subanalysis excluding CTs on CML given its myeloproliferative nature and observed similar results as with the whole group analysis (supplemental Table 4).
Ninety-three percent of CTs leading to the regulatory approval of the drug were published in scientific journals (Figure 1G). In contrast, our extensive search could not find full-length manuscripts for 21.8% of trials that did not lead to regulatory approval. Furthermore, CT abstracts including drugs and regimens that led to regulatory approval were published in journals with higher impact factors (Figure 1H-I).
The number of patients, CT phase, leukemia type, number of authors in the abstract, number of institutions involved, and international studies were associated with regulatory approval (Table 1). Multivariate analysis showed that studies targeting the treatment of ALL, CLL, or CML were more likely to result in approvals than those CTs which aimed at the management of AML.
Univariate and multivariate adjusted OR and 95% CI for regulatory approval of the abstracts reporting CTs according to their study design and characteristics
| Variable . | Univariate OR (95% CI) . | P value . | Multivariate OR (95% CI) . | P value . |
|---|---|---|---|---|
| Number of patients | 1.003 (1.002-1.005) | <.001 | 1.002 (0.999-1.004) | .154 |
| Year | 1.073 (0.889-1.296) | .464 | 0.977 (0.791-1.256) | .978 |
| CT phase | ||||
| Phase 1 | Reference | Reference | ||
| Phase 2 | 1.452 (0.797-2.647) | .223 | 1.405 (0.692-2.856) | .347 |
| Phase 3 | 7.167 (3.246-15.823) | <.001 | 2.843 (0.854-9.470) | .089 |
| Age group | ||||
| Adults (>17 y) | Reference | Reference | ||
| Older adults (>59 y) | 0.321 (0.123-0.836) | .020 | 0.498 (0.159-1.557) | .231 |
| Pediatric | 1.284 (0.396-4.169) | .677 | 0.670 (0.146-3.069) | .606 |
| Type of leukemia | ||||
| AML | Reference | Reference | ||
| ALL | 4.000 (1.788-8.948) | <.001 | 4.999 (1.882-13.282) | .001 |
| CML | 28.333 (9.785-82.039) | <.001 | 21.632 (6.455-72.479) | <.001 |
| CLL | 2.784 (1.427-5.428) | .003 | 2.842 (1.294-6.241) | .009 |
| Disease status | ||||
| Untreated | Reference | Reference | ||
| R and/or R | 0.943 (0.533-1.667) | .839 | 1.442 (0.644-3.230) | .373 |
| Untreated, R and/or R | 0.757 (0.353-1.621) | .473 | 2.655 (0.982-7.172) | .054 |
| No. of authors in the abstract | 1.070 (1.024-1.118) | .003 | 1.043 (0.971-1.121) | .250 |
| No. of institutions involved | 1.064 (1.026-1.104) | <.001 | 0.989 (0.924-1.059) | .756 |
| Multicenter trials | 3.219 (1.416-7.319) | .005 | 1.928 (0.713-5.212) | .196 |
| International study | 3.859 (2.265-6.577) | <.001 | 1.826 (0.888-3.752) | .101 |
| Variable . | Univariate OR (95% CI) . | P value . | Multivariate OR (95% CI) . | P value . |
|---|---|---|---|---|
| Number of patients | 1.003 (1.002-1.005) | <.001 | 1.002 (0.999-1.004) | .154 |
| Year | 1.073 (0.889-1.296) | .464 | 0.977 (0.791-1.256) | .978 |
| CT phase | ||||
| Phase 1 | Reference | Reference | ||
| Phase 2 | 1.452 (0.797-2.647) | .223 | 1.405 (0.692-2.856) | .347 |
| Phase 3 | 7.167 (3.246-15.823) | <.001 | 2.843 (0.854-9.470) | .089 |
| Age group | ||||
| Adults (>17 y) | Reference | Reference | ||
| Older adults (>59 y) | 0.321 (0.123-0.836) | .020 | 0.498 (0.159-1.557) | .231 |
| Pediatric | 1.284 (0.396-4.169) | .677 | 0.670 (0.146-3.069) | .606 |
| Type of leukemia | ||||
| AML | Reference | Reference | ||
| ALL | 4.000 (1.788-8.948) | <.001 | 4.999 (1.882-13.282) | .001 |
| CML | 28.333 (9.785-82.039) | <.001 | 21.632 (6.455-72.479) | <.001 |
| CLL | 2.784 (1.427-5.428) | .003 | 2.842 (1.294-6.241) | .009 |
| Disease status | ||||
| Untreated | Reference | Reference | ||
| R and/or R | 0.943 (0.533-1.667) | .839 | 1.442 (0.644-3.230) | .373 |
| Untreated, R and/or R | 0.757 (0.353-1.621) | .473 | 2.655 (0.982-7.172) | .054 |
| No. of authors in the abstract | 1.070 (1.024-1.118) | .003 | 1.043 (0.971-1.121) | .250 |
| No. of institutions involved | 1.064 (1.026-1.104) | <.001 | 0.989 (0.924-1.059) | .756 |
| Multicenter trials | 3.219 (1.416-7.319) | .005 | 1.928 (0.713-5.212) | .196 |
| International study | 3.859 (2.265-6.577) | <.001 | 1.826 (0.888-3.752) | .101 |
Bold values indicate statistical significance.
ALL, acute lymphocytic leukemia; CI, confidence interval; CLL, chronic lymphocytic leukemia; OR, odds ratio; R and/or R, relapsed and/or refractory.
Our study revealed that a considerable focus of research was on AML, which is evident from the greater number of CTs targeting this condition. However, despite this emphasis, the likelihood of approval of new drugs for AML remained disproportionately low. The first-line therapy for AML has remained unchanged for over 5 decades, relying still on cytarabine and daunorubicin (“7+3” regimen).5 Nevertheless, the recognition of the genetic and molecular complexities of AML has triggered considerable interest in the development of targeted therapeutic interventions at an accelerated pace.6 Despite many encouraging early leads, many new drugs (some targeted and some chemotherapeutic agents) have failed to confirm clinical benefit in definitive trials. With the drugs that have shown benefit and that have been approved, modest improvements in overall outcomes have been achieved. Thus, there is still much research required to improve the outcomes of the large percentage of patients that still succumb to AML.
Despite most abstracts reporting phase 1 CTs indicated a favorable safety profile, only half of them moved to subsequent phases. Similarly, most trials in phase 2 and phase 3 concluded that the agents under investigation exhibited efficacy, but only a quarter of them led to approvals. This suggests a potential tendency to overestimate early results presented in scientific conferences.7
We could not identify the publication of 21.8% of trials that did not result in regulatory approval. Notably, the overall publication rate of the abstracts that we analyzed (82.0%) was higher than that reported in the literature for other fields (1.3%-52.4%8-10). Our findings are consistent with existing literature, indicating a bias toward publishing studies with positive results.11 It underscores the importance of raising awareness among investigators and stakeholders regarding the value of reporting negative or nonsignificant results from well-designed studies.
Some limitations of this study must be acknowledged. First, only CTs presented at the ASCO, ASH, and/or EHA annual meetings were analyzed, potentially resulting in some being missed. Second, hand searches were conducted to identify phase transitions, possibly resulting in the underestimation of phase transition rate. However, we believe that these issues, although important and possibly influencing the actual rates, do not detract from the main observation of the publication bias and a relative limited approval rate of these trials (as defined for this analysis, although knowledge can be gained from well-designed trials even if results are negative or do not lead to regulatory approval).
In conclusion, AML persists as the most challenging type of leukemia in terms of drug development, indicating potential areas for further exploration. Publication bias also mirrors the outcomes of these trials which may encounter challenges in dissemination within the scientific community. Moving forward, addressing these challenges and leveraging identified factors associated with regulatory approval (which ultimately translates into availability for use in practice) can inform strategies to streamline drug development processes and ultimately improve outcomes for patients with leukemia.
Contribution: All authors contributed substantially to the manuscript; K.C.T. and M.M.-G. collected, analyzed, and interpreted data, performed statistical analysis, and wrote the manuscript; K.M., T.F., M.S., B.S., and C.G.-L. collected data and reviewed the manuscript; and J.E.C. designed research, analyzed and interpreted data, wrote the manuscript.
Conflict-of-interest disclosure: J.E.C. reports consulting for Novartis, Pfizer, Sun Pharma, Nerviano, Bio-Path Holdings, Rigel, and Gilead; and research support from Novartis, Ascentage Pharma, Sun Pharma, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Jorge Cortes, Georgia Cancer Center, 1410 Laney Walker Blvd, CN2222, Augusta, GA 3091; email: jorge.cortes@augusta.edu.
References
Author notes
K.C.T. and M.M.-G. contributed equally to this study.
Presented in abstract form at the 65th annual meeting of the American Society of Hematology, San Diego, CA, 10 December 2023.
The data generated or analyzed during this study are included in this manuscript and its supplementary Methods. All other relevant data to the current study are available on reasonable request from the corresponding author, Jorge E. Cortes (cortes@jcortes@augusta.edu).
The full-text version of this article contains a data supplement.