Key Points
In the phase 3 SELECT-MDS-1 trial, CR was similar between arms in pts with higher-risk MDS.
RARA gene overexpression/RARα activation in myeloid malignancies warrants further investigation as biomarker-driven studies are feasible.
Visual Abstract
Higher-risk myelodysplastic syndrome (HR-MDS) with RARA gene overexpression is a subset of patients (pts) with an actionable target for tamibarotene, an oral and a selective retinoic acid receptor-α (RAR-α) agonist. Tamibarotene with azacitidine (AZA) showed complete remission (CR) rates in myeloid leukemia. SELECT-MDS-1 was a phase 3 study comparing the activity of tamibarotene + AZA to placebo + AZA in these pts with newly diagnosed HR-MDS with RARA overexpression. Eligible pts had confirmed RARA overexpression, untreated MDS with higher-risk features by revised International Prognostic Scoring System (IPSS-R), and marrow blast count >5%. Pts were randomized 2:1 to receive tamibarotene + AZA or placebo + AZA, respectively. A total of 246 participants were randomized with 164 and 82 in the tamibarotene + AZA and placebo + AZA groups, respectively. Baseline characteristics included: 69.9% male; median age 75 years (range, 38-93); primary MDS, 89.8%; MDS-excess blasts-1, 48% and MDS-excess blasts-2, 52%; and IPSS-R risk category intermediate (25.5%), high (35.7%), and very high (38.9%). The study did not meet the primary end point of CR, with a P value of .2084 for the treatment effect in the tamibarotene + AZA group. The CR rates were 23.81% and 18.75% in the tamibarotene + AZA and placebo + AZA groups, respectively. The use of tamibarotene-based therapy to target RAR-α as a novel approach in pts with HR-MDS with RARA gene overexpression is not a paradigm, which can augment response rates beyond AZA monotherapy. Further explorations of alternative approaches, including those with a biomarker, to alter the natural history of this disease are warranted. This trial was registered at www.clinicaltrials.gov as #NCT04797780.
Introduction
Myelodysplastic neoplasms (alternatively known as myelodysplastic syndromes and abbreviated MDS regardless) encompass a group of clonal myeloid malignancies with a broad range of natural histories and treatment outcomes.1 Higher-risk MDS (HR-MDS) is one end of this spectrum, typically with greater transfusion and therapeutic needs than lower-risk disease. The continuum between HR-MDS and acute myeloid leukemia (AML) is well recognized given the arbitrariness of bone marrow blast percentage cutoffs for diagnostic classification (currently, <20% for MDS and at least 20% for AML), but some biological differences with AML are also acknowledged.2,3 Although efforts to better prognosticate, classify, and assess treatment responses in HR-MDS have accelerated, drug development efforts in randomized trials have not yielded promising results beyond hypomethylating agent–based approaches.4,5
SY-1425 (tamibarotene6) is an orally available, synthetic retinoid approved in Japan (Amnolake tablets) since April 2005 for the treatment of relapsed or refractory acute promyelocytic leukemia,7 which is characterized by the presence of the t(15;17) translocation or expression of the PML-RARα gene.8 Tamibarotene was designed to be a more potent and selective retinoic acid receptor α (RARα) agonist with significantly improved in vivo pharmacologic properties compared with all-trans retinoic acid (ATRA). In vitro, tamibarotene is ∼10-fold more potent than ATRA. It has a lower affinity for cellular retinoic acid binding protein, the overexpression of which is associated with resistance to ATRA, and is not subject to the predominant route of retinoid metabolism by Cyp26A1. These 2 features likely contribute to the sustained plasma levels of tamibarotene with repeated dosing. Furthermore, tamibarotene is a selective RARα agonist,9 which may provide tamibarotene a distinct safety, tolerability, and efficacy profile.
The combination of oral tamibarotene plus azacitidine was evaluated in a phase 2 clinical study (ClinicalTrials.gov identifier: NCT02807558) in 51 patients (pts), unfit and with newly diagnosed AML identified as RARA+ (n = 22) or RARA− (n = 29) for RARA messenger RNA overexpression in peripheral blasts using a blood-based biomarker test.10 In 18 response-evaluable pts who are RARA+, complete remission (CR)/CR with incomplete hematologic recovery rate was 61%, and CR rate was 50%, and this supported further study of the regimen in HR-MDS.
The pivotal phase 3 SELECT-MDS-1 study of tamibarotene + azacitidine vs placebo + azacitidine had hoped to change the paradigm for frontline treatment of HR-MDS. The use of tamibarotene-based combination therapy with azacitidine to target RARα, is a novel biomarker-driven approach, in pts with HR-MDS with RARA gene overexpression. SELECT-MDS-1 enrollment supported the analysis of the primary end point of CR. The results from the planned analyses are presented here, placed in context of a negative study and the next steps for the HR-MDS field.
Methods
Pts
Eligible pts were aged ≥18 years with morphologically confirmed MDS by World Health Organization (2016) and higher-risk disease features, including >5% bone marrow myeloblasts and very high-, high-, or intermediate-risk disease according to the revised International Prognostic Scoring System (IPSS-R). Pts had to be positive for RARA gene overexpression based on a quantitative reverse transcription polymerase chain reaction investigational assay performed at a central laboratory. This test was positive or negative and not used quantitatively. Full eligibility criteria are in the supplemental Appendix (Protocol).
Trial design and treatment
The trial was conducted in accordance with the Declaration of Helsinki and the good clinical practice guidelines of the International Council for Harmonisation. The study was conducted at 135 sites in 13 countries. The trial safety was monitored by an independent data monitoring committee. Institutional review board approval was received at all clinical sites.
Pts were randomized (2:1) to receive oral tamibarotene 6 mg twice per day (orally) or placebo each day on days 8 through 28 of each 28-day treatment cycle (full details in the supplemental Appendix), with all randomized pts to receive IV or subcutaneous azacitidine 75 mg/m2 on days 1 to 7 or days 1 to 5, 8, and 9 in 28-day cycles. The randomization scheme was generated by an independent statistician, with treatment assignment via an interactive web response system. Pts were stratified by the IPSS-R risk group (intermediate, high, and very high risk) and by geographical region (North America, Western Europe, and Israel vs Eastern Europe).
Somatic mutation testing was performed centrally when available but not required at baseline. DNA was isolated from whole blood collected before treatment, and when material remained after RARA testing, next-generation sequencing was performed. The assay was the Oncomine Myeloid Assay GX version 2. The DNA panel included 28 hot spot gene targets available in the supplemental Appendix (ANKRD26, ABL1, BRAF, CBL, CSF3R, DDX41, DNMT3A, FLT3 [ITD + TKD], GATA2, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, MYD88, NPM1, NRAS, PPM1D, PTPN11, SMC1A, SMC3, SETBP1, SF3B1, SRSF2, U2AF1, and WT1) and 17 genes that were fully sequenced (ASXL1, BCOR, CALR, CEBPA, ETV6, EZH2, IKZF1, NF1, PHF6, PRPF8, RB1, RUNX1, SH2B3, STAG2, TET2, TP53, and ZRSR). The prevalence of mutations with variant allele frequency of >2% and the number of mutations in each patient was tabulated.
End points and assessments
The primary end point was the proportion of pts who achieved CR rate of tamibarotene plus azacitidine vs placebo plus azacitidine by cycle 7, day 1 (after 6 cycles of therapy.) The key secondary end point was overall survival (OS). Other secondary end points are included in the protocol supplemental.
Response was assessed by the investigator (not by central review) based on the local laboratory results per the modified International Working Group (IWG) 2006 MDS criteria.11 Data analyses were not planned by the IWG 202312 based on time of study design, which preceded publication of the IWG 2023. Bone marrow aspirates were collected to measure response on day 1 of cycles 2 and 4, followed by every third cycle (7, 10, 13, etc), with bone marrow aspirates collected at other times as clinically determined based upon clinical findings or changes in peripheral blood counts. Core bone marrow biopsies were collected as clinically determined by the investigator for response. Deaths on treatment included deaths that occurred between date of randomization and up to ∼30 days after the last administration of drug.
Statistical analysis
There were 2 planned interim futility analyses, 1 for the CR and 1 for OS, respectively; the CR analysis was passed. OS futility was to be performed only if the primary efficacy CR rate was positive but since it was negative, the OS futility was actually not performed. The interim CR futility analysis results were based on the first 95 randomized pts followed for 6 months on treatment and were within the prespecified statistical thresholds with no concerning safety signals noted; hence, the study’s Independent Data Monitoring Committee recommended continuing with the study as planned. Enrollment for the efficacy analysis of the primary end point of CR was completed in March 2024.
The CR rate was evaluated in the intent-to-treat (ITT) population (patient populations are defined in the supplemental Appendix). CR rate and 95% exact binomial confidence intervals (CIs) were calculated by treatment group. The stratified Cochran-Mantel-Haenszel test was applied to compare the CR rates between the 2 treatment groups. The point estimate of the difference of the proportions for CR rate between the 2 treatment groups was provided along with 95% CIs. OS was to be evaluated in the ITT population. Because CR differences were not significant, the planned interim analysis for OS was not performed, no statistical testing could be performed on secondary end points, and the P values provided are descriptive only. Because event-free survival is strongly correlated with OS and because OS was the key secondary end point, these analyses were not performed. The α spending was set up so that if CR was positive, OS would be analyzed after ∼550 pts were enrolled based on the projected occurrence of ∼25% of events. The absolute difference between the 2 arms that was needed for success was dependent on the performance of the control arm in this study design. The end point was designed to demonstrate a statistically significant difference between the observed CR rates, with a 1-sided P value of 0.025 (clinically, this likely correlated to an ∼13%-15% improvement in CR [with assumptions of the CR of the control arm] that would have been needed to be demonstrated to show statistical significance). However, because CR was negative, OS was not analyzed. The multiple exploratory analyses planned as part of this study were thought to have the most value in the setting of positive efficacy data. Thus, without that positive CR read-out and with the rapid closure of the study and the company, some data that would have been forthcoming in the setting of a positive is simply not available.
The safety analysis set includes all participants who have received any amount of study drug (tamibarotene, placebo, or azacitidine). The statistical analysis plan is provided as a supplemental.
Results
Pts
The study opened in February 2021. Nearly 1200 pts were screened for this trial, with ∼500 being RARA− and another 499 RARA+, but of these pts, 142 individuals were not eligible for the trial. Supplemental Figure 1 reviews all disposition. Approximately 50% of pts with HR-MDS were positive for the biomarker, with nearly half of those pts going forward with randomization in the study. The more common reasons for lack of eligibility were marrow blasts that were ≤5% or, at time of marrow evaluation, MDS was not present or pts had AML. There were >175 pts for whom no RARA test was confirmed because of failed assay.
Ultimately, a total of 246 pts with HR-MDS and RARA overexpression were randomized across 13 countries and enrolled at 135 sites, comprising the ITT population as of 28 May 2024. From initiation, 246 participants were randomized to the study, with 164 and 82 in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively. Safety analyses were performed on all 245 treated pts. We report all safety data. The modified ITT analysis set included the first 190 randomized participants, which formed the analysis set for primary data analyses.
Table 1 reviews the baseline demographics, which were generally comparable across treatment groups and representative of a patient population with MDS with higher-risk features. Key baseline characteristics for the ITT population include: 69.9% male; median age of 75 years (range, 38-93); 89.8% with primary MDS; World Health Organization classification MDS-EB-1, 48% and MDS-EB-2 of 52%; median bone marrow blasts of 9.0%; IPSS-R risk category of intermediate (25.5%), high (35.7%), very high (38.9%); and cytogenetic risk status of very good (2.8%), good (42.1%), intermediate (14.8%), poor (12.5%), and very poor (21.8%). However, baseline demographics that were not balanced between the 2 groups included Eastern Cooperative Oncology Group performance status and bone marrow blast percentage as shown in Table 1. Baseline samples for central next-generation sequencing testing were available for 118 pts. Table 2 shows the descriptive analysis of the cytogenetic and molecular characteristics of treated pts. There was no ability to calculate IPSS-M in this study; these have not been correlated with RARA overexpression.
Demographics and baseline characteristics of pts treated
| . | Tamibarotene + azacitidine (n = 126) . | Placebo + azacitidine (n = 64) . | Total (N = 190) . |
|---|---|---|---|
| Age, y∗ | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 73.873 (8.4984) | 75.346 (8.4145) | 74.369 (8.4767) |
| Median | 74.955 | 75.530 | 74.975 |
| Min, max | 37.65, 91.50 | 47.95, 92.86 | 37.65, 92.86 |
| Age group, n (%), y | |||
| ≥18 to <65 | 13 (10.32) | 6 (9.38) | 19 (10.00) |
| ≥65 to <75 | 52 (41.27) | 25 (39.06) | 77 (40.53) |
| ≥75 | 61 (48.41) | 33 (51.56) | 94 (49.47) |
| ≥65 | 113 (89.68) | 58 (90.63) | 171 (90.00) |
| Sex, n (%) | |||
| Male | 86 (68.25) | 46 (71.88) | 132 (69.47) |
| Female | 40 (31.75) | 18 (28.13) | 58 (30.53) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 2 (1.59) | 0 (0.00) | 2 (1.05) |
| Not Hispanic or Latino | 44 (34.92) | 21 (32.81) | 65 (34.21) |
| Not Reported | 3 (2.38) | 2 (3.13) | 5 (2.63) |
| Missing† | 77 (61.11) | 41 (64.06) | 118 (62.11) |
| Race, n (%) | |||
| American Indian or Alaska Native | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Asian | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Black or African American | 4 (3.17) | 1 (1.56) | 5 (2.63) |
| Native Hawaiian or other Pacific Islander | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| White | 42 (33.33) | 20 (31.25) | 62 (32.63) |
| Other | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Multiple | 1 (0.79) | 0 (0.00) | 1 (0.53) |
| Not reported | 2 (1.59) | 2 (3.13) | 4 (2.11) |
| Missing† | 77 (61.11) | 41 (64.06) | 118 (62.11) |
| Body mass index, kg/m2‡ | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 26.659 (4.7545) | 26.609 (4.2877) | 26.642 (4.5912) |
| Median | 26.025 | 25.930 | 25.970 |
| Min, max | 16.06, 50.55 | 17.57, 38.01 | 16.06, 50.55 |
| Geographical region, n (%) | |||
| North America, Western Europe plus Israel | 122 (96.83) | 62 (96.88) | 184 (96.84) |
| Eastern Europe | 4 (3.17) | 2 (3.13) | 6 (3.16) |
| ECOG Performance Status, n (%) | |||
| 0 | 57 (45.24) | 31 (48.44) | 88 (46.32) |
| 1 | 61 (48.41) | 26 (40.63) | 87 (45.79) |
| 2 | 7 (5.56) | 7 (10.94) | 14 (7.37) |
| 3 | 1 (0.79) | 0 (0.00) | 1 (0.53) |
| Time since high-risk diagnosis of MDS, mo | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 2.898 (6.4125) | 4.452 (16.6073) | 3.421 (10.9395) |
| Median | 1.150 | 1.410 | 1.220 |
| Min, max | 0.07, 43.86 | 0.03, 131.19 | 0.03, 131.19 |
| Type of MDS, n (%) | |||
| Primary or not-treatment-related MDS | 111 (88.10) | 56 (87.50) | 167 (87.89) |
| Secondary or treatment-related MDS | 15 (11.90) | 8 (12.50) | 23 (12.11) |
| IPSS-R risk group, n (%) | |||
| Intermediate | 34 (26.98) | 16 (25.00) | 50 (26.32) |
| High | 40 (31.75) | 21 (32.81) | 61 (32.11) |
| Very high | 52 (41.27) | 27 (42.19) | 79 (41.58) |
| Bone marrow blasts (%) | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 9.87 (3.670) | 9.29 (3.558) | 9.67 (3.634) |
| Median | 10.00 | 8.00 | 9.00 |
| Min, max | 3.0, 19.0 | 5.2, 18.0 | 3.0, 19.0 |
| Bone marrow blast category, n (%) | |||
| <5% | 2 (1.59) | 0 (0.00) | 2 (1.05) |
| ≥5% to ≤10% | 73 (57.94) | 44 (68.75) | 117 (61.58) |
| >10% to ≤15% | 42 (33.33) | 14 (21.88) | 56 (29.47) |
| >15% | 9 (7.14) | 6 (9.38) | 15 (7.89) |
| >5% | 121 (96.03) | 64 (100.00) | 185 (97.37) |
| ≤10% | 75 (59.52) | 44 (68.75) | 119 (62.63) |
| >10% | 51 (40.48) | 20 (31.25) | 71 (37.37) |
| . | Tamibarotene + azacitidine (n = 126) . | Placebo + azacitidine (n = 64) . | Total (N = 190) . |
|---|---|---|---|
| Age, y∗ | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 73.873 (8.4984) | 75.346 (8.4145) | 74.369 (8.4767) |
| Median | 74.955 | 75.530 | 74.975 |
| Min, max | 37.65, 91.50 | 47.95, 92.86 | 37.65, 92.86 |
| Age group, n (%), y | |||
| ≥18 to <65 | 13 (10.32) | 6 (9.38) | 19 (10.00) |
| ≥65 to <75 | 52 (41.27) | 25 (39.06) | 77 (40.53) |
| ≥75 | 61 (48.41) | 33 (51.56) | 94 (49.47) |
| ≥65 | 113 (89.68) | 58 (90.63) | 171 (90.00) |
| Sex, n (%) | |||
| Male | 86 (68.25) | 46 (71.88) | 132 (69.47) |
| Female | 40 (31.75) | 18 (28.13) | 58 (30.53) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 2 (1.59) | 0 (0.00) | 2 (1.05) |
| Not Hispanic or Latino | 44 (34.92) | 21 (32.81) | 65 (34.21) |
| Not Reported | 3 (2.38) | 2 (3.13) | 5 (2.63) |
| Missing† | 77 (61.11) | 41 (64.06) | 118 (62.11) |
| Race, n (%) | |||
| American Indian or Alaska Native | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Asian | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Black or African American | 4 (3.17) | 1 (1.56) | 5 (2.63) |
| Native Hawaiian or other Pacific Islander | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| White | 42 (33.33) | 20 (31.25) | 62 (32.63) |
| Other | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Multiple | 1 (0.79) | 0 (0.00) | 1 (0.53) |
| Not reported | 2 (1.59) | 2 (3.13) | 4 (2.11) |
| Missing† | 77 (61.11) | 41 (64.06) | 118 (62.11) |
| Body mass index, kg/m2‡ | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 26.659 (4.7545) | 26.609 (4.2877) | 26.642 (4.5912) |
| Median | 26.025 | 25.930 | 25.970 |
| Min, max | 16.06, 50.55 | 17.57, 38.01 | 16.06, 50.55 |
| Geographical region, n (%) | |||
| North America, Western Europe plus Israel | 122 (96.83) | 62 (96.88) | 184 (96.84) |
| Eastern Europe | 4 (3.17) | 2 (3.13) | 6 (3.16) |
| ECOG Performance Status, n (%) | |||
| 0 | 57 (45.24) | 31 (48.44) | 88 (46.32) |
| 1 | 61 (48.41) | 26 (40.63) | 87 (45.79) |
| 2 | 7 (5.56) | 7 (10.94) | 14 (7.37) |
| 3 | 1 (0.79) | 0 (0.00) | 1 (0.53) |
| Time since high-risk diagnosis of MDS, mo | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 2.898 (6.4125) | 4.452 (16.6073) | 3.421 (10.9395) |
| Median | 1.150 | 1.410 | 1.220 |
| Min, max | 0.07, 43.86 | 0.03, 131.19 | 0.03, 131.19 |
| Type of MDS, n (%) | |||
| Primary or not-treatment-related MDS | 111 (88.10) | 56 (87.50) | 167 (87.89) |
| Secondary or treatment-related MDS | 15 (11.90) | 8 (12.50) | 23 (12.11) |
| IPSS-R risk group, n (%) | |||
| Intermediate | 34 (26.98) | 16 (25.00) | 50 (26.32) |
| High | 40 (31.75) | 21 (32.81) | 61 (32.11) |
| Very high | 52 (41.27) | 27 (42.19) | 79 (41.58) |
| Bone marrow blasts (%) | |||
| N | 126 | 64 | 190 |
| Mean (SD) | 9.87 (3.670) | 9.29 (3.558) | 9.67 (3.634) |
| Median | 10.00 | 8.00 | 9.00 |
| Min, max | 3.0, 19.0 | 5.2, 18.0 | 3.0, 19.0 |
| Bone marrow blast category, n (%) | |||
| <5% | 2 (1.59) | 0 (0.00) | 2 (1.05) |
| ≥5% to ≤10% | 73 (57.94) | 44 (68.75) | 117 (61.58) |
| >10% to ≤15% | 42 (33.33) | 14 (21.88) | 56 (29.47) |
| >15% | 9 (7.14) | 6 (9.38) | 15 (7.89) |
| >5% | 121 (96.03) | 64 (100.00) | 185 (97.37) |
| ≤10% | 75 (59.52) | 44 (68.75) | 119 (62.63) |
| >10% | 51 (40.48) | 20 (31.25) | 71 (37.37) |
ECOG, Eastern Cooperative Oncology Group; max, maximum; min, minimum; SD, standard deviation.
Age is calculated as the time from the date of birth to the date of main informed consent.
Missing includes data not collected and data not solicited because collection was not allowed per local regulations.
Body mass index (kg/m2) = weight (kg)/[height (cm)/100]2.
Cytogenetic and molecular characteristics of the treated pts
| . | Tamibarotene + azacitidine (N = 126) . | Placebo + azacitidine (N = 64) . | Total (N = 190) . |
|---|---|---|---|
| Cytogenetic risk status by IPSS-R, n (%) | |||
| Very good | 5 (3.97) | 1 (1.56) | 6 (3.16) |
| Good | 57 (45.24) | 27 (42.19) | 84 (44.21) |
| Intermediate | 17 (13.49) | 10 (15.63) | 27 (14.21) |
| Poor | 13 (10.32) | 9 (14.06) | 22 (11.58) |
| Very poor | 33 (26.19) | 16 (25.00) | 49 (25.79) |
| Missing | 1 (0.79) | 1 (1.56) | 2 (1.05) |
| Low hemoglobin (<11 g/dL) | |||
| Yes | 102 (80.95) | 53 (82.81) | 155 (81.58) |
| No | 24 (19.05) | 11 (17.19) | 35 (18.42) |
| Low platelets (<100 × 109/L) | |||
| Yes | 90 (71.43) | 46 (71.88) | 136 (71.58) |
| No | 36 (28.57) | 18 (28.13) | 54 (28.42) |
| Low absolute neutrophil count (<1.0 × 109/L) | |||
| Yes | 58 (46.03) | 26 (40.63) | 84 (44.21) |
| No | 67 (53.17) | 37 (57.81) | 104 (54.74) |
| Missing | 1 (0.79) | 1 (1.56) | 2 (1.05) |
| Molecular abnormalities for genes commonly associated with MDS∗, n (%) | |||
| Participants with central NGS data | 76 (60.32) | 42 (65.63) | 118 (62.11) |
| ASXL1 mutated | 21 (27.63) | 14 (33.33) | 35 (29.66) |
| TET2 mutated | 21 (27.63) | 10 (23.81) | 31 (26.27) |
| TP53 mutated | 18 (23.68) | 8 (19.05) | 26 (22.03) |
| SRSF2 mutated | 11 (14.47) | 10 (23.81) | 21 (17.80) |
| RUNX1 mutated | 9 (11.84) | 8 (19.05) | 17 (14.41) |
| SF3B1 mutated | 11 (14.47) | 6 (14.29) | 17 (14.41) |
| STAG2 mutated | 7 (9.21) | 4 (9.52) | 11 (9.32) |
| DNMT3A mutated | 7 (9.21) | 3 (7.14) | 10 (8.47) |
| U2AF1 mutated | 6 (7.89) | 3 (7.14) | 9 (7.63) |
| U2AF1L5 mutated | 5 (6.58) | 3 (7.14) | 8 (6.78) |
| BCOR mutated | 6 (7.89) | 0 (0.00) | 6 (5.08) |
| CBL mutated | 3 (3.95) | 2 (4.76) | 5 (4.24) |
| IDH2 mutated | 4 (5.26) | 1 (2.38) | 5 (4.24) |
| NRAS mutated | 4 (5.26) | 1 (2.38) | 5 (4.24) |
| EZH2 mutated | 2 (2.63) | 2 (4.76) | 4 (3.39) |
| PPM1D mutated | 3 (3.95) | 1 (2.38) | 4 (3.39) |
| DDX41 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| IDH1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| NF1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| SETBP1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| CEBPA mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| ETV6 mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| FLT3 mutated | 0 (0.00) | 2 (4.76) | 2 (1.69) |
| KRAS mutated | 0 (0.00) | 2 (4.76) | 2 (1.69) |
| PTPN11 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| RB1 mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| WT1 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| ZRSR2 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| BRAF mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| GATA2 mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| JAK2 mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| SMC1A mutated | 0 (0.00) | 1 (2.38) | 1 (0.85) |
| ASXL1 wildtype/TET2 mutated | 14 (18.42) | 5 (11.90) | 19 (16.10) |
| ASXL1 wildtype/TET2 wildtype | 41 (53.95) | 23 (54.76) | 64 (54.24) |
| TP53 multi-hit | 6 (7.89) | 3 (7.14) | 9 (7.63) |
| TP53 single-hit∗ | 12 (15.79) | 5 (11.90) | 17 (14.41) |
| Number of mutated genes at baseline | |||
| 0 | 8 (10.53) | 7 (16.67) | 15 (12.71) |
| 1 | 16 (21.05) | 6 (14.29) | 22 (18.64) |
| 2 | 25 (32.89) | 9 (21.43) | 34 (28.81) |
| ≥3 | 27 (35.53) | 20 (47.62) | 47 (39.83) |
| . | Tamibarotene + azacitidine (N = 126) . | Placebo + azacitidine (N = 64) . | Total (N = 190) . |
|---|---|---|---|
| Cytogenetic risk status by IPSS-R, n (%) | |||
| Very good | 5 (3.97) | 1 (1.56) | 6 (3.16) |
| Good | 57 (45.24) | 27 (42.19) | 84 (44.21) |
| Intermediate | 17 (13.49) | 10 (15.63) | 27 (14.21) |
| Poor | 13 (10.32) | 9 (14.06) | 22 (11.58) |
| Very poor | 33 (26.19) | 16 (25.00) | 49 (25.79) |
| Missing | 1 (0.79) | 1 (1.56) | 2 (1.05) |
| Low hemoglobin (<11 g/dL) | |||
| Yes | 102 (80.95) | 53 (82.81) | 155 (81.58) |
| No | 24 (19.05) | 11 (17.19) | 35 (18.42) |
| Low platelets (<100 × 109/L) | |||
| Yes | 90 (71.43) | 46 (71.88) | 136 (71.58) |
| No | 36 (28.57) | 18 (28.13) | 54 (28.42) |
| Low absolute neutrophil count (<1.0 × 109/L) | |||
| Yes | 58 (46.03) | 26 (40.63) | 84 (44.21) |
| No | 67 (53.17) | 37 (57.81) | 104 (54.74) |
| Missing | 1 (0.79) | 1 (1.56) | 2 (1.05) |
| Molecular abnormalities for genes commonly associated with MDS∗, n (%) | |||
| Participants with central NGS data | 76 (60.32) | 42 (65.63) | 118 (62.11) |
| ASXL1 mutated | 21 (27.63) | 14 (33.33) | 35 (29.66) |
| TET2 mutated | 21 (27.63) | 10 (23.81) | 31 (26.27) |
| TP53 mutated | 18 (23.68) | 8 (19.05) | 26 (22.03) |
| SRSF2 mutated | 11 (14.47) | 10 (23.81) | 21 (17.80) |
| RUNX1 mutated | 9 (11.84) | 8 (19.05) | 17 (14.41) |
| SF3B1 mutated | 11 (14.47) | 6 (14.29) | 17 (14.41) |
| STAG2 mutated | 7 (9.21) | 4 (9.52) | 11 (9.32) |
| DNMT3A mutated | 7 (9.21) | 3 (7.14) | 10 (8.47) |
| U2AF1 mutated | 6 (7.89) | 3 (7.14) | 9 (7.63) |
| U2AF1L5 mutated | 5 (6.58) | 3 (7.14) | 8 (6.78) |
| BCOR mutated | 6 (7.89) | 0 (0.00) | 6 (5.08) |
| CBL mutated | 3 (3.95) | 2 (4.76) | 5 (4.24) |
| IDH2 mutated | 4 (5.26) | 1 (2.38) | 5 (4.24) |
| NRAS mutated | 4 (5.26) | 1 (2.38) | 5 (4.24) |
| EZH2 mutated | 2 (2.63) | 2 (4.76) | 4 (3.39) |
| PPM1D mutated | 3 (3.95) | 1 (2.38) | 4 (3.39) |
| DDX41 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| IDH1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| NF1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| SETBP1 mutated | 2 (2.63) | 1 (2.38) | 3 (2.54) |
| CEBPA mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| ETV6 mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| FLT3 mutated | 0 (0.00) | 2 (4.76) | 2 (1.69) |
| KRAS mutated | 0 (0.00) | 2 (4.76) | 2 (1.69) |
| PTPN11 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| RB1 mutated | 1 (1.32) | 1 (2.38) | 2 (1.69) |
| WT1 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| ZRSR2 mutated | 2 (2.63) | 0 (0.00) | 2 (1.69) |
| BRAF mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| GATA2 mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| JAK2 mutated | 1 (1.32) | 0 (0.00) | 1 (0.85) |
| SMC1A mutated | 0 (0.00) | 1 (2.38) | 1 (0.85) |
| ASXL1 wildtype/TET2 mutated | 14 (18.42) | 5 (11.90) | 19 (16.10) |
| ASXL1 wildtype/TET2 wildtype | 41 (53.95) | 23 (54.76) | 64 (54.24) |
| TP53 multi-hit | 6 (7.89) | 3 (7.14) | 9 (7.63) |
| TP53 single-hit∗ | 12 (15.79) | 5 (11.90) | 17 (14.41) |
| Number of mutated genes at baseline | |||
| 0 | 8 (10.53) | 7 (16.67) | 15 (12.71) |
| 1 | 16 (21.05) | 6 (14.29) | 22 (18.64) |
| 2 | 25 (32.89) | 9 (21.43) | 34 (28.81) |
| ≥3 | 27 (35.53) | 20 (47.62) | 47 (39.83) |
NGS, next-generation sequencing; WHO, World Health Organization.
Time since initial/high-risk diagnosis = (date of randomization - date of initial/high-risk diagnosis of MDS + 1) / 30.4375.
Participants may be counted in multiple categories. Mutation counts based on central NGS data.
Efficacy
At the time of analysis, 63 pts (38.41%) and 37 pts (45.12%) remained on therapy in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively, and 100 (60.98%) and 45 (54.88%) had discontinued tamibarotene or placebo or azacitidine alone, respectively.
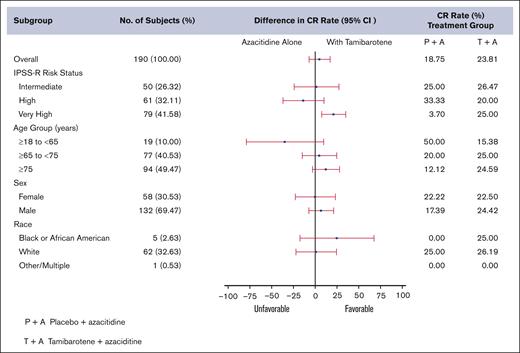
In the first 190 enrolled pts, the CR rate by ITT in the tamibarotene + azacitidine treatment arm was 23.8% whereas it was only 18.8% in the azacitidine control arm. Although higher in the experimental arm, the difference across the arms was not statistically significant (P = .2084). The study, therefore, did not meet the primary end point of CR (Table 3). The CR rates were 23.81% (95% CI, 16.7-32.2) and 18.75% (95% CI, 10.1-30.5) in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively. The OS and even-free survival analyses were not performed. Figure 1 shows the CR rates by subgroups. The very high-risk group by IPSS-R showed more favorable benefit from addition of tamibarotene. A longer median time to CR of participants was observed in the tamibarotene + azacitidine group compared with the placebo + azacitidine group (4.8 vs 3.6 months). The duration of CR of participants in the placebo + azacitidine group was not estimable (NE). The Kaplan-Meier (K-M) estimated medians (in months) and the corresponding 95% CIs in the tamibarotene + azacitidine and the placebo + azacitidine group are 15.7 (95% CI, 6.9 to NE) and NE (95% CI, 16.6 to NE), respectively.
Response rates and secondary end points
| . | Tamibarotene + azacitidine . | Placebo + azacitidine . |
|---|---|---|
| CR, n (%) | 30 (23.1) | 12 (18.75) |
| Median duration of CR, (95% CI) | 15.7 (6.9 to NE) | NE |
| ORR, n (%) | 90 (71.43) | 45 (70.31) |
| Median duration of ORR, (95% CI), mo | 12 (7.9-22.1) | 19.4 (5.8 to NE) |
| Median time to initial response, (95% CI), mo | 1.0 | 1.1 |
| Achieved red cell TD at baseline n (%) | 35 (79.55) Of 44 TD at baseline | 16 (57.14) Of 28 TD at baseline |
| Patient who maintained red cell TI at baseline n (%) | 51 (89.47) Of 57 TI at baseline | 23 (82.14) Of 28 TI at baseline |
| . | Tamibarotene + azacitidine . | Placebo + azacitidine . |
|---|---|---|
| CR, n (%) | 30 (23.1) | 12 (18.75) |
| Median duration of CR, (95% CI) | 15.7 (6.9 to NE) | NE |
| ORR, n (%) | 90 (71.43) | 45 (70.31) |
| Median duration of ORR, (95% CI), mo | 12 (7.9-22.1) | 19.4 (5.8 to NE) |
| Median time to initial response, (95% CI), mo | 1.0 | 1.1 |
| Achieved red cell TD at baseline n (%) | 35 (79.55) Of 44 TD at baseline | 16 (57.14) Of 28 TD at baseline |
| Patient who maintained red cell TI at baseline n (%) | 51 (89.47) Of 57 TI at baseline | 23 (82.14) Of 28 TI at baseline |
ORR, overall response rate; TD, transfusion independence when dependent.
Forest plot of CR rate (%) by subgroups of pts treated on SELECT-MDS-1.
A similar median time to initial response of participants was observed in the tamibarotene + azacitidine group compared with the placebo + azacitidine group (1.0 vs 1.1 months). A similar proportion of participants in the tamibarotene + azacitidine group achieved overall response of CR, partial response, and/or hematologic improvement compared with the placebo + azacitidine group. A shorter K-M estimate of median duration of overall response of participants was observed in the tamibarotene + azacitidine group compared with the placebo + azacitidine group (12.0 vs 19.4 months). A significantly higher proportion of participants in the tamibarotene + azacitidine group achieved transfusion independence compared with the placebo + azacitidine group. The analyses results are in Table 3.
In the absence of the OS analysis in a mature data set, we believe the available data support similar survival between the arms at the time of the primary analysis. We note that there is a limitation in the “on-treatment” death summary in that it does not include deaths in pts that discontinued treatment but remained on the study in survival follow-up. The primary reason for study discontinuation because of death was comparable between the 2 treatment arms and numerically higher in the tamibarotene + azacitidine arm. Also, grade 5 adverse event (AEs) were similar, to slightly higher, in the tamibarotene + azacitidine arm. Therefore, we assert than we can reliably conclude that survival was similar or worse in the tamibarotene + azacitidine arm. These supportive data are illustrative in the absence of a formal K-M OS analysis.
Subgroup analyses were completed when feasible. There was a significantly higher CR rate in the IPSS-R very-high group treated with tamibarotene + azacitidine 25% (14%, 38.9%) compared to placebo/azacitidine 3.7% (0.1%, 19%) but we do not know the molecular characteristics of these pts given the small numbers sequenced. Younger pts had higher rates of CR than older pts using azacitidine alone. Those aged 18 to 64 years demonstrated 50% CR (11.8%, 88.2%) with azacitidine alone but 15% (1.9%, 45.5%) with tamibarotene + azacitidine, whereas those aged 65 to 74 years had a CR of 25% (14%, 38.9%) in the tamibarotene + azacitidine arm and participants aged ≥75 years showed a CR of 24.6% (14.5%, 37.3%) in the tamibarotene + azacitidine arm but only 4% with azacitidine single agent. Male and female pts responded with CR at essentially the same rates as did those of all ethnic backgrounds. In those pts with blasts between 10% and 14% there was a higher CR rate with tamibarotene + azacitidine (21.4%; 10%, 36.8%) compared with placebo + azacitidine (7%; 0.2%, 33.9%) CR rates between the 2 arms did not greatly differ by RARA overexpression and there were consistently responders by CR in all quartiles in both cohorts, suggesting that RARA overexpression may predict for response with less ability to distinguish by therapy.
Safety
Table 4 reviews the safety events. A total of 163 of 164 (99.39%) participants randomized to the experimental arm were treated with tamibarotene + azacitidine , and 82 (100%) participants were treated with placebo + azacitidine. The mean duration of exposure of tamibarotene was 6.2 months. The mean duration of exposure of azacitidine was 6.1 months in the tamibarotene + azacitidine group and 6.3 months in the placebo + azacitidine group. The median number of cycles of tamibarotene was 5. The median number of cycles of azacitidine was 5 in the tamibarotene + azacitidine group and 5 in the placebo + azacitidine group, with these numbers slightly lower than corresponding duration of exposure because of patient delay for start of next cycle. The rates of discontinuation from treatment were 60.98% and 54.88% for the tamibarotene + azacitidine and placebo + azacitidine groups, respectively. Overall, the most common reasons for treatment discontinuation were progressive disease (17.48%), alternate therapy (transplant [9.35%]), and death (8.13%), which was similar across the arms. Also, 153 (95.63%) and 81 (95.29%) participants in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively, had treatment-emergent AEs. Fourteen participants (5.69%) had AE as the primary reason for treatment discontinuation, with 12 (7.32%) and 2 (2.44%) in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively. The proportion of participants with serious AEs was higher in the tamibarotene + azacitidine group than the placebo + azacitidine group, as shown in Table 4. Notably, 20 (12.50%) and 8 (9.41%) on-treatment deaths were reported in the tamibarotene + azacitidine and placebo + azacitidine groups, respectively. AEs that led to death were similar in both arms at 10.63% and 8.24%, respectively.
Overall safety profile, and most common any-grade and grade 3 or above TEAEs occurring in at least 10% (any grade) or at least 10% (grade 3 or above) of pts in the safety population
| . | Tamibarotene + azacitidine . | Placebo + azacitidine . |
|---|---|---|
| Median duration of study treatment, mo | 5.1 (0.03-30.55) | 5 (0.03-29.98) |
| Participants with interrupted doses | 91 (56.88) | 39 (45.88) |
| Participants with missed doses | 99 (61.88) | 53 (62.35) |
| Participants with decreased doses | 16 (10) | 2 (2.35) |
| Incidence of TEAEs, n (%) | ||
| Any TEAE | 153 (95.63) | 81 (95.29) |
| Any drug-related TEAE | 94 (58.75) | 35 (41.18) |
| Any grade ≥3 TEAE | 37 (23.13) | 17 (20.00) |
| Any drug-related grade ≥3 TEAE | 84 (52.50) | 29 (34.12) |
| TEAE leading to discontinuation of tami/placebo, n (%) | 31 (19.38) | 6 (7.06) |
| TEAE leading to discontinuation of AZA, n (%) | 28 (17.50) | 8 (9.41) |
| On-study deaths, n (%) | 20 (12.50) | 8 (9.41) |
| Cause of death | ||
| Progressive disease | 3 (1.88) | 1 (1.18) |
| AE | 17 (10.63) | 7 (8.24) |
| Most common any-grade TEAEs (≥10% of pts) | ||
| Constipation | 65 (41) | 36 (42.3) |
| Nausea | 43 (26.9) | 18 (21.2) |
| Diarrhea | 31 (19.4) | 16 (18.9) |
| Vomiting | 21 (13.1) | 13 (15.3) |
| Anemia | 51 (31.9) | 31 (36.5) |
| Neutropenia | 51 (31.9) | 26 (30.6) |
| Thrombocytopenia | 43 (26.9) | 18 (21.2) |
| Febrile neutropenia | 29 (13.1) | 12 (14) |
| Asthenia | 20 (31.2) | 13 (15.3) |
| Pyrexia | 43 (26.9) | 13 (15.3) |
| Fatigue | 16 (10) | 8 (9.4) |
| Pneumonia | 27 (16.9) | 8 (9.4) |
| Hypertriglyceridemia | 55 (34.4) | 2 (2.4) |
| Decreased appetite | 28 (17.5) | 14 (16.5) |
| Rash | 34 (21.3) | 2 (2.4) |
| Pruritis | 25 (15.63) | 7 (8.24) |
| Alopecia | 23 (14.4) | 1 (1.2) |
| Arthralgia | 24 (15) | 6 (7.1) |
| Most common grade 3/4 TEAEs (≥10% of pts) | ||
| Anemia | 99 (61.9) | 24 (28.2) |
| Neutropenia | 40 (25) | 24 (28.2) |
| Thrombocytopenia | 37 (23.1) | 16 (18.8) |
| Febrile neutropenia | 29 (18.1) | 12 (14.1) |
| Pneumonia | 18 (11.3) | 7 (8.2) |
| Hypertriglyceridemia | 22 (13.75) | 0 |
| . | Tamibarotene + azacitidine . | Placebo + azacitidine . |
|---|---|---|
| Median duration of study treatment, mo | 5.1 (0.03-30.55) | 5 (0.03-29.98) |
| Participants with interrupted doses | 91 (56.88) | 39 (45.88) |
| Participants with missed doses | 99 (61.88) | 53 (62.35) |
| Participants with decreased doses | 16 (10) | 2 (2.35) |
| Incidence of TEAEs, n (%) | ||
| Any TEAE | 153 (95.63) | 81 (95.29) |
| Any drug-related TEAE | 94 (58.75) | 35 (41.18) |
| Any grade ≥3 TEAE | 37 (23.13) | 17 (20.00) |
| Any drug-related grade ≥3 TEAE | 84 (52.50) | 29 (34.12) |
| TEAE leading to discontinuation of tami/placebo, n (%) | 31 (19.38) | 6 (7.06) |
| TEAE leading to discontinuation of AZA, n (%) | 28 (17.50) | 8 (9.41) |
| On-study deaths, n (%) | 20 (12.50) | 8 (9.41) |
| Cause of death | ||
| Progressive disease | 3 (1.88) | 1 (1.18) |
| AE | 17 (10.63) | 7 (8.24) |
| Most common any-grade TEAEs (≥10% of pts) | ||
| Constipation | 65 (41) | 36 (42.3) |
| Nausea | 43 (26.9) | 18 (21.2) |
| Diarrhea | 31 (19.4) | 16 (18.9) |
| Vomiting | 21 (13.1) | 13 (15.3) |
| Anemia | 51 (31.9) | 31 (36.5) |
| Neutropenia | 51 (31.9) | 26 (30.6) |
| Thrombocytopenia | 43 (26.9) | 18 (21.2) |
| Febrile neutropenia | 29 (13.1) | 12 (14) |
| Asthenia | 20 (31.2) | 13 (15.3) |
| Pyrexia | 43 (26.9) | 13 (15.3) |
| Fatigue | 16 (10) | 8 (9.4) |
| Pneumonia | 27 (16.9) | 8 (9.4) |
| Hypertriglyceridemia | 55 (34.4) | 2 (2.4) |
| Decreased appetite | 28 (17.5) | 14 (16.5) |
| Rash | 34 (21.3) | 2 (2.4) |
| Pruritis | 25 (15.63) | 7 (8.24) |
| Alopecia | 23 (14.4) | 1 (1.2) |
| Arthralgia | 24 (15) | 6 (7.1) |
| Most common grade 3/4 TEAEs (≥10% of pts) | ||
| Anemia | 99 (61.9) | 24 (28.2) |
| Neutropenia | 40 (25) | 24 (28.2) |
| Thrombocytopenia | 37 (23.1) | 16 (18.8) |
| Febrile neutropenia | 29 (18.1) | 12 (14.1) |
| Pneumonia | 18 (11.3) | 7 (8.2) |
| Hypertriglyceridemia | 22 (13.75) | 0 |
AZA, azacitidine; tami, tamibarotene; TEAE, treatment-emergent AE.
Discussion
Sobering is the panoply of drugs for HR-MDS that once appeared promising but have not held up on later-phase randomized studies. Eprenetapopt13,14 (p53-refolding agent), pevonedistat15 (NEDD8 inhibitor), sabatolimab,16 and magrolimab17 (anti-CD47 monoclonal antibody promoting macrophage-induced phagocytosis) were all once drugs that many hematologists had pinned hopes on to alter the course of disease beyond our current limited armamentarium currently available for HR-MDS. Unfortunately, response rates were either lower than expected in phase 3 trials or primary end points were simply not met. We must learn from each study as to how to improve the next.
The success of the SELECT-MDS-1 trial was as an efficiently accrued international phase 3 trial that was biomarker driven, the first of its kind in MDS. Although the doublet combination tested in SELECT-MDS-1 with tamibarotene failed to meet the primary end point, it is noteworthy in a trial conducted at so many sites, to demonstrate an azacitidine monotherapy CR rate similar to that of modern azacitidine control arms. There was neither overperformance nor underperformance, suggesting that our azacitidine dosing is aligned with that of real-world settings. These data set may also be useful in the future for its molecular characterization for the international world of HR MDS. There was a higher proportion of pts in the combination arm achieving red blood cell transfusion independence (RBC TI; 78% of those who were transfusion dependent at baseline), suggesting the potential for a disease-modulating biology that drives anemia in MDS.
In the safety analysis of all randomized and treated pts (n = 245), tamibarotene + azacitidine appeared to be generally well tolerated, with an AE profile that was similar to that seen in earlier sponsored studies. The comparable proportions of pts who achieved and maintained platelet TI and who maintained RBC TI suggest that there was little, if any, additive bone marrow suppression for the combination treatment compared with the azacitidine monotherapy arm. There was also no increase in grade 3 or 4 neutropenia or episodes of febrile neutropenia between the arms for the same reason. There was greater grade 3 and 4 anemia in the tamibarotene-treated arm (62% of pts compared with 28% in the placebo) although more TI (RBC TI). This metric is challenging in a hematology study because identifying hematologic AEs requires the investigator to assess the anemia as clinically significant. There is likely site variability in this practice that limits further interpretation. Of note, the exploratory analyses proposed to better understand RBC TI were not planned. These would be difficult to conduct in such a large, multinational study and would have added feasibility challenges and cost to the study in advance of knowing that RBC TI would be improved in the absence of a CR, but this is valuable lesson for future statistical planning in studies of this nature.
There are limited data available on subsequent anti-MDS therapies including allogeneic stem cell transplantation. However, the discontinuation rate because of AEs specifically, although generally low across both arms with comparable time on treatment, was more than twice as high in the tamibarotene arm. This may have limited the ability for those pts to achieve CRs and thus move ahead with transplant if a lower blast percentage was achieved. It is unknown whether the results would have been different if there was central pathology review. However, data review and queries were used to ensure that the investigator’s response assessment was consistent with the reported peripheral blood and marrow data. Furthermore, bias was minimized by the use of placebo and double-blind design. Therefore, we speculate that the results would not have been different with central review, but this is another valuable consideration for future trials.
Although the time to initial response in both cohorts was comparable at 1 month, reasons leading to a nearly 2-month longer time to CR with the combination differentiating therapy are not clear. There was a difference in the 2 arms for time from diagnosis, with pts entering the azacitidine monotherapy arm having nearly twice the time from diagnosis to start of therapy. Perhaps these pts had more favorable disease biology allowing similar responses, substantiated by higher blast counts in the tamibarotene arm. Lastly, the mechanistic synergy of tamibarotene, a retinoid with differentiating effects,18 and azacitidine may require a longer time to achieve a CR in HR-MDS with RARA overexpression.
Furthermore, discontinuation or lack of time on treatment has been a challenge in many trials of this nature.19-22 We had observed a generally well-tolerated safety profile throughout these previous studies, leading to a plausible conclusion that it was not drug intolerance or AEs leading to discontinuation but possibly investigator bias. Astute clinical investigators often develop experience of knowledge allowing for one’s understanding of “blinded” treatment assignment. For example, observation of hypertriglyceridemia, bony aches, or low-grade skin erythema may have unmasked the investigator to treatment with tamibarotene. This knowledge or bias may have led such investigators who observed these and then saw no CR after the cycle-3 marrow biopsy to discontinuation before a future time when longer time on study could have allowed CR. It will always remain challenging to truly blind a study when pts need more time on active therapy to attain their response.15,22 As has been noted in other studies,4 a small phase 2 data set conducted at a limited number of centers is hard to replicate in a global study. The lack of data on OS as well as use of a less current IWG over IWG 2023 may have precluded a better understanding of the clinical benefit of the tamibarotene + azacitidine combination for pts with HR-MDS and RARA overexpression.
The lack of improved efficacy here does beg the question of how to approach the next biomarker-driver study in this disease. We should acknowledge that this trial did demonstrate feasibility of central biomarker testing and still allowed an enrollment rate comparable with any other nonbiomarker study in MDS. With perhaps less “druggable” mutations, transcriptional programs may offer a space for therapies and we will continue to learn from other such programs in the myeloid field.23 As such, the biology of RARA overexpression would seem to be relevant in both MDS and AML, and therefore there was a rationale for RARα as a target. RARA positivity had been studied in AML24 but not formally validated in HR-MDS in advance, leading to the hypothesis that lower peripheral blast counts in the blood of pts with MDS may have introduced some bias in the ability of the validated biomarker assay to select for tamibarotene response in MDS. We should acknowledge that although this validated assay was associated with higher responses in pts with low-blast-count AML who were RARA+ vs RARA−, the assay was not formally tested in pts with HR-MDS with blasts of <20%. Although the assay appeared to select for a subset of pts with MDS with unique RARA-related biology, the lack of a clear additive effect with the addition of tamibarotene may be because of a more dominant effect of the higher-risk MDS features. Also, the different underlying marrow failure biology in MDS may prevent translation of AML results.10,24 Decades ago, the attempts to treat MDS with retinoids, alone or in combination, held some suggestions of possible efficacy.25,26
As the authors and study investigators, we have tried here to speculate as to the reasons for the unsuccessful trial, in particular, given the previous positive data in newly diagnosed unfit AML24 including those with low-blast-count AML, as noted earlier. It is critical to review these issues for future improvements. One key observation was the improved transfusion rates and TI in the tamibarotene arm, which may lead future investigators to focus on lower-risk MDS in combination with azacitidine. Nonetheless, this is very disappointing news for pts with HR-MDS who need better therapies. We are grateful to the investigators, study staff, and pts who have participated in the trial. Only through well-designed clinical trials, such as this trial, and thoroughly analyzed, can we try to improve on the treatment options available for pts. We staunchly encourage the field to continue these valuable efforts.
Acknowledgments
The authors thank all of the patients and their families and the investigators and staff at all clinical sites for participating in the trial. No editorial support was used in manuscript preparation.
This study was sponsored by Syros Pharmaceuticals, Inc.
Authorship
Contribution: A.E.D., D.A.R., V.M.K., and M.J.K. wrote the first draft of the manuscript; and all authors conceived and/or designed the work that led to the submission, acquired the data, and/or played a key role in interpreting the results, revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work.
Conflict-of-interest disclosure: A.E.D. participated in advisory boards and/or had a consultancy with and received honoraria from Bristol Myers Squibb (BMS), Agios, and Novartis, and served on clinical trial committees or data safety monitoring boards for Novartis, Agios, AbbVie, Kura, Geron, Servier, Keros, Shattuck Labs, and BMS. S.T. participated in advisory boards or was a speaker for BMS, AbbVie, Astellas, and Novartis. A.P. participated in advisory board and/or received honoraria from Alexion, Pfizer, Novartis, Takeda, Grifols, Sobi, and Sanofi. D.D. received grants and/or research support from Alexion, Sobi, Amgen, Novartis, and Roche, and honoraria or fees for giving a talk from Daiichi-Sankyo, Incyte, Sobi, Novartis, Alexion, Amgen, and Roche. J.-M.T.-D. was a consultant on advisory boards for Novartis, SELLAS Life Sciences, BMS, Alexion, and Incyte; received travel funds from Alexion, Novartis, Sellas, and Pfizer; and received honoraria for consultancy from SELLAS Life Sciences. G.M. was a consultant, participated in advisory boards, or was a speaker for AbbVie, Astellas, AstraZeneca, Enable Life Sciences, Immunogen, Jazz, Janssen, Menarini/Stemline, Pfizer, Ryvu, Servier, Syros, Takeda, and UK Neqas, and received research support from AbbVie, Astellas, AstraZeneca, Daiichi Sankyo, and Pfizer. A.J. participated in advisory boards and/or reports consultancy with BMS, AbbVie, Novartis, and GlaxoSmithKline (GSK). A.M.Z. participated on advisory boards, consulted for and participated in clinical trial committees, and/or received honoraria from AbbVie, Akesobio, Agios, Amgen, Astellas, BioCryst, BeiGene, Boehringer-Ingelheim, Celgene/BMS, Chiesi, Daiichi Sankyo, Dr Reddy, Epizyme, Fibrogen, GSK, Glycomimetics, Genentech, Gilead, Geron, Janssen, Jasper, Karyopharm, Kyowa Kirin, Keros, Kura, Novartis, Notable, Orum, Otsuka, Pfizer, Regeneron, Rigel, Seattle Genetics, Schrodinger, Syros, Syndax, Servier, Takeda, Treadwell, Taiho, Vincerx, and Zentalis. S.D.-S. participated on advisory boards with Pfizer and reports travel grants with AbbVie. M.D.C. received honoraria/speaker fees from BMS, Novartis, Keros, and AbbVie, and participated on advisory boards with BMS, Agios, Hemavant, Syros, Keros, GSK, Curis, Astex/Otsuka, and Ascentage. W.C.-H. participated on advisory boards with BMS, Ascentage, and Replimune, and participated in clinical trials with AbbVie, Agios, Akesobio, BMS, Gilead, Incyte, Novartis, Syros, Shattuck Labs, and Sun Pharma. L.S. participated on advisory boards with BMS, AbbVie, Gilead, Novartis, Amgen, Pfizer, Takeda, Daiichi Sankyo, Servier, and Jazz. Y.O. reports consultancy with AbbVie and Medison, and participated on advisory boards with GSK, BMS, Astellas Gilead, and Novartis. P.K. received honoraria/conference support/advisory board fees from AbbVie, Astellas, Jazz, Gilead, Stemline-Menarini, Pfizer, Johnson & Johnson, and BMS-Celgene. M.L. participated on advisory boards for AbbVie, Astex Pharmaceuticals, Imago BioSciences, Janssen, Otsuka, and Syros, and received research support to institution from Janssen. G.B. reports speakers' bureau participation with, and travel support from, Novartis, Sanofi, AbbVie, Jazz, and Gilead. H.E.C. participated in clinical trial committees and/or received honoraria for consultancy with AbbVie, Agios, Amgen, BMS, Daiichi Sankyo, Geron, Kura, Jazz, Rigel, Novartis, Stemline, and Servier, and received research funding from Celgene. D.A.S. reports consultancy with AbbVie, Agios, Debiopharm, Janssen, Johnson & Johnson, Molecular Partners, and Novartis; served on advisory boards with AvenCell, Astellas, bluebird bio, BMS, Dark Blue Therapeutics, Geron, Shattuck Labs, Servier, Syndax, Syros, and Taiho Oncology; and received research funding from Aprea and Jazz. U.B. is a consultant for Novartis, AbbVie, Genentech, Incyte, Daiichi Sankyo, Sumitomo, Rigel, Astellas, Incyte, BMS, and Servier Scientific; participated on an advisory board with Vincerx Pharma; participated on a steering committee for BeiGene, Servier, and Sumitomo; and participated on a data monitoring committee for Takeda. V.S. participated on advisory boards for AbbVie, Ascentage, BMS, Geron, GSK, Keros, Jazz, Novartis, Otsuka, Syros, and Servier and received travel support from Janssen. J.M.Z. reports honoraria from Roche, Takeda, AbbVie, and Sobi, and received research support from BMS and Takeda. D.A.R., S.P., P.R., M.J.K., A.K., J.C., T.A.M., and V.M.K. are all employees of Syros Pharmaceuticals, Inc. T.C. reports consulting and board membership for Syros, AbbVie, BMS, and Novartis.
Correspondence: Amy E. DeZern, Hematologic Malignancies, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, 1650 Orleans St, CRBI Room 2M08, Baltimore, MD 21287; email: adezern1@jhmi.edu.
References
Author notes
Data are available on request from the corresponding author, Amy E. DeZern (adezern1@jhmi.edu).
The full-text version of this article contains a data supplement.