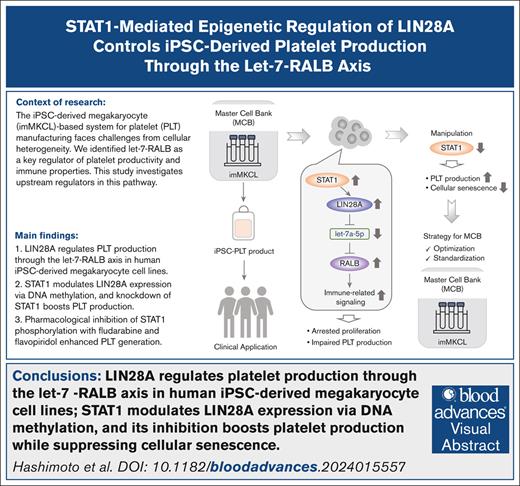
Key Points
LIN28A regulates PLT production through the let-7 microRNA–RAS-like proto-oncogene B axis in human iPSC–derived megakaryocyte cell lines.
STAT1 modulates LIN28A expression via DNA methylation, and its inhibition boosts PLT production while suppressing cellular senescence.
Visual Abstract
Ex vivo platelet (PLT) production from induced pluripotent stem cells (iPSCs) represents a potential solution to address the limitations of donor-dependent PLT transfusion therapy. Although our established immortalized megakaryocyte progenitor cell lines (imMKCLs) from iPSCs enable large-scale production of functional iPSC-derived PLT products, cellular heterogeneity and senescence remain significant challenges for robust industrial-scale manufacturing. We recently identified RAS-like proto-oncogene B as a key regulator of immune properties and PLT productivity of imMKCLs, acting downstream of the let-7a-5p microRNA. This study aims to identify the upstream regulators of let-7a-5p in this context. Herein, we demonstrate that the expression of Lin-28 homolog A (LIN28A), which negatively regulates let-7a-5p, is controlled in imMKCLs through DNA methylation–dependent mechanisms. Analysis of the LIN28A locus revealed distinct methylation patterns between let-7high and let-7low populations within an intronic CpG island. Overexpression of LIN28A upregulated immune-related signaling and diminished PLT production from imMKCLs. We further examined for transcriptional regulators by motif enrichment analysis and small interfering RNA–mediated knockdown, identifying STAT1 as an upstream regulator of LIN28A. Knockdown of STAT1 led to the suppression of immune-related gene expression, resulting in increased PLT production. Inhibition of STAT1 phosphorylation with fludarabine and flavopiridol enhanced PLT generation, uncovering a novel role in PLT generation beyond their established functions in cell cycle arrest and apoptosis. In conclusion, our findings unveil the modulating roles of immune and senescence signaling in imMKCLs to optimize cell and culture conditions for large-scale PLT manufacturing.
Introduction
Platelets, an essential cellular component in transfusion medicine, play crucial roles in hemostasis, thrombosis, and maintenance of vascular integrity. Since James Blundell’s primitive success with human blood transfusion in 1818,1 the current dependency on donor-derived platelets has substantial challenges, including limited shelf life, risk of bacterial contamination, alloimmune-mediated reactions, and anticipated supply shortages due to aging societies.2 Ex vivo blood production from induced pluripotent stem cells (iPSCs) has been proposed as a solution to these issues, offering the potential for consistent platelet supply for transfusion therapy. Several research groups, including ours, have made substantial progress in developing methods for producing platelet products ex vivo from human iPSCs (iPSC-PLTs).3-5 However, scaling up to achieve clinically relevant yields was difficult.
To overcome this limitation, we previously established immortalized megakaryocyte progenitor cell lines (imMKCLs) by introducing doxycycline (DOX)-inducible c-MYC, BMI1, and BCL-XL transgenes into human iPSC–derived hematopoietic progenitor cells.6,7 imMKCLs demonstrate sustained proliferative capacity over several months in the presence of DOX and produce agonist-responsive CD41+CD42b (GPIbα)+ functional iPSC-PLTs upon DOX removal. A breakthrough was achieved by implementing turbulent flow-based bioreactors, enabling the generation of clinically relevant quantities (1011 level) of such functional iPSC-PLTs.8 These discoveries enabled not only the first-in-human clinical trial by autologous iPSC-PLTs9-11 but also a robust in vitro system for investigating fundamental mechanisms of megakaryocyte development and thrombopoiesis.12-14
Despite these achievements, several critical issues were raised, particularly associated with the quality control of both imMKCL master cells and their derived iPSC-PLTs. In that context, we recently identified let-7 microRNA (miRNA) and its downstream target, RAS like proto-oncogene B (RALB), as critical regulators of immune-biased megakaryocyte subpopulations.15 These subpopulations, characterized by lower let-7 activity, exhibited immune-related signal activation, particularly interferon (IFN) signaling, and compromised imMKCL quality, evidenced by arrested proliferation and impaired iPSC-PLT production. That study provided indispensable insights into the standardization of iPSC-PLT generation toward industrial-scale manufacturing.15 However, the upstream regulators of the let-7–RALB axis and the mechanisms driving cellular heterogeneity in imMKCLs have remained elusive.
The RNA-binding protein LIN28, which exists as 2 paralogs (LIN28A and LIN28B), has been identified as a key regulator of stem cell maintenance and differentiation.16 The LIN28-mediated regulation of let-7 miRNA maturation has significant implications for stem cell biology, glucose metabolism, and various human diseases.17,18 Although the role of LIN28 proteins in early development is known, their potential involvement in megakaryocyte development and thrombopoiesis remains largely unexplored. This study focuses on elucidating the upstream regulatory mechanisms controlling iPSC-PLT production capability in imMKCLs, with an emphasis on the epigenetic and transcriptional regulations of the LIN28-let-7–RALB axis. Our findings demonstrate that LIN28A expression is regulated through DNA methylation–dependent mechanisms mediated by STAT1, ultimately influencing platelet production efficiency. This study uncovers a novel STAT1–LIN28A-let-7 regulatory network that enhances iPSC-PLT production capacity while suppressing immune-skewed properties linked to cellular senescence in imMKCLs. These findings offer valuable insights for strategizing optimization toward an improved ex vivo iPSC-PLT manufacturing system.
Materials and methods
Cells
Human iPSC lines, TkDN-Sev2 and T-1, were established in-house.19 imMKCLs (clone 7, clone 7-3, and M35-1) were induced by DOX-inducible defined factors from human iPSCs and were used in previous studies.7,8,15 The use of all cells was approved by the ethics committees at Kyoto University and Chiba University. imMKCLs (clone 7, clone 7-3, and M35-1) were cultured as described before.8 DOX was used to control the proliferation and differentiation stages of imMKCLs.
Overexpression of human LIN28A in imMKCLs
Viral vector use was approved by committees at Kyoto University and Chiba University. The full-length coding sequence of human LIN28A was cloned into the lentiviral vector LV-TRE-mOKS-Ubc-tTA-I2G.20 Lentiviral production using the 293T system was described previously.6 imMKCLs (clone 7) were transduced with either the resultant or control (MOCK) viruses.6 After transduction, green fluorescent protein (GFP)–positive imMKCLs were sorted using a BD FACS-Aria IIIu.
Reverse transcription and real-time quantitative PCR
Total RNA was extracted using the microRNeasy Micro Kit or microRNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed using SuperScript VILO Master Mix (Thermo Fisher Scientific) or ReverTra Ace qPCR RT Master Mix with genomic DNA Remover (Toyobo, Osaka, Japan). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed with the SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) using the StepOnePlus system (Thermo Fisher Scientific). GAPDH was used as the internal control. Primer sets used are listed in supplemental Table 1. Let-7a-5p miRNA reverse transcription was performed using a TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) and miRNA-specific stem-loop RT primers according to the manufacturer’s instructions. RT-qPCR was performed with the TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific) using the StepOnePlus system. Let-7 expression was determined relative to RNU6B using specific TaqMan probes.
Flow cytometry
Cells were suspended in a staining medium, incubated for 30 minutes with appropriate antibodies on ice in the dark, and sorted or analyzed using a BD FACSAria IIIu. Platelet counts were determined as previously described.8 The following antibodies were used for the analysis: allophycocyanin (APC)-conjugated anti-CD41a (integrin αIIb; HIP8 clone; BioLegend, San Diego, CA) and phycoerythrin (PE)-conjugated anti-CD42b (GPIbα; eBioscience, San Diego, CA). Samples were analyzed using a BD FACSVerse.
DNA methylation assay
Genomic DNA was extracted using the DNeasy Blood & Tissue Kit (Qiagen) and treated with sodium bisulfite using the MetylEasy Xceed Rapid DNA Bisulfite Modification Kit (Takara Bio). Bisulfite-treated DNA was amplified using EpiTaq HS (Takara Bio). Primers targeting CpG islands in the LIN28A region were designed by MethPrimer21 and are listed in supplemental Table 2. PCR amplicons were cloned into the pMD20-T vector using the Mighty TA-cloning Kit (Takara Bio) and transformed into DH5α competent cells. The plasmid from each clone was sequenced by Macrogen Japan Corp (Kyoto, Japan). Methylation status analysis was performed using QUMA (quantification tool for m ethylation analysis; RIKEN, Wako, Japan).22 Examples of sequence logos were generated based on the matrix ID. The discrepancy in the number of CpG sites may arise from differences in the sequencing read coverage and quality between let-7high and let-7low cells.
Expression of a dominant-negative C/EBP
The retroviral vector pGCsam of dominant-negative CCAAT/enhancer binding protein (C/EBP) with an internal ribosomal entry site-enhanced GFP (EGFP) was generously provided by Atsushi Iwama.23 A dominant-negative form of C/EBP, followed by internal ribosomal entry site-EGFP were subcloned into a retroviral vector pGCsam (A-C/EBP) Retroviral production was performed as previously described.23 imMKCLs (clone 7) were transduced with the resultant or control viruses. After transduction, EGFP-positive imMKCLs were sorted using a BD FACSAria IIIu.
siRNA-mediated STAT1 gene knockdown
The Dharmacon siGENOME small interfering RNA (siRNA) SMARTpool designed against human STAT1 (siRNAs targeting STAT1 [siSTAT1]), comprising 4 siRNAs targeting different sites, and a nontargeting control siRNA (siNT) were purchased from Horizon Discovery Ltd. Gene silencing was performed according to the Dharmacon reverse transfection protocol with minor modifications. Briefly, siRNA at 25-nM final concentration was diluted in the transfection buffer from the Stemfect RNA Transfection Kit (ReproCELL), with the transfection reagent diluted in an equal volume of buffer. The diluted siRNA and transfection reagent were combined and incubated at room temperature for 15 minutes. Subsequently, 12.5 μL per well of the transfection mixture was added to the wells of a 96-well plate, followed by the addition of 2 × 105 imMKCLs (clone 7; DOX-ON) in 100-μL medium to each well. After a 4-hour incubation period, cells were washed with phosphate-buffered saline and subjected to either proliferation or differentiation culture conditions. To validate the gene knockdown efficiency, total RNA was isolated and analyzed 48 hours after the transfection.
Intracellular flow cytometry
The intracellular flow cytometry assay was optimized for imMKCLs as follows. Cells were fixed in 4% paraformaldehyde for 15 minutes at room temperature, followed by methanol permeabilization (15 minutes on ice). After 2 washes with phosphate-buffered saline, cells were incubated with an anti-STAT1 polyclonal antibody (Proteintech; 10144-2-AP; 0.4 μg/100 μL) for 1 hour on ice. Subsequently, cells were washed and incubated with a Multi-rAb CoraLite Plus 647-Goat Anti-Rabbit Recombinant Secondary Antibody (H+L; Proteintech; RGAR005; 1:1000) on ice for 30 minutes. Stained cells and nonstaining controls were then analyzed using a BD FACSVerse.
Analysis of cytokine secretion by imMKCLs
Cytokine secretion in culture supernatants was measured using the human inflammatory cytokine cytometric bead array kit (BD Biosciences) according to the manufacturer’s guidelines. Briefly, bead populations with distinct fluorescence intensities coated with capture antibody proteins were mixed with PE-conjugated detection antibodies and recombinant standards or test samples and then incubated to form sandwich complexes. After acquiring sample data by flow cytometry, cytokine concentrations were calculated using the FCAP Array software (BD Biosciences).
Pharmaceutical inhibition of STAT1 phosphorylation by fludarabine and flavopiridol in imMKCLs
The effects of 2 STAT1 phosphorylation inhibitors, fludarabine and flavopiridol, on iPSC-PLT production from imMKCLs were evaluated. Briefly, imMKCLs (clone 7) were cultured under differentiation conditions (DOX-OFF) in the absence or presence of fludarabine (0 to ∼5 μM) or flavopiridol (0 to ∼100 nM). Dimethyl sulfoxide was used as a vehicle control in all conditions.
PAC-1 binding and P-selectin expression of iPSC-PLTs
Platelet activation complex (PAC)-1 binding and P-selectin levels were measured according to the protocol outlined in our previous study.10 Briefly, culture suspensions (iPSC-PLTs) were stimulated with or without phorbol-12-myristate-13-acetate (0.2 μM), adenosine triphosphate plus thrombin receptor activator peptide 6 (100 μM and 40 μM, respectively), or thrombin (1 U/mL). Subsequently, mixtures were incubated with BV421 mouse anti-human CD62P (BD Biosciences), APC-anti-human CD41a (BioLegend), and fluorescein isothiocyanate-PAC-1 (BD Biosciences) antibodies. After a 30-minute incubation at room temperature, samples were diluted with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Tyrode buffer and analyzed using a BD FACSVerse.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism software (GraphPad Software, La Jolla, CA). Data are expressed as the mean ± standard error of the mean. P values < .05 were considered significant. The total percentage of methylated CpGs in each sample was calculated by QUMA software.22 Methylation pattern results were dichotomized. The Fisher exact test was used to evaluate the presence of any significant differences. Details of the sample size used, statistics, and statistical significance are indicated in each figure legend.
Results
LIN28A regulates iPSC-PLT production through the let-7 miRNA–RALB axis in imMKCLs
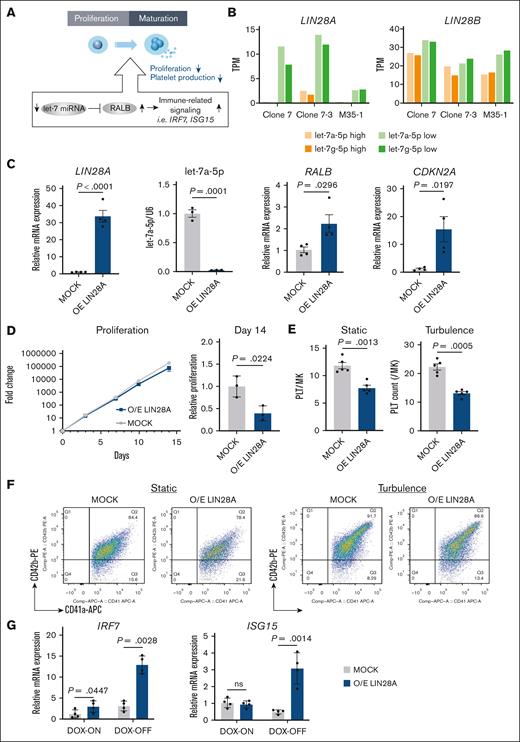
Our previous study established that the let-7 miRNA–RALB axis is a major regulator in determining immune-biased imMKCL subpopulations. Specifically, reduced let-7a-5p activity leads to elevated RALB expression and subsequent activation of immune-related signaling, particularly IFN signals. Dysregulation of these subpopulations compromises the overall imMKCL population quality, as exemplified by reduced proliferation rate, leading to impaired iPSC-PLT production (Figure 1A).15 In this study, we further investigated the regulatory mechanism upstream of the let-7–RALB axis. Our analysis focused on LIN28, a well-established developmental regulator of the let-7 family, that functions through reciprocal regulation: LIN28 proteins inhibit let-7 family maturation, whereas the let-7 family downregulates the expression of LIN28 proteins.17,18 Examination of our bulk RNA sequencing (RNA-seq) data obtained from our previous report15 demonstrated that LIN28A, but not LIN28B, was significantly upregulated in imMKCL subpopulations with lower let-7 activity (let-7low) compared to let-7high cells (Figure 1B). To examine the regulatory role of LIN28A, we tested lentiviral-mediated LIN28A overexpression, which resulted in increased messenger RNA expression of LIN28A and RALB and decreased levels of let-7a-5p (Figure 1C). These findings positioned LIN28A as an upstream regulator of the let-7–RALB axis in imMKCLs. Consistent with our hypothesis, LIN28A overexpression significantly increased cyclin-dependent kinase (CDK) inhibitor 2A (CDKN2A) expression (Figure 1C), a well-known marker of cellular senescence,24 impeded proliferation (Figure 1D), and subsequently impaired iPSC-PLT production under both static or turbulent flow conditions (Figure 1E-F). These were accompanied by the activation of IFN signaling genes, which serve as markers of immune-biased imMKCL subpopulation (Figure 1G). Collectively, these results suggest that LIN28A functions as a key regulator of the let-7 miRNA–RALB axis in imMKCLs, thereby modulating iPSC-PLT production capacity.
LIN28A regulates the let-7 miRNA–RALB axis in imMKCLs. (A) A schematic illustration depicting the let-7 miRNA–RALB axis regulating immune-related signaling, which alters the proliferation and platelet production capacity of imMKCLs. (B) LIN28A but not LIN28B shows increased expression in let-7low cells compared to let-7high cells. Bar graphs show TPM values of the indicated genes from 3 distinct imMKCL clones. (C) The lentiviral-mediated overexpression (O/E) of LIN28A significantly increased LIN28A and RALB messenger RNA (mRNA) expression while suppressing let-7a-5p expression. (D) Growth curve and relative fold change of the cell count on day 14 of MOCK and LIN28A O/E imMKCLs (clone 7). (E) LIN28A overexpression led to deficient iPSC-PLT generation by imMKCLs (clone 7). (F) Representative flow cytometry plots of iPSC-PLTs generated from MOCK or LIN28A O/E imMKCLs in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (G) LIN28A overexpression induced mRNA expression of representative immune-related genes in imMKCLs. Data are expressed as the mean ± standard error of the mean (SEM) from 3 to 4 independent experiments. Unpaired 2-tailed Student t tests were used to assess statistical significance. TPM, transcripts per kilobase million.
LIN28A regulates the let-7 miRNA–RALB axis in imMKCLs. (A) A schematic illustration depicting the let-7 miRNA–RALB axis regulating immune-related signaling, which alters the proliferation and platelet production capacity of imMKCLs. (B) LIN28A but not LIN28B shows increased expression in let-7low cells compared to let-7high cells. Bar graphs show TPM values of the indicated genes from 3 distinct imMKCL clones. (C) The lentiviral-mediated overexpression (O/E) of LIN28A significantly increased LIN28A and RALB messenger RNA (mRNA) expression while suppressing let-7a-5p expression. (D) Growth curve and relative fold change of the cell count on day 14 of MOCK and LIN28A O/E imMKCLs (clone 7). (E) LIN28A overexpression led to deficient iPSC-PLT generation by imMKCLs (clone 7). (F) Representative flow cytometry plots of iPSC-PLTs generated from MOCK or LIN28A O/E imMKCLs in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (G) LIN28A overexpression induced mRNA expression of representative immune-related genes in imMKCLs. Data are expressed as the mean ± standard error of the mean (SEM) from 3 to 4 independent experiments. Unpaired 2-tailed Student t tests were used to assess statistical significance. TPM, transcripts per kilobase million.
Epigenetic regulation of LIN28A expression via differential DNA methylation
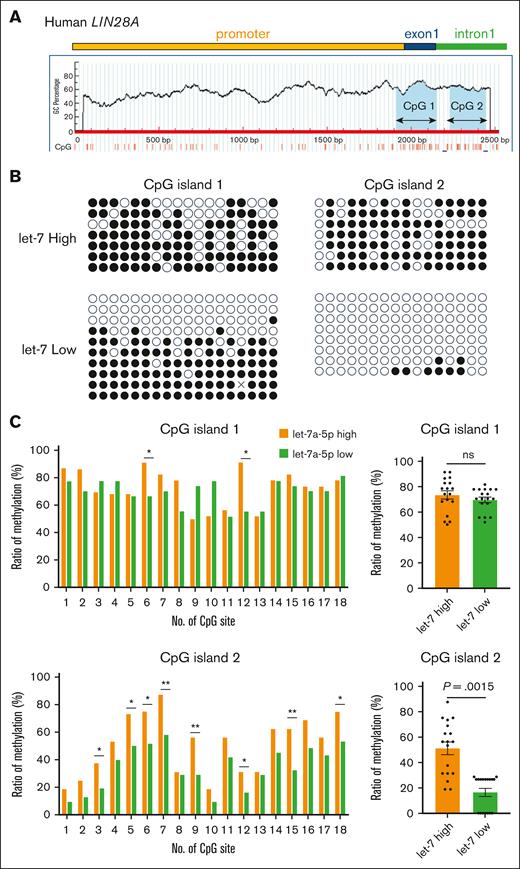
Previous studies have demonstrated that LIN28A expression is regulated by epigenetic mechanisms, including DNA methylation25 and histone modification.26 In this study, we analyzed predicted CpG islands in the LIN28A promoter region using the MethPrimer software.21 The criteria for prediction included an island size >100, GC content >50.0, and an observed-to-expected number of CpG pattern ratio >0.6. Two CpG islands were identified: one (239 bp) located in exon 1 and the other (212 bp) within intron 1 (Figure 2A). To investigate the methylation status of these regions in imMKCLs, we performed bisulfite sequencing of both CpG islands in imMKCLs. Comparative analysis of individual cytosine and guanine dinucleotides revealed distinct methylation patterns exclusively in CpG island 2, but not CpG island 1, between let-7low and let-7high cells. The total methylation rate in let-7high cells was substantially higher (51.05%) than let-7low cells (16.42%; Figure 2B-C). Specifically, we identified 8 specific CpG dinucleotides that exhibited hypomethylation in let-7low cells but were consistently hypermethylated in let-7high cells (Figure 2C). This differential methylation pattern strongly suggests that LIN28A expression in imMKCLs is regulated, at least in part, through epigenetic mechanisms, particularly related to let-7 activity.
Distinct DNA methylation at human LIN28A CpG island 2 in let-7 low and let-7 high imMKCLs. (A) A schematic representation of CpG islands in the human LIN28A gene. Two CpG islands sequences were identified using the MethPrimer platform. Bisulfite PCR was performed, and the methylation status of each CpG island was evaluated. (B) The methylation status of individual CpG dinucleotides in human LIN28A CpG islands was analyzed by bisulfite sequencing in let-7high and let-7low cells. Results are shown by methylated (closed) or unmethylated (open) circles. (C) Individual and total methylation rates of 2 CpG islands are shown. Fisher exact tests were used to assess statistical significance by QUMA software. ∗P < .05; ∗∗P < .01. ns, not significant.
Distinct DNA methylation at human LIN28A CpG island 2 in let-7 low and let-7 high imMKCLs. (A) A schematic representation of CpG islands in the human LIN28A gene. Two CpG islands sequences were identified using the MethPrimer platform. Bisulfite PCR was performed, and the methylation status of each CpG island was evaluated. (B) The methylation status of individual CpG dinucleotides in human LIN28A CpG islands was analyzed by bisulfite sequencing in let-7high and let-7low cells. Results are shown by methylated (closed) or unmethylated (open) circles. (C) Individual and total methylation rates of 2 CpG islands are shown. Fisher exact tests were used to assess statistical significance by QUMA software. ∗P < .05; ∗∗P < .01. ns, not significant.
TF binding site analysis of CpG island 2 in the human LIN28A gene
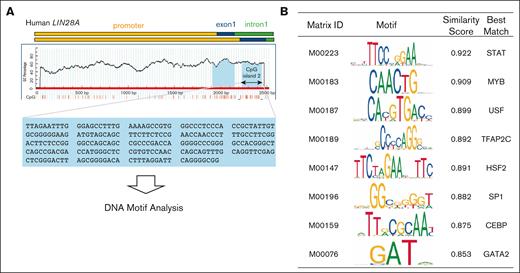
To identify transcriptional factors (TFs) that might regulate LIN28A expression involving epigenetic mechanisms, we conducted an in-depth analysis of potential binding sites within the methylation-sensitive CpG island 2 of LIN28A (212 bp; Figure 3A). Using the TFBIND algorithm,27 we identified multiple candidate TF binding motifs (Figure 3B) and confirmed the expression of candidate genes using our RNA-seq data (supplemental Figure 1). Among identified genes, STAT and C/EBP families were strong candidates given their known roles in hematopoietic development28,29 and immune system regulation.30,31 We hypothesized that members of the STAT or C/EBP families might manipulate the expression of LIN28A via DNA methylation–dependent mechanisms, in which their loss of function could potentially suppress LIN28A expression, subsequently dampening immune signaling and improving iPSC-PLT production capacity. However, investigating C/EBP family function was particularly challenging due to the extensive functional redundancy among family members in myeloid cells, in which a single gene disruption often fails to reveal their physiological roles.32 In this context, we used a dominant-negative C/EBP approach capable of antagonizing all C/EBP family members simultaneously.23 Contrary to our expectations, expression of the dominant-negative C/EBP led to increased expression of LIN28A, IRF7, and ISG15 during the proliferation stage of imMKCLs. Notably, this intervention did not affect RALB expression, imMKCL proliferation, or subsequent platelet production (supplemental Figure 2A-C). These unexpected results may reflect the complex roles of the C/EBP family in megakaryocyte development and thrombopoiesis, but further studies are needed to clarify these functions.
TF binding motif analysis of CpG island 2 in the human LIN28A gene and functional enrichment of candidate transcription factors. (A) A schematic representation of CpG island 2 in the human LIN28A gene. TF binding sites of CpG island 2 were identified using the TFBIND software. (B) The top motifs identified are listed in the table.
TF binding motif analysis of CpG island 2 in the human LIN28A gene and functional enrichment of candidate transcription factors. (A) A schematic representation of CpG island 2 in the human LIN28A gene. TF binding sites of CpG island 2 were identified using the TFBIND software. (B) The top motifs identified are listed in the table.
STAT1 knockdown enhances iPSC-PLT production through modulation of the LIN28A/let-7–RALB axis
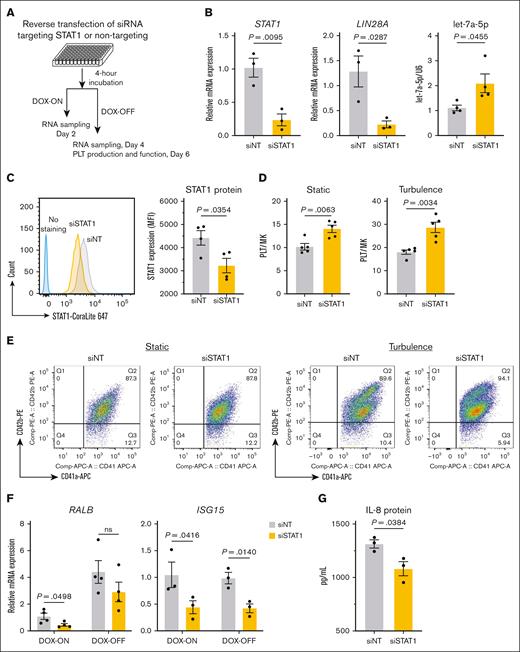
Our RNA-seq analysis revealed that STAT1 and STAT3 are expressed in imMKCLs (supplemental Figure 1). We first investigated the regulatory role of STAT1 using siRNA-mediated knockdown. A pool of siSTAT1 and siNT was used here (Figure 4A). The knockdown effectively reduced STAT1 expression at both the messenger RNA (Figure 4B) and protein levels (Figure 4C), leading to concurrent suppression of LIN28A expression and elevated let-7a-5p levels (Figure 4B). These results led us to conclude that STAT1 is a potential regulator of the LIN28A/let-7 pathway in imMKCLs. Meanwhile, cell proliferation was slightly reduced in STAT1 knockdown cells (supplemental Figure 3A), consistent with a previous report on megakaryocyte proliferation.33 The knockdown of STAT1 notably enhanced iPSC-PLT production under both static and turbulent flow conditions (Figure 4D-E), likely due to modulation of RALB and IFN signaling gene expression (Figure 4F; supplemental Figure 3B). Importantly, the functionality of iPSC-PLTs remained intact after STAT1 knockdown, as evidenced by comparable levels of annexin V binding, PAC-1 binding, and P-selectin expression between the siSTAT1 and siNT groups (supplemental Figure 3C-D). Interestingly, STAT1 knockdown also reduced the expression of CDKN2A and secretion of interleukin 8 (IL-8), a critical component of senescence-associated secretory phenotype known to diminish imMKCL quality (supplemental Figure 3B; Figure 4G).15 We also validated our key findings in the M35-1 clone, the patient-derived imMKCL used in the iPLAT1 clinical trial.9 STAT1 knockdown in M35-1 clone led to a significant improvement in iPSC-PLT production (supplemental Figure 3E-F). These findings suggest a novel connection between the STAT1 regulatory network and the senescent and aging features of imMKCLs.
STAT1 is the upstream regulator of LIN28A in imMKCLs. The siRNA SMARTpool designed against human STAT1 (siSTAT1) and a siNT were transfected into imMKCLs. (A) A schematic illustration of the experimental procedure of siRNA-mediated STAT1 knockdown in imMKCLs. (B) STAT1 knockdown significantly decreased STAT1 and LIN28A mRNA expression and increased let-7a-5p expression. (C) Intracellular protein levels of STAT1 (mean fluorescence intensity detected in siNT or siSTAT1 imMKCLs by intracellular flow cytometry. STAT1 expression was compared using a histogram overlay in the left panel. (D) STAT1 knockdown improved iPSC-PLT generation by imMKCLs (clone 7). (E) Representative flow cytometry plots of iPSC-PLTs generated from siNT or siSTAT1 imMKCLs in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (F) STAT1 knockdown decreased mRNA expression of representative immune-related genes in imMKCLs. (G) The secretion of IL-8 from clone 7 and clone 7-3 during the maturation stage (DOX-OFF day 6). Data are expressed as the mean ± SEM from 3 to 4 independent experiments. Unpaired 2-tailed Student t tests were used to assess statistical significance. IL-8, interleukin 8; MK, megakaryocyte; ns, not significant.
STAT1 is the upstream regulator of LIN28A in imMKCLs. The siRNA SMARTpool designed against human STAT1 (siSTAT1) and a siNT were transfected into imMKCLs. (A) A schematic illustration of the experimental procedure of siRNA-mediated STAT1 knockdown in imMKCLs. (B) STAT1 knockdown significantly decreased STAT1 and LIN28A mRNA expression and increased let-7a-5p expression. (C) Intracellular protein levels of STAT1 (mean fluorescence intensity detected in siNT or siSTAT1 imMKCLs by intracellular flow cytometry. STAT1 expression was compared using a histogram overlay in the left panel. (D) STAT1 knockdown improved iPSC-PLT generation by imMKCLs (clone 7). (E) Representative flow cytometry plots of iPSC-PLTs generated from siNT or siSTAT1 imMKCLs in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (F) STAT1 knockdown decreased mRNA expression of representative immune-related genes in imMKCLs. (G) The secretion of IL-8 from clone 7 and clone 7-3 during the maturation stage (DOX-OFF day 6). Data are expressed as the mean ± SEM from 3 to 4 independent experiments. Unpaired 2-tailed Student t tests were used to assess statistical significance. IL-8, interleukin 8; MK, megakaryocyte; ns, not significant.
Pharmacological inhibition of STAT1 enhances iPSC-PLT production under turbulent flow conditions
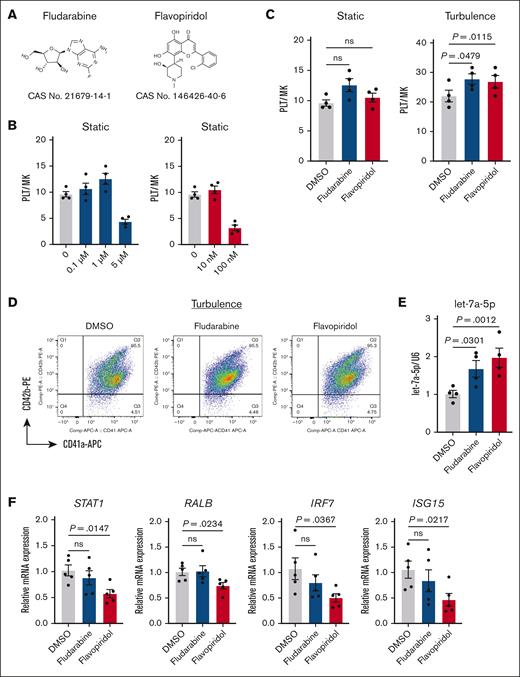
Given that STAT1 activity is primarily regulated by posttranslational modifications, particularly phosphorylation of STAT1 tyrosine and serine residues,34 we hypothesized that pharmacological inhibition of STAT1 phosphorylation could augment iPSC-PLT production. To test this, we evaluated the effects of 2 known STAT1 phosphorylation inhibitors, fludarabine35,36 and flavopiridol37 (Figure 5A). Dose optimization experiments were performed across a range of concentrations (0 to ∼5 μM for fludarabine and 0 to ∼100 nM for flavopiridol), with maximal efficacy on iPSC-PLT production observed at 1-μM fludarabine and 10-nM flavopiridol (Figure 5B). Although both inhibitors elicited only modest, nonsignificant increases in iPSC-PLT yield under static cultures, they significantly enhanced production under turbulent flow conditions (Figure 5C-D). Further analysis of their effects on gene expression revealed distinct mechanism. Although flavopiridol treatment upregulated let-7a-5p and downregulated STAT1, RALB, and IFN signaling, which aligns well with our hypothesis, fludarabine did not, suggesting an alternative mechanism (Figure 5E-F). Nevertheless, our findings establish STAT1 as a critical upstream regulator of the LIN28A/let-7/RALB axis, modulating iPSC-PLT production.
Pharmacological inhibition of STAT1 enhances iPSC-PLT production under turbulent flow conditions. (A) Chemical structures of fludarabine and flavopiridol, the 2 established inhibitors for STAT1 phosphorylation. (B) Dose optimization on iPSC-PLT production of fludarabine (0 to ∼5 μM) and flavopiridol (0 to ∼100 nM). (C) iPSC-PLT generation by imMKCLs (clone 7) in the absence and presence of fludarabine (1 μM) or flavopiridol (10 nM) under static or turbulent flow conditions. (D) Representative flow cytometry plots of iPSC-PLTs generated in the absence and presence of fludarabine (1 μM) or flavopiridol (10 nM) in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (E) Fludarabine (1 μM) or flavopiridol (10 nM) treatments elevated let-7a-5p expression. (F) Flavopiridol (10 nM) treatment reduced mRNA expression of representative immune-related genes in imMKCLs at the maturation stage. Data are expressed as the mean ± SEM from 4 to 5 independent experiments. Statistical significance was assed using 1-way analysis of variance or unpaired 2-tailed Student t tests. DMSO, dimethyl sulfoxide.
Pharmacological inhibition of STAT1 enhances iPSC-PLT production under turbulent flow conditions. (A) Chemical structures of fludarabine and flavopiridol, the 2 established inhibitors for STAT1 phosphorylation. (B) Dose optimization on iPSC-PLT production of fludarabine (0 to ∼5 μM) and flavopiridol (0 to ∼100 nM). (C) iPSC-PLT generation by imMKCLs (clone 7) in the absence and presence of fludarabine (1 μM) or flavopiridol (10 nM) under static or turbulent flow conditions. (D) Representative flow cytometry plots of iPSC-PLTs generated in the absence and presence of fludarabine (1 μM) or flavopiridol (10 nM) in static or turbulent flow conditions. CD41+CD42b+ platelets were identified from a platelet-sized gate. (E) Fludarabine (1 μM) or flavopiridol (10 nM) treatments elevated let-7a-5p expression. (F) Flavopiridol (10 nM) treatment reduced mRNA expression of representative immune-related genes in imMKCLs at the maturation stage. Data are expressed as the mean ± SEM from 4 to 5 independent experiments. Statistical significance was assed using 1-way analysis of variance or unpaired 2-tailed Student t tests. DMSO, dimethyl sulfoxide.
Discussion
We initiated the first-in-human clinical trial of transfusion by autologous iPSC-PLTs.9 The trial involved a 52-year-old patient suffering from aplastic anemia and allogeneic platelet transfusion refractoriness, who had been treated with standard medicine. We generated iPSCs from the patient’s circulating T cells and derived autologous imMKCLs.9,10 Through 3 cohort clinical trials and further in-depth study, we recognized distinct differences between the aged donor–derived M35-1 imMKCL clone and our control clone, clone 7 (established from neonate fibroblast–derived iPSCs), in terms of both iPSC-PLT production and gene expression profile.15 Based on such points, we identified a novel regulatory mechanism controlling iPSC-PLT production capacity, with a particular focus on the regulation of the LIN28A-let-7–RALB axis. We demonstrate that LIN28A expression, regulated by DNA methylation and STAT1, acts as a critical upstream modulator of the let-7–RALB pathway, ultimately influencing platelet production capacity from imMKCLs. Our findings provide new insights into how TFs and epigenetic modifications determine imMKCL differentiation, senescence, and subsequent iPSC-PLT emergence and quality, with significant implications for optimizing iPSC-PLT manufacturing.
One of the major findings of this study is the identification of LIN28A as a key regulator of the let-7 miRNA–RALB axis in imMKCLs (Figure 1). Both LIN28A and LIN28B are critical pluripotency genes highly expressed in undifferentiated embryonic stem cells and iPSCs.26 Human hematopoietic development at the transition stage from fetal liver to adult bone marrow is marked by let-7 upregulation and accompanied by LIN28B downregulation.38 In murine thrombopoiesis, Lin28b also exhibits high expression in fetal megakaryocytes and negatively regulates the levels of platelet P-selectin.39 Moreover, the developmentally restricted expression of Lin28b suppresses a megakaryocyte-biased hematopoietic stem cell pool.40 We previously demonstrated that suppressed endogenous let-7a-5p activity leads to RALB upregulation, thereby accelerating the development of immune-biased imMKCL subpopulations that compromise the overall quality of imMKCLs.15 Our imMKCLs, which mimic early embryonic megakaryocyte development, represent a heterogeneous population with varying let-7 expression and activity, resulting in distinct iPSC-PLT production capacities and senescence phenotypes.15 We found difference in the expression of LIN28A, but not LIN28B, between let-7low and let-7high cells (Figure 1), and sequential analysis of these populations revealed that the differential expression of LIN28A to be epigenetically regulated by DNA methylation (Figure 2). LIN28A overexpression experiments demonstrated its role as a key regulator controlling the senescence phenotype in imMKCLs that recapitulate embryonic development. Altogether, our findings demonstrate that epigenetic modulation of LIN28A expression, particularly through DNA methylation, contributes to cellular heterogeneity within imMKCLs.
Meanwhile, our investigation of TF regulation revealed STAT1 as a key modulator of LIN28A expression, whereas the C/EBP family's involvement was more intricate than initially hypothesized (Figure 3; supplemental Figure 2). The STAT family has been extensively studied as central mediators for IFNs and innate inflammatory response, with established roles in hematopoiesis.30,41 In particular, thrombopoietin, the primary megakaryocyte-potentiating factor, activates multiple STAT proteins (STAT1, STAT3, and STAT5) through its receptor c-Mpl in megakaryocytes and other hematopoietic cells.42 Previous studies demonstrated that Stat3 activation promotes the expansion of megakaryocytic progenitor cells,43 whereas Stat1 can rescue multiple defects in Gata1-deficient megakaryopoiesis in mice.33 Moreover, Stat1 deletion in mice shifts hematopoietic balance by reducing megakaryopoiesis while favoring erythropoiesis.44 In our ex vivo human iPSC–PLT manufacturing system, STAT1 knockdown yielded different results, because it suppressed LIN28A expression and increased let-7a-5p levels, leading to significantly enhanced iPSC-PLT production without compromising platelet functionality, as evidenced by preserved activation markers (PAC-1 and P-selectin; supplemental Figure 3). Intriguingly, STAT1 depletion reduced the expression of cellular senescence markers, including CDKN2A and the key senescence-associated secretory phenotype factor, IL8. Although coupregulation of STAT1 and CDKN2A has been observed in senescent cells and cancers, their regulatory relationship appears to be context dependent.45 These findings highlight critical and evident differences between murine and human hematopoiesis. We previously demonstrated that IFN-α, an upstream regulator of STAT1, impairs both imMKCL proliferation and iPSC-PLT generation. However, RNA-seq analysis revealed no detectable expression of the IFN family in imMKCLs.15 Alternative STAT1 activation pathways, including damage-associated molecular patterns via toll-like receptor signaling,46 the cGAS/STING pathway, and interleukin 6 signaling,47 may be involved. We hypothesize that senescent imMKCLs may release damage-associated molecular patterns and interleukins, suggesting that identifying STAT1 activators could lead to optimized culture conditions, preventing imMKCL quality decline. These findings indicate that STAT1 inhibition could benefit clinical applications by enhancing PLT production while preventing cellular senescence, a major obstacle to maintaining the long-term stability and quality of imMKCLs.
Fludarabine, a purine analog, and flavopiridol, a CDK inhibitor, are both clinically applied agents for treating leukemias.48,49 In our study, pharmacological inhibition of STAT1 phosphorylation by these compounds exhibited promotive effects on iPSC-PLT production from imMKCLs via distinct mechanisms (Figure 5). Although primarily recognized for their roles in inducing cell cycle arrest and apoptosis,36 our findings reveal an unexpected functional benefit of these compounds in the context of ex vivo platelet production. Given their pleiotropic effects and cytotoxicity, further studies are needed to clarify their mechanisms, optimize dosing, and assess safety for potential clinical translation.
In summary, our study identifies key regulatory networks involving STAT1, LIN28A, and the let-7–RALB axis that determine iPSC-PLT production capacity and quality and provides new insights into the reality of cellular heterogeneity in imMKCLs. These findings advance not only the comprehensive understanding of platelet biogenesis but also practical approaches for optimizing platelet manufacturing.
Acknowledgments
The authors thank A. Iwama (Tokyo University) for sharing the retroviral vector pGCsam of dominant-negative C/EBP; K. Tsujimura and K. Nonomura for technical support; Y. Nakata (Chiba University) for critical discussion; A. Matsumura, A. Hinoshita, and M. Watanabe for secretary support; and K. Hui (Kyoto Univeristy) for critical reading.
This study was supported, in part, by the Project for Core Center for iPS Cell Research (JP23bm1323001; K.E. and N.S.) and the Project for Regenerative/Cellular Medicine and Gene Therapies under grant numbers JP22bm0704051 (K.E.) and JP23bm1123028 (N.S. and S.J.C.) from Japan Agency for Medical Research and Development; a grant-in-aid for scientific research (S) (21H05047; K.E.), a grant-in-aid for early-career scientists (22K13124; S.J.C.), a grant-in-aid for challenging research (23K18299; K.E.), and a grant-in-aid for scientific research (C) (25K10397; S.J.C.) from the Japan Society for the Promotion of Science; grants from SENSHIN Medical Research Foundation (S.J.C.) and SHIONOGI Infectious Disease Research Promotion Foundation (2024N087; S.J.C.); and a grant from the Canon Foundation (K.E.) and the research and development grant from the New Energy and Industrial Technology Development Organization (JPNP23028; K.E.).
Authorship
Contribution: K.H. and S.J.C. designed and performed experiments, analyzed data, interpreted results, prepared figures, and wrote the manuscript; K.F. and A.K. assisted with experiments; N.S. and M.E. provided intellectual contributions and edited the manuscript; N.T. contributed to the experimental design, motif analysis, and data interpretation and edited the manuscript; and K.E. managed the overall project, contributed to the funding acquisition, experimental design, data interpretation, and edited the manuscript.
Conflict-of-interest disclosure: K.E. was a founder of Megakaryon Co, Ltd but has no stock currently, and the interests of K.E. were reviewed and managed by Kyoto University in accordance with its conflict-of-interest policies. The remaining authors declare no competing financial interests.
Correspondence: Si Jing Chen, Department of Regenerative Medicine, Graduate School of Medicine, Chiba University, 1-8-1, Inohana, Chuo-ku, Chiba-shi, Chiba 260-0856 Japan; email: sjchen@chiba-u.jp; and Koji Eto, Department of Clinical Application, Center for iPS Cell Research and Application, Kyoto University, 53 Kawaharacho, Shogoin, Sakyoku, Kyoto 606-8507, Japan; email: eto.koji.8a@kyoto-u.ac.jp.
References
Author notes
K.H. and S.J.C. contributed equally to this study.
The raw data sets were deposited in the DNA Data Bank of Japan database (association codes DRA016355).
The RNA sequencing (RNA-seq) data sets were reanalyzed and generated using the RNA-seq data from our previous work.
The full-text version of this article contains a data supplement.