Key Points
Clinically significant extravascular hemolysis affects 20%–25% of patients with PNH receiving treatment with ravulizumab or eculizumab.
Fatigue was mostly mild and remained stable during treatment in patients with clinically significant extravascular hemolysis.
Visual Abstract
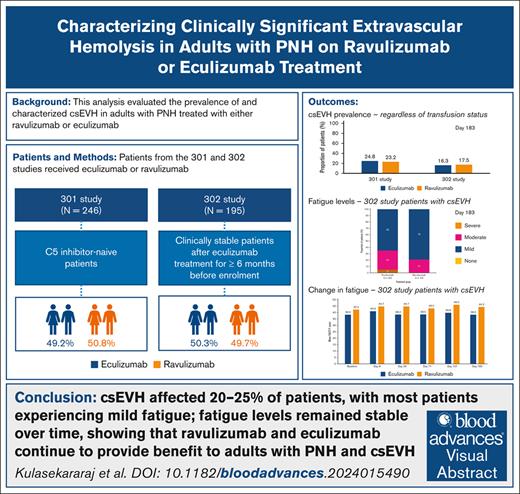
In patients with paroxysmal nocturnal hemoglobinuria (PNH), the complement component 5 (C5) inhibitors ravulizumab and eculizumab control terminal complement activity and intravascular hemolysis, which are drivers of morbidity and mortality. During C5 inhibitor treatment, ongoing C3 fragment deposition on surviving PNH red blood cells may cause clinically significant extravascular hemolysis (csEVH) in some patients. csEVH is not thought to be life threatening but may cause symptomatic anemia and need for transfusion. This post hoc analysis of studies 301 (NCT02946463) and 302 (NCT03056040) evaluated the prevalence of csEVH (symptomatic anemia [hemoglobin levels <9.5 g/dL] with absolute reticulocyte count ≥120 × 109/L) in adult patients with PNH treated with ravulizumab or eculizumab for 6 months (183 days). The association between csEVH and fatigue (measured using the Functional Assessment of Chronic Illness Therapy - Fatigue [FACIT-F] scale) was also evaluated in study 302 patients with stable disease. On day 183 of studies 301 and 302, csEVH prevalence was 23.2% (29/125) and 20.2% (19/94) with ravulizumab and 24.8% (30/121) and 21.3% (20/94) with eculizumab, respectively. All patients in study 302 with csEVH experienced fatigue, which was mostly mild (ravulizumab, 79%; eculizumab, 65%) or moderate (ravulizumab, 21%; eculizumab, 30%). FACIT-F scores remained stable from baseline (ravulizumab, 42.2; eculizumab, 38.3) to day 183 (ravulizumab, 44.3; eculizumab, 38.3), close to general population normative values. This analysis demonstrated that csEVH affected 20% to 25% of patients with PNH, most of whom experienced mild fatigue, with fatigue remaining stable during treatment. Ravulizumab and eculizumab continue to provide benefit to adults with PNH with csEVH.
Introduction
Uncontrolled terminal complement activation on the surface of red (RBCs) and white blood cells and platelets plays a critical role in paroxysmal nocturnal hemoglobinuria (PNH), a rare and chronic hematologic disorder.1,2 In PNH, an acquired somatic mutation of the PIGA gene affects the expression of glycosylphosphatidylinositol (GPI), resulting in the absence of or a decrease in GPI-anchored proteins CD55 and CD59 on hematopoietic progenitor cells and their progeny blood cells.1,3 RBCs lacking GPI-anchored proteins are susceptible to complement-mediated lysis by the membrane attack complex3 and to the activation of platelets and white blood cells, ultimately resulting in intravascular hemolysis (IVH), increased risk of thrombosis, organ damage, and death.1,3,4
The aim of most treatments for patients with PNH is to achieve control of terminal complement activity and IVH to prevent thrombosis and organ damage, thereby reducing morbidity and mortality. Currently, there are multiple approved treatment options that may be considered for patients with PNH, including the complement component 5 (C5) inhibitors ravulizumab (the current standard of care, where available) and eculizumab. Eculizumab is a first-generation C5 inhibitor that received US Food and Drug Administration approval for the treatment of PNH in 2007,5 which is administered via IV infusion every 2 weeks. Ravulizumab, engineered through select modifications to eculizumab, was approved in 2018.6 Ravulizumab has a weight-based dosing regimen and fourfold longer terminal half-life than eculizumab, allowing for immediate, complete, and sustained terminal complement blockade. This results in a more favorable pharmacokinetic/pharmacodynamic profile than the previous treatment, with a less frequent regimen for IV infusion (every 8 weeks).6-8
The approval of ravulizumab was supported by 2 pivotal studies. The phase 3 clinical study 301 (NCT02946463) compared ravulizumab vs eculizumab in patients with PNH who were previously naive to C5 inhibitors.9 Meanwhile, the phase 3 clinical study 302 (NCT03056040) enrolled stable, eculizumab-experienced adults with PNH who were then treated with either ravulizumab or eculizumab.10 Both studies demonstrated that ravulizumab was noninferior to eculizumab.9,10 Four-year data from the 302 study demonstrated effective long-term control of terminal complement activity and IVH, along with maintained patient survival, with a 98.4% survival rate (0.6 deaths per 100 patient-years).11 Outcomes from another clinical study and a real-world registry12-14 have provided evidence for the efficacy of ravulizumab or eculizumab for the treatment of patients with PNH and improvements in morbidity and mortality.15-18
If terminal complement inhibition is incomplete during C5 inhibitor treatment, there is a risk that patients will experience breakthrough hemolysis, characterized by the return of IVH and reappearance of PNH symptoms (fatigue, hemoglobinuria, and abdominal pain) in the presence of a lactate dehydrogenase (LDH) level ≥2 × upper limit of normal (ULN; 246 U/L); in C5 inhibitor–naive patients, these events must take place after a prior reduction of LDH level to <1.5 × ULN.19 Alternatively, during treatment with C5 inhibitors, patients with PNH may experience extravascular hemolysis (EVH). EVH arises from the absence of CD55 (a regulator of C3 convertase) on the surface of RBCs.20 This causes ongoing C3 deposition on surviving PNH RBCs, making them susceptible to phagocytosis in the liver or spleen.3,20,23 Because RBCs are destroyed expeditiously by terminal complement activation-driven IVH, EVH is negligible in patients with PNH not receiving C5 inhibitor treatment.21 EVH is not thought to be life threatening4 and many patients do not experience EVH symptoms or require specific treatment for EVH.22 Some patients experience clinically significant EVH (csEVH), and these patients may experience symptomatic anemia and possibly require blood transfusions.22 A symptom associated with EVH-related anemia is fatigue,4,23 which can be disabling in patients with PNH, negatively affecting patients’ quality of life (QoL).4,24 However, fatigue is often disproportionate to the level of hemoglobin and is more closely associated with IVH and terminal complement activity.4,21,23 This has been supported by evidence from clinical studies that demonstrated that eculizumab and ravulizumab treatment improved fatigue levels in patients with PNH as a result of immediate reduction in LDH (a marker of IVH) levels, whereas hemoglobin levels remained stable.25,26
The aim of this analysis was to evaluate the prevalence of csEVH in adults with PNH treated with a C5 inhibitor (ravulizumab or eculizumab) from the 301 and 302 studies. Additionally, the association between csEVH and fatigue was evaluated in patients with stable disease from the 302 study.
Methods
Study design
The 301 (NCT02946463) and 302 (NCT03056040) clinical studies were phase 3, randomized, open-label, active-controlled, noninferiority, multicenter studies, as previously described.9,10 Throughout the 26-week primary evaluation period of the 301 and 302 studies, patients were randomly assigned to receive weight-based ravulizumab dosing (every 8 weeks) or eculizumab maintenance dosing (every 2 weeks) (supplemental Material). The primary evaluation period was followed by an extension period of up to 6 or 4 years (301 and 302 studies, respectively), during which all patients received ravulizumab.
Patients
As previously detailed, patients were aged ≥18 years and had a confirmed diagnosis of PNH in both the 301 and 302 studies.9,10 In the 301 study, C5 inhibitor–naive patients experienced PNH-related signs or symptoms within 3 months of screening, including fatigue, hemoglobinuria, or anemia.9 In contrast, patients in the 302 study were clinically stable after receiving eculizumab for ≥6 months before enrollment.11 All participants or their legal representative provided informed consent.10
Outcomes
csEVH
This post hoc analysis reports the prevalence of csEVH in patients with PNH who were either naive to C5 inhibitors and experienced PNH symptoms (301 study) or clinically stable after a minimum of 6 months on eculizumab (302 study). Owing to the lack of a universal definition, for the purposes of this analysis, csEVH was defined as symptomatic anemia (hemoglobin levels < 9.5 g/dL) with absolute reticulocyte count ≥120 × 109/L at day 183, with or without blood transfusion, a definition used previously in the ALPHA study, a clinical trial in patients with PNH.27 Additionally, to determine whether the prevalence of csEVH changed over time in patients with stable disease from the 302 study, the proportion of patients who met the hemoglobin levels and reticulocyte count criteria was also evaluated at baseline and week 52 in this population.
Fatigue
Fatigue levels on day 183 were recorded for patients experiencing csEVH in the 302 study using the Functional Assessment of Chronic Illness Therapy - Fatigue (FACIT-F) score. The FACIT-F score ranges from 0 to 52, with fatigue levels classified as mild (≥40), moderate (≤20 to <40), or severe (<20). Change in fatigue levels was assessed over time from baseline to day 183 in patients from the 302 study. The association between FACIT-F score and hemoglobin levels was assessed in patients with baseline hemoglobin <9.5 g/dL.
Fatigue levels were also evaluated in patients with csEVH from the 302 trial who received transfusions any time between baseline and day 183 and who had LDH levels between >1 × ULN and ≤1.5 × ULN, or >1.5 × ULN on day 183.
QoL
QoL was evaluated in patients with csEVH from the 302 trial, measured over time from baseline to day 183 using the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire - Core 30 Scale (EORTC QLQ-C30).28 The EORTC QLQ-C30 was used to assess global health status/QoL, physical functioning, emotional functioning, cognitive functioning, social functioning, role functioning, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. All scales and single-item measures range from 1 to 100. High scores for global health status/QoL and functional scales indicate a high level of QoL and functioning, whereas high scores for symptoms indicate a high level of symptomatology.
Statistical analysis
Given the post hoc nature of the analyses, descriptive statistics are presented for all end points.
Results
Patient demographics and clinical characteristics
As previously detailed, 246 patients were included in the primary evaluation period of the 301 study; 125 (50.8%) received ravulizumab and 121 (49.2%) eculizumab (Table 1). In the 302 study, of the 195 patients included in the primary evaluation period, 97 (49.7%) received ravulizumab and 98 (50.3%) eculizumab. Patient characteristics were generally similar between the treatment groups (Table 2).
Patient demographics and clinical characteristics at baseline in the 301 study
| Variable . | Eculizumab (n = 121) . | Ravulizumab (n = 125) . | Total (N = 246) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 69 (57.0) | 65 (52.0) | 134 (54.5) |
| Female | 52 (43.0) | 60 (48.0) | 112 (45.5) |
| Age at PNH diagnosis, mean ± SD (range), y | 39.6 ± 16.7 (13-82) | 37.9 ± 14.9 (15-81) | 38.7 ± 15.8 (13-82) |
| Age at first infusion, mean ± SD (range), y | 46.2 ± 16.24 (18-86) | 44.8 ± 15.16 (18-83) | 45.5 ± 15.69 (18-86) |
| Race, n (%) | |||
| White | 50 (41.3) | 43 (34.4) | 93 (37.8) |
| Asian | 57 (47.1) | 72 (57.6) | 129 (52.4) |
| African American | 4 (3.3) | 2 (1.6) | 6 (2.4) |
| American Indian or Alaska Native | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Not reported | 5 (4.1) | 3 (2.4) | 8 (3.3) |
| Other | 4 (3.3) | 4 (3.2) | 8 (3.3) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 101 (83.5) | 116 (92.8) | 217 (88.2) |
| Hispanic or Latino | 13 (10.7) | 5 (4.0) | 18 (7.3) |
| Not reported | 4 (3.3) | 2 (1.6) | 6 (2.4) |
| Missing/unknown | 3 (2.5) | 2 (1.6) | 5 (2.0) |
| Baseline weight, mean ± SD (range), kg | 69.2 ± 14.86 (45-113) | 68.2 ± 15.58 (40-115) | 68.7 ± 15.21 (40-115) |
| LDH, mean ± SD (range), U/L | 1578.3 ± 727.1 (423.5-3139.5) | 1633.5 ± 778.8 (378.0-3759.5) | 1606.4 ± 752.7 (378.0-3759.5) |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 13.7 ± 17.7 (0.1-95.3) | 12.4 ± 20.5 (0.1-99.5) | 13.0 ± 19.2 (0.1-99.5) |
| RBC type III | 25.2 ± 16.9 (0.4-75.6) | 26.3 ± 17.2 (0.1-82.0) | 25.8 ± 17.1 (0.1-82.0) |
| Total RBC | 38.7 ± 23.2 (2.2-98.0) | 38.4 ± 23.7 (3.0-99.6) | 38.6 ± 23.4 (2.2-99.6) |
| Granulocytes | 85.3 ± 19.0 (8.0-100.0) | 84.2 ± 21.0 (4.2-99.9) | 84.7 ± 20.0 (4.2-100.0) |
| Monocytes | 89.2 ± 15.2 (17.0-99.9) | 86.9 ± 18.1 (8.5-99.9) | 88.0 ± 16.7 (8.5-99.9) |
| Patients with a history of pRBC/whole blood transfusion, n (%)∗ | 100 (82.6) | 103 (82.4) | 203 (82.5) |
| Number of pRBC/whole blood transfusions, total, mean ± SD (range)∗ | 572 5.7 ± 5.53 (1-28) | 677 6.6 ± 6.04 (1-28) | 1249 6.2 ± 5.80 (1-28) |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 861 8.6 ± 7.9 (1-32) | 925 9.0 ± 7.7 (1-44) | 1786 8.8 ± 7.8 (1-44) |
| History of PNH-associated conditions, n (%) | |||
| Aplastic anemia | 38 (31.4) | 41 (32.8) | 79 (32.1) |
| Myelodysplastic syndrome | 6 (5.0) | 7 (5.6) | 13 (5.3) |
| Variable . | Eculizumab (n = 121) . | Ravulizumab (n = 125) . | Total (N = 246) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 69 (57.0) | 65 (52.0) | 134 (54.5) |
| Female | 52 (43.0) | 60 (48.0) | 112 (45.5) |
| Age at PNH diagnosis, mean ± SD (range), y | 39.6 ± 16.7 (13-82) | 37.9 ± 14.9 (15-81) | 38.7 ± 15.8 (13-82) |
| Age at first infusion, mean ± SD (range), y | 46.2 ± 16.24 (18-86) | 44.8 ± 15.16 (18-83) | 45.5 ± 15.69 (18-86) |
| Race, n (%) | |||
| White | 50 (41.3) | 43 (34.4) | 93 (37.8) |
| Asian | 57 (47.1) | 72 (57.6) | 129 (52.4) |
| African American | 4 (3.3) | 2 (1.6) | 6 (2.4) |
| American Indian or Alaska Native | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Not reported | 5 (4.1) | 3 (2.4) | 8 (3.3) |
| Other | 4 (3.3) | 4 (3.2) | 8 (3.3) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 101 (83.5) | 116 (92.8) | 217 (88.2) |
| Hispanic or Latino | 13 (10.7) | 5 (4.0) | 18 (7.3) |
| Not reported | 4 (3.3) | 2 (1.6) | 6 (2.4) |
| Missing/unknown | 3 (2.5) | 2 (1.6) | 5 (2.0) |
| Baseline weight, mean ± SD (range), kg | 69.2 ± 14.86 (45-113) | 68.2 ± 15.58 (40-115) | 68.7 ± 15.21 (40-115) |
| LDH, mean ± SD (range), U/L | 1578.3 ± 727.1 (423.5-3139.5) | 1633.5 ± 778.8 (378.0-3759.5) | 1606.4 ± 752.7 (378.0-3759.5) |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 13.7 ± 17.7 (0.1-95.3) | 12.4 ± 20.5 (0.1-99.5) | 13.0 ± 19.2 (0.1-99.5) |
| RBC type III | 25.2 ± 16.9 (0.4-75.6) | 26.3 ± 17.2 (0.1-82.0) | 25.8 ± 17.1 (0.1-82.0) |
| Total RBC | 38.7 ± 23.2 (2.2-98.0) | 38.4 ± 23.7 (3.0-99.6) | 38.6 ± 23.4 (2.2-99.6) |
| Granulocytes | 85.3 ± 19.0 (8.0-100.0) | 84.2 ± 21.0 (4.2-99.9) | 84.7 ± 20.0 (4.2-100.0) |
| Monocytes | 89.2 ± 15.2 (17.0-99.9) | 86.9 ± 18.1 (8.5-99.9) | 88.0 ± 16.7 (8.5-99.9) |
| Patients with a history of pRBC/whole blood transfusion, n (%)∗ | 100 (82.6) | 103 (82.4) | 203 (82.5) |
| Number of pRBC/whole blood transfusions, total, mean ± SD (range)∗ | 572 5.7 ± 5.53 (1-28) | 677 6.6 ± 6.04 (1-28) | 1249 6.2 ± 5.80 (1-28) |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 861 8.6 ± 7.9 (1-32) | 925 9.0 ± 7.7 (1-44) | 1786 8.8 ± 7.8 (1-44) |
| History of PNH-associated conditions, n (%) | |||
| Aplastic anemia | 38 (31.4) | 41 (32.8) | 79 (32.1) |
| Myelodysplastic syndrome | 6 (5.0) | 7 (5.6) | 13 (5.3) |
pRBC, packed RBC; SD, standard deviation.
In the 12 months before the first study dose of ravulizumab or eculizumab.
Patient demographics and clinical characteristics at baseline in the 302 study
| Variable . | Eculizumab (n = 98) . | Ravulizumab (n = 97) . | Total (N = 195) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 48 (49.0) | 50 (51.5) | 98 (50.3) |
| Female | 50 (51.0) | 47 (48.5) | 97 (49.7) |
| Age at PNH diagnosis, mean ± SD (range), y | 36.8 ± 14.14 (11-74) | 34.1 ± 14.41 (6-73) | 35.5 ± 14.30 (6-74) |
| Age at first eculizumab infusion, mean ± SD (range), y | 43.2 ± 13.93 (14-74) | 40.7 ± 14.30 (14-74) | 42.0 ± 14.14 (14-74) |
| Race, n (%) | |||
| White | 61 (62.2) | 50 (51.5) | 111 (56.9) |
| Asian | 19 (19.4) | 23 (23.7) | 42 (21.5) |
| African American | 3 (3.1) | 5 (5.2) | 8 (4.1) |
| Not reported | 13 (13.3) | 13 (13.4) | 26 (13.3) |
| Other | 1 (1.0) | 2 (2.1) | 3 (1.5) |
| Unknown | 1 (1.0) | 3 (3.1) | 4 (2.1) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 77 (78.6) | 76 (78.4) | 153 (78.5) |
| Hispanic or Latino | 4 (4.1) | 3 (3.1) | 7 (3.6) |
| Not reported | 17 (17.3) | 15 (15.5) | 32 (16.4) |
| Missing/unknown | 0 | 3 (3.1) | 3 (1.5) |
| Baseline weight, mean ± SD (range), kg | 73.4 ± 14.6 (44-104) | 72.4 ± 16.8 (45-129) | 72.9 ± 15.7 (44-129) |
| LDH, mean ± SD (range), U/L | 235.2 ± 49.7 (100.0-365.5) | 228.0 ± 48.7 (135.0-383.5) | 231.64 ± 49.2 (100.0-383.5) |
| Hemoglobin, mean ± SD (range), g/dL | 10.9 ± 1.8 (4.8-15.5) | 11.1 ± 1.8 (7.5-16.1) | 11.0 ± 1.8 (4.8-16.1) |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 16.3 ± 23.6 (0.1-91.9) | 14.9 ± 19.6 (0.1-80.9) | 15.6 ± 21.6 (0.1-80.9) |
| RBC type III | 43.5 ± 29.7 (0.9-99.4) | 44.6 ± 30.5 (0.0-98.8) | 44.0 ± 30.0 (0.0-99.4) |
| Total RBC | 59.5 ± 31.4 (1.5-99.6) | 60.7 ± 32.5 (0.1-99.8) | 60.1 ± 31.9 (0.1-99.8) |
| Granulocytes | 84.0 ± 21.4 (3.3-99.8) | 82.6 ± 23.6 (7.4-99.9) | 83.3 ± 22.5 (3.3-99.9) |
| Monocytes | 86.1 ± 19.7 (12.1-99.9) | 85.6 ± 20.5 (11.2-99.9) | 85.9 ± 20.0 (11.2-99.9) |
| Patients with a history of pRBC/whole blood transfusion, n (%)∗ | 12 (12.2) | 13 (13.4) | 25 (12.8) |
| Number of pRBC/whole blood transfusions, total, mean ± SD (range)∗ | 30 2.5 ± 2.3 (1-8) | 64 4.9 ± 5.5 (1-17) | 94 3.8 ± 4.4 (1-17) |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 50 4.2 ± 3.8 (2.0-15.0) | 103 7.9 ± 8.8 (1.0-32.0) | 153 6.1 ± 7.0 (1-32) |
| FACIT-F score, mean ± SD (range) | 40.7 ± 9.5 (16.0-52.0) | 42.5 ± 9.4 (1.0-52.0) | 41.6 ± 9.5 (1.0-52.0) |
| History of PNH-associated conditions, n (%) | |||
| Aplastic anemia | 39 (39.8) | 34 (35.1) | 73 (37.4) |
| Myelodysplastic syndrome | 6 (6.1) | 3 (3.1) | 9 (4.6) |
| Variable . | Eculizumab (n = 98) . | Ravulizumab (n = 97) . | Total (N = 195) . |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 48 (49.0) | 50 (51.5) | 98 (50.3) |
| Female | 50 (51.0) | 47 (48.5) | 97 (49.7) |
| Age at PNH diagnosis, mean ± SD (range), y | 36.8 ± 14.14 (11-74) | 34.1 ± 14.41 (6-73) | 35.5 ± 14.30 (6-74) |
| Age at first eculizumab infusion, mean ± SD (range), y | 43.2 ± 13.93 (14-74) | 40.7 ± 14.30 (14-74) | 42.0 ± 14.14 (14-74) |
| Race, n (%) | |||
| White | 61 (62.2) | 50 (51.5) | 111 (56.9) |
| Asian | 19 (19.4) | 23 (23.7) | 42 (21.5) |
| African American | 3 (3.1) | 5 (5.2) | 8 (4.1) |
| Not reported | 13 (13.3) | 13 (13.4) | 26 (13.3) |
| Other | 1 (1.0) | 2 (2.1) | 3 (1.5) |
| Unknown | 1 (1.0) | 3 (3.1) | 4 (2.1) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 77 (78.6) | 76 (78.4) | 153 (78.5) |
| Hispanic or Latino | 4 (4.1) | 3 (3.1) | 7 (3.6) |
| Not reported | 17 (17.3) | 15 (15.5) | 32 (16.4) |
| Missing/unknown | 0 | 3 (3.1) | 3 (1.5) |
| Baseline weight, mean ± SD (range), kg | 73.4 ± 14.6 (44-104) | 72.4 ± 16.8 (45-129) | 72.9 ± 15.7 (44-129) |
| LDH, mean ± SD (range), U/L | 235.2 ± 49.7 (100.0-365.5) | 228.0 ± 48.7 (135.0-383.5) | 231.64 ± 49.2 (100.0-383.5) |
| Hemoglobin, mean ± SD (range), g/dL | 10.9 ± 1.8 (4.8-15.5) | 11.1 ± 1.8 (7.5-16.1) | 11.0 ± 1.8 (4.8-16.1) |
| PNH clone size, mean ± SD (range) | |||
| RBC type II | 16.3 ± 23.6 (0.1-91.9) | 14.9 ± 19.6 (0.1-80.9) | 15.6 ± 21.6 (0.1-80.9) |
| RBC type III | 43.5 ± 29.7 (0.9-99.4) | 44.6 ± 30.5 (0.0-98.8) | 44.0 ± 30.0 (0.0-99.4) |
| Total RBC | 59.5 ± 31.4 (1.5-99.6) | 60.7 ± 32.5 (0.1-99.8) | 60.1 ± 31.9 (0.1-99.8) |
| Granulocytes | 84.0 ± 21.4 (3.3-99.8) | 82.6 ± 23.6 (7.4-99.9) | 83.3 ± 22.5 (3.3-99.9) |
| Monocytes | 86.1 ± 19.7 (12.1-99.9) | 85.6 ± 20.5 (11.2-99.9) | 85.9 ± 20.0 (11.2-99.9) |
| Patients with a history of pRBC/whole blood transfusion, n (%)∗ | 12 (12.2) | 13 (13.4) | 25 (12.8) |
| Number of pRBC/whole blood transfusions, total, mean ± SD (range)∗ | 30 2.5 ± 2.3 (1-8) | 64 4.9 ± 5.5 (1-17) | 94 3.8 ± 4.4 (1-17) |
| Units of pRBC/whole blood transfusion, total, mean ± SD (range) | 50 4.2 ± 3.8 (2.0-15.0) | 103 7.9 ± 8.8 (1.0-32.0) | 153 6.1 ± 7.0 (1-32) |
| FACIT-F score, mean ± SD (range) | 40.7 ± 9.5 (16.0-52.0) | 42.5 ± 9.4 (1.0-52.0) | 41.6 ± 9.5 (1.0-52.0) |
| History of PNH-associated conditions, n (%) | |||
| Aplastic anemia | 39 (39.8) | 34 (35.1) | 73 (37.4) |
| Myelodysplastic syndrome | 6 (6.1) | 3 (3.1) | 9 (4.6) |
pRBC, packed RBC; SD, standard deviation.
In the 12 months before the first study dose of ravulizumab or eculizumab.
Prevalence of csEVH
After the same duration of C5 inhibition, the prevalence of csEVH was similar between the treatment groups in the 301 and 302 studies. Irrespective of transfusion status, the prevalence of csEVH on day 183 in patients from the 301 study was 23.2% (29/125) in the ravulizumab group and 24.8% (30/121) in the eculizumab group. Irrespective of transfusion status, in the 302 study, the proportion of patients who met the hemoglobin levels and reticulocyte count criteria at baseline was 17.5% (17/97) in the ravulizumab group and 16.3% (16/98) in the eculizumab group. In patients from the 302 study with nonmissing hemoglobin and absolute reticulocyte count data on day 183, the prevalence of csEVH was 20.2% (19/94) in the ravulizumab group and 21.3% (20/94) in the eculizumab group. In this same study, the prevalence of csEVH with need for transfusion was 5.3% (5/94) in the ravulizumab group and 9.6% (9/94) in the eculizumab group. At week 52 of the 302 study, the proportion of patients meeting the csEVH criteria, irrespective of transfusion status, was 21.1% (20/95) in the ravulizumab-to-ravulizumab group and 14.3% (13/91) in the eculizumab-to-ravulizumab group. The prevalence of csEVH with need for transfusion at week 52 was 5.3% (5/94) and 9.9% (9/91) for the same respective groups.
Demographics and clinical characteristics among patients with csEVH
At baseline, clone sizes were similar between all patients and those with csEVH in the 301 trial (Tables 1 and 3). In the 302 trial, there were some differences at baseline between all patients and those with csEVH regarding clone sizes, mainly for RBC type III and total RBC sizes, which were larger in patients with csEVH than for all patients (Tables 2 and 4). In patients from the 302 trial, baseline mean LDH levels in those treated with eculizumab and ravulizumab were <1.5 × ULN (243.5 U/L and 246.7 U/L, respectively), indicating effective control of IVH.
Demographics and clinical characteristics at baseline of patients with csEVH in the 301 study
| Variable . | Eculizumab (n = 30) . | Ravulizumab (n = 29) . |
|---|---|---|
| Age at PNH diagnosis, n, mean ± SD (range), y | 30 40.3 ± 17.0 (15-82) | 28 40.5 ± 16.6 (17-73) |
| PNH clone size, mean ± SD (range) | ||
| RBC type II | 11.1 ± 13.7 (0.3-59.1) | 11.1 ± 14.3 (0.3-50.2) |
| RBC type III | 22.7 ± 14.2 (2.4-63.3) | 21.1 ± 15.1 (2.9-75.9) |
| Total RBC | 33.8 ± 22.3 (3.3-98.0) | 32.3 ± 20.1 (9.2-78.0) |
| Granulocytes | 93.6 ± 5.3 (80.2-99.6) | 92.2 ± 12.2 (43.9-99.9) |
| Monocytes | 94.7 ± 5.0 (83.3-99.9) | 92.8 ± 14.0 (28.6-99.9) |
| Variable . | Eculizumab (n = 30) . | Ravulizumab (n = 29) . |
|---|---|---|
| Age at PNH diagnosis, n, mean ± SD (range), y | 30 40.3 ± 17.0 (15-82) | 28 40.5 ± 16.6 (17-73) |
| PNH clone size, mean ± SD (range) | ||
| RBC type II | 11.1 ± 13.7 (0.3-59.1) | 11.1 ± 14.3 (0.3-50.2) |
| RBC type III | 22.7 ± 14.2 (2.4-63.3) | 21.1 ± 15.1 (2.9-75.9) |
| Total RBC | 33.8 ± 22.3 (3.3-98.0) | 32.3 ± 20.1 (9.2-78.0) |
| Granulocytes | 93.6 ± 5.3 (80.2-99.6) | 92.2 ± 12.2 (43.9-99.9) |
| Monocytes | 94.7 ± 5.0 (83.3-99.9) | 92.8 ± 14.0 (28.6-99.9) |
SD, standard deviation.
Demographics and clinical characteristics at baseline of patients with csEVH in the 302 study
| Variable . | Eculizumab (n = 20) . | Ravulizumab (n = 19) . |
|---|---|---|
| Age at PNH diagnosis, mean ± SD (range), y | 44.5 ± 14.3 (22-74) | 36.8 ± 16.34 (6-65) |
| Age at first eculizumab infusion, mean ± SD (range), y | 50.6 ± 14.2 (24-74) | 42.9 ± 15.1 (19-68) |
| LDH, mean ± SD (range), U/L | 243.5 ± 50.9 (153.3-365.5) | 246.7 ± 51.1 (146.0-330.5) |
| PNH clone size, mean ± SD (range), n | ||
| RBC type II | 19 10.4 ± 17.9 (0.1-74.4) | 17 8.0 ± 8.5 (0.5-29.3) |
| RBC type III | 20 61.9 ± 29.2 (1.2-99.2) | 19 66.1 ± 20.3 (26.1-98.8) |
| Total RBC | 19 71.7 ± 24.4 (26.8-99.6) | 17 76.0 ± 23.6 (26.6-99.6) |
| Granulocytes | 20 93.6 ± 7.2 (73.1-99.6) | 19 95.9 ± 6.4 (71.4-99.9) |
| Monocytes | 20 94.9 ± 7.4 (68.6-99.6) | 19 96.1 ± 6.7 (70.3-99.9) |
| Variable . | Eculizumab (n = 20) . | Ravulizumab (n = 19) . |
|---|---|---|
| Age at PNH diagnosis, mean ± SD (range), y | 44.5 ± 14.3 (22-74) | 36.8 ± 16.34 (6-65) |
| Age at first eculizumab infusion, mean ± SD (range), y | 50.6 ± 14.2 (24-74) | 42.9 ± 15.1 (19-68) |
| LDH, mean ± SD (range), U/L | 243.5 ± 50.9 (153.3-365.5) | 246.7 ± 51.1 (146.0-330.5) |
| PNH clone size, mean ± SD (range), n | ||
| RBC type II | 19 10.4 ± 17.9 (0.1-74.4) | 17 8.0 ± 8.5 (0.5-29.3) |
| RBC type III | 20 61.9 ± 29.2 (1.2-99.2) | 19 66.1 ± 20.3 (26.1-98.8) |
| Total RBC | 19 71.7 ± 24.4 (26.8-99.6) | 17 76.0 ± 23.6 (26.6-99.6) |
| Granulocytes | 20 93.6 ± 7.2 (73.1-99.6) | 19 95.9 ± 6.4 (71.4-99.9) |
| Monocytes | 20 94.9 ± 7.4 (68.6-99.6) | 19 96.1 ± 6.7 (70.3-99.9) |
SD, standard deviation.
Fatigue
Fatigue levels on day 183 in patients experiencing csEVH in the 302 study
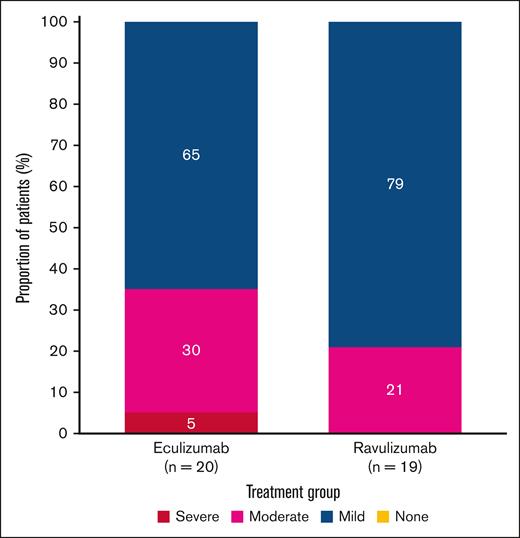
On day 183, all patients with csEVH experienced some fatigue (Figure 1). In most of these patients, fatigue was mild: 15 of 19 patients (79%) treated with ravulizumab and 13 of 20 (65%) of those treated with eculizumab. Moderate fatigue was experienced by 4 of 19 patients (21%) treated with ravulizumab and 6 of 20 (30%) treated with eculizumab. No patients treated with ravulizumab experienced severe fatigue, and 1 of 20 (5%) treated with eculizumab experienced severe fatigue.
Distribution of fatigue levels in study 302 at day 183 based on FACIT-F score in patients with csEVH. Mild indicates FACIT-F score ≥40; moderate, FACIT-F score ≥20 to <40; severe, FACIT-F score <20.
Distribution of fatigue levels in study 302 at day 183 based on FACIT-F score in patients with csEVH. Mild indicates FACIT-F score ≥40; moderate, FACIT-F score ≥20 to <40; severe, FACIT-F score <20.
Change in fatigue levels over time in patients experiencing csEVH in the 302 study
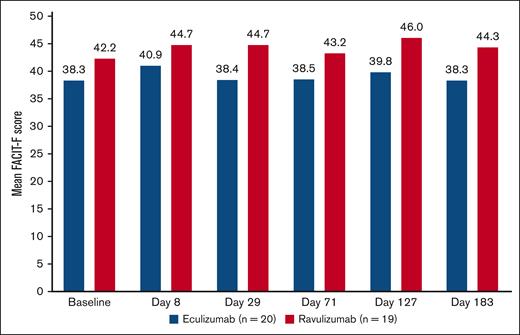
Patient fatigue levels were stable from baseline to day 183, with small numerical improvements (Figure 2). The percentage change from baseline in FACIT-F score was slightly higher in patients treated with ravulizumab than eculizumab. The mean percentage change from baseline was 11.8% (standard deviation, 36.1) in patients treated with ravulizumab and 1.8% (standard deviation, 23.6) in those treated with eculizumab.
Change in mean fatigue score over time using FACIT-F score in patients with csEVH from study 302. FACIT-F score ranges from 0 to 52, with a higher score indicating less fatigue.
Change in mean fatigue score over time using FACIT-F score in patients with csEVH from study 302. FACIT-F score ranges from 0 to 52, with a higher score indicating less fatigue.
Association between fatigue and hemoglobin levels in patients experiencing csEVH in the 302 study
In 40 patients who had baseline hemoglobin levels <9.5 g/dL, no correlation was identified between hemoglobin levels and FACIT-F score (r = 0.14; P = .3832).
Fatigue levels in patients with csEVH according to need for transfusion and LDH levels in the 302 study
Of the patients with csEVH treated with ravulizumab, 5 of 19 (26.3%) received a transfusion between baseline and day 183. Among these 5 patients, 2 (40.0%) had LDH levels between >1 × ULN and ≤1.5 × ULN, with one (50.0% [1/2]) experiencing mild fatigue and the other (50.0% [1/2]) moderate. Of the patients with csEVH treated with eculizumab, 9 of 20 (45.0%) received a transfusion between baseline and day 183. Among these 9 patients, 4 (44.4%) had LDH levels between >1 × ULN and ≤1.5 × ULN; 2 of these patients (50.0%) experienced mild fatigue, 1 (25.0%) moderate and 1 (25.0%) severe. None of the patients in either treatment group who received transfusions between baseline and day 183 had LDH levels >1.5 × ULN.
QoL
Mean global health status/QoL scores remained stable from baseline to day 183 for patients treated with ravulizumab (70.6 and 70.2, respectively) and eculizumab (62.5 and 57.1, respectively; Table 5). Mean functional scale scores also remained stable over time in patients treated with ravulizumab and eculizumab, with scores >75 at baseline and day 183 for all scales. Symptomatic scores remained <20 at baseline and day 183 for all symptoms, with the exception of dyspnea and insomnia in the eculizumab treatment group (dyspnea, 26.7 and 28.3, respectively; insomnia, 23.3 and 28.3, respectively).
Change in QoL over time in patients with csEVH in the 302 study based on the EORTC QLQ-C30
| Measure, mean ± SD (range) . | Eculizumab (n = 20) . | Ravulizumab (n = 19) . | ||
|---|---|---|---|---|
| Baseline . | Day 183 . | Baseline . | Day 183 . | |
| Global health status/QoL | 62.5 ± 15.4 (41.7-100.0) | 57.1 ± 25.4 (8.3-100.0) | 70.6 ± 18.9 (33.3-100.0) | 70.2 ± 15.3 (33.3-100.0) |
| Physical functioning | 81.3 ± 15.1 (53.3-100.0) | 81.0 ± 15.2 (40.0-100.0) | 82.8 ± 21.7 (33.3-100.0) | 88.4 ± 16.3 (46.7-100.0) |
| Emotional functioning | 81.3 ± 17.9 (41.7-100.0) | 85.8 ± 16.2 (50.0-100.0) | 88.2 ± 15.8 (50.0-100.0) | 91.7 ± 15.7 (41.7-100.0) |
| Cognitive functioning | 83.3 ± 23.6 (0.0-100.0) | 86.7 ± 20.7 (33.3-100.0) | 89.5 ± 18.6 (33.3-100.0) | 92.1 ± 14.0 (50.0-100.0) |
| Social functioning | 76.7 ± 28.3 (0.0-100.0) | 88.3 ± 15.4 (50.0-100.0) | 85.1 ± 26.0 (0.0-100.0) | 92.1 ± 16.1 (50.0-100.0) |
| Role functioning | 80.0 ± 20.0 (33.3-100.0) | 76.7 ± 26.2 (16.7-100.0) | 82.5 ± 28.6 (0.0-100.0) | 90.4 ± 17.0 (50.0-100.0) |
| Nausea and vomiting | 3.3 ± 8.7 (0.0-33.3) | 3.3 ± 10.3 (0.0-33.3) | 7.0 ± 14.0 (0.0-50.0) | 2.6 ± 6.3 (0.0-16.7) |
| Pain | 10.8 ± 19.7 (0.0-66.7) | 12.5 ± 24.7 (0.0-100.0) | 11.4 ± 18.5 (0.0-50.0) | 6.1 ± 11.4 (0.0-33.3) |
| Dyspnea | 26.7 ± 25.6 (0.0-100.0) | 28.3 ± 31.1 (0.0-100.0) | 12.3 ± 19.0 (0.0-66.7) | 8.8 ± 15.1 (0.0-33.3) |
| Insomnia | 23.3 ± 30.8 (0.0-100.0) | 28.3 ± 31.1 (0.0-100.0) | 14 ± 25.6 (0.0-66.7) | 8.8 ± 15.1 (0.0-33.3) |
| Appetite loss | 6.7 ± 17.4 (0.0-66.7) | 16.7 ± 29.6 (0.0-66.7) | 14.0 ± 20.2 (0.0-66.7) | 7.0 ± 14.0 (0.0-33.3) |
| Constipation | 18.3 ± 29.6 (0.0-100.0) | 13.3 ± 27.4 (0.0-100.0) | 8.8 ± 18.7 (0.0-66.7) | 5.3 ± 12.5 (0.0-33.3) |
| Diarrhea | 5.0 ± 12.2 (0.0-33.3) | 1.7 ± 7.5 (0.0-33.3) | 7.0 ± 14.0 (0.0-33.3) | 3.5 ± 10.5 (0.0-33.3) |
| Financial difficulties | 15.0 ± 31.5 (0.0-100.0) | 5.0 ± 12.2 (0.0-33.3) | 8.8 ± 18.7 (0.0-66.7) | 7.0 ± 14.0 (0.0-33.3) |
| Measure, mean ± SD (range) . | Eculizumab (n = 20) . | Ravulizumab (n = 19) . | ||
|---|---|---|---|---|
| Baseline . | Day 183 . | Baseline . | Day 183 . | |
| Global health status/QoL | 62.5 ± 15.4 (41.7-100.0) | 57.1 ± 25.4 (8.3-100.0) | 70.6 ± 18.9 (33.3-100.0) | 70.2 ± 15.3 (33.3-100.0) |
| Physical functioning | 81.3 ± 15.1 (53.3-100.0) | 81.0 ± 15.2 (40.0-100.0) | 82.8 ± 21.7 (33.3-100.0) | 88.4 ± 16.3 (46.7-100.0) |
| Emotional functioning | 81.3 ± 17.9 (41.7-100.0) | 85.8 ± 16.2 (50.0-100.0) | 88.2 ± 15.8 (50.0-100.0) | 91.7 ± 15.7 (41.7-100.0) |
| Cognitive functioning | 83.3 ± 23.6 (0.0-100.0) | 86.7 ± 20.7 (33.3-100.0) | 89.5 ± 18.6 (33.3-100.0) | 92.1 ± 14.0 (50.0-100.0) |
| Social functioning | 76.7 ± 28.3 (0.0-100.0) | 88.3 ± 15.4 (50.0-100.0) | 85.1 ± 26.0 (0.0-100.0) | 92.1 ± 16.1 (50.0-100.0) |
| Role functioning | 80.0 ± 20.0 (33.3-100.0) | 76.7 ± 26.2 (16.7-100.0) | 82.5 ± 28.6 (0.0-100.0) | 90.4 ± 17.0 (50.0-100.0) |
| Nausea and vomiting | 3.3 ± 8.7 (0.0-33.3) | 3.3 ± 10.3 (0.0-33.3) | 7.0 ± 14.0 (0.0-50.0) | 2.6 ± 6.3 (0.0-16.7) |
| Pain | 10.8 ± 19.7 (0.0-66.7) | 12.5 ± 24.7 (0.0-100.0) | 11.4 ± 18.5 (0.0-50.0) | 6.1 ± 11.4 (0.0-33.3) |
| Dyspnea | 26.7 ± 25.6 (0.0-100.0) | 28.3 ± 31.1 (0.0-100.0) | 12.3 ± 19.0 (0.0-66.7) | 8.8 ± 15.1 (0.0-33.3) |
| Insomnia | 23.3 ± 30.8 (0.0-100.0) | 28.3 ± 31.1 (0.0-100.0) | 14 ± 25.6 (0.0-66.7) | 8.8 ± 15.1 (0.0-33.3) |
| Appetite loss | 6.7 ± 17.4 (0.0-66.7) | 16.7 ± 29.6 (0.0-66.7) | 14.0 ± 20.2 (0.0-66.7) | 7.0 ± 14.0 (0.0-33.3) |
| Constipation | 18.3 ± 29.6 (0.0-100.0) | 13.3 ± 27.4 (0.0-100.0) | 8.8 ± 18.7 (0.0-66.7) | 5.3 ± 12.5 (0.0-33.3) |
| Diarrhea | 5.0 ± 12.2 (0.0-33.3) | 1.7 ± 7.5 (0.0-33.3) | 7.0 ± 14.0 (0.0-33.3) | 3.5 ± 10.5 (0.0-33.3) |
| Financial difficulties | 15.0 ± 31.5 (0.0-100.0) | 5.0 ± 12.2 (0.0-33.3) | 8.8 ± 18.7 (0.0-66.7) | 7.0 ± 14.0 (0.0-33.3) |
SD, standard deviation.
Discussion
After the approval of eculizumab and ravulizumab, the prognosis for patients with PNH improved considerably. Both treatments have been shown to control terminal complement activity, IVH, and thrombosis, and significantly improve survival. Control of terminal complement activity and IVH, as demonstrated by sustained reduction of LDH <1.5 × ULN, should remain the primary aim for patients with PNH. The presence of csEVH and its impact on patients with PNH treated with C5 inhibitors had not been investigated before this study. Our analysis has shown that csEVH affected 20% to 25% of patients with PNH receiving C5 inhibitors, and its prevalence was the same for ravulizumab or eculizumab. Notably, this was observed for patients with and without stable disease. Furthermore, in patients from the 302 study, the prevalence of csEVH was similar at baseline and day 183, with no change after switching to ravulizumab. It is important to highlight that there is currently no established consensus definition of EVH in guidelines; therefore, the definition used here was that used in a prior study of patients receiving PNH treatment.27
In patients with PNH not treated with C5 inhibitors, fatigue has been reported in >80% of cases, with median FACIT-F scores of 34.0.29 Additionally, fatigue was often found to be disproportionate to hemoglobin levels. In untreated patients, IVH and terminal complement activity contribute more to the symptoms of fatigue than anemia,30 as demonstrated by previous studies in patients with PNH, in which eculizumab and ravulizumab treatment reduced fatigue as a result of a reduction in LDH from ∼10 × ULN to near-normal levels in all patients, independent of hemoglobin levels.26 Therefore, it remains critical to control terminal complement activity and IVH. This reduces morbidity and mortality of patients with PNH, while improving fatigue. This is important, because fatigue can have a substantial negative impact on QoL29,31 and affect daily life and cognitive function, including concentration and memory.32 In this analysis, fatigue was evaluated only in patients in the 302 study, because these patients had clinically stable disease. In patients from the 302 study treated with C5 inhibitors who had csEVH, the severity of fatigue was lower than what has been reported for untreated patients with PNH.29 Our analysis showed that the proportion of patients with mild fatigue on day 183 was 79% with ravulizumab and 65% with eculizumab, and the proportion of patients with moderate fatigue was 21% with ravulizumab and 30% with eculizumab.
Baseline mean FACIT-F scores across both treatment groups from the 302 study ranged from 38.3 to 42.2 and were close to the general US population mean score of 43.5,33 indicating that these eculizumab-experienced patients who were identified as experiencing csEVH did not have excessive fatigue at the start of the study. Furthermore, baseline LDH levels were <1.5 × ULN, indicating that patients with csEVH did not experience residual IVH at that time point. A previous study using data from the International PNH Registry estimated that a change from baseline of 5 points in FACIT-F score constitutes a clinically important change in patients with PNH.34 In our analysis, fatigue remained relatively consistent over time in both treatment groups, with less than a 5-point change on average from baseline to day 183; however, because the participants had stable disease and were on treatment at enrollment, a clinically meaningful improvement in fatigue would not be expected.
Evaluating the association between hemoglobin levels and fatigue in patients with PNH who are experiencing csEVH may help to identify patients who could benefit further from treatments to specifically address their anemia and associated symptoms. Although previous evidence in patients with PNH has indicated that hemoglobin levels <10.5 g/dL were associated with a higher frequency of fatigue than hemoglobin levels ≥10.5 g/dL,35 another study reported that improved hemoglobin levels were not a significant predictor of reductions in fatigue.26 Consistent with the latter study, in this analysis, there was no significant association between baseline hemoglobin levels and FACIT-F scores in patients with csEVH with hemoglobin levels <9.5 g/dL. Fatigue is multifactorial, and these data are in line with other studies reporting that factors other than hemoglobin can influence it, including age, sex, and comorbid conditions.29,36 Other evidence has shown a correlation between LDH levels and fatigue in PNH, with a reduction in levels predicting improvements in fatigue.26 Our analysis also evaluated the need for transfusion and levels of LDH and fatigue, which showed that among patients who received a transfusion none had LDH >1.5 × ULN, and most of those with LDH between >1 × ULN and ≤1.5 × ULN experienced either mild or moderate fatigue. These results may indicate that residual IVH was not the cause of fatigue in patients with transfusions, although this finding was based on a small sample size.
Regarding the QoL of patients with csEVH and stable PNH disease, the results showed that EORTC QLQ-C30 scores remained relatively stable over time, with patients demonstrating high functional scale scores (>75; indicating a high level of functioning) and low symptomatic scale scores (<20; indicating low symptomatology). Furthermore, the values observed in this analysis were comparable to those reported in the general population, based on a survey from 13 countries across Europe, Canada, and the United States.37
A limitation of this analysis includes the fact that in the absence of a universal definition of csEVH, this was described as symptomatic anemia (hemoglobin levels < 9.5 g/dL) with absolute reticulocyte count ≥120 × 109/L, a definition that was used as inclusion criteria in the ALPHA study.27
The findings of this post hoc analysis show that, after C5 inhibitor treatment, csEVH affected 20% to 25% of patients receiving PNH treatment, with similar prevalence between patients receiving ravulizumab and eculizumab. Moreover, in patients with csEVH, fatigue remained close to levels in the general population during treatment. Some differences in fatigue severity between the treatment groups were noted, with higher proportions of mild fatigue and lower proportions of moderate fatigue in the ravulizumab group. Overall, these results show that the C5 inhibitors ravulizumab and eculizumab provide benefit to adults with PNH with csEVH.
Acknowledgments
Medical writing support was provided by Rebecca Spencer Martín and Rebecca Hornby of Oxford PharmaGenesis, Oxford, United Kingdom, with funding from Alexion, AstraZeneca Rare Disease.
Authorship
Contribution: A.G.K. and J.W.L. contributed to research design; A.G.K., A.R., C.I.P., and J.W.L. performed the research; A.R., J.W.L., and W.B. collected the data; J.Y. performed statistical analysis; and all authors analyzed and interpreted the data and developed and/or revised the manuscript.
Conflict-of-interest disclosure: A.G.K. has received honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Celgene/Bristol Myers Squibb (BMS), Novartis, and Ra Pharma; is on the board of directors or advisory boards for Alexion, AstraZeneca Rare Disease, Amgen, Celgene/BMS, Novartis, Pfizer, Roche, and Ra Pharma; and has received consultancy fees from Achillion, Akari Therapeutics, Alexion, AstraZeneca Rare Disease, BioCryst, Celgene/BMS, Janssen Pharmaceuticals, Novartis, Novo Nordisk, Pfizer, Roche, and Samsung. J.W.L. has received research funding from Achillion and Alexion, AstraZeneca Rare Disease; served as advisory board member for and received honoraria from Alexion, AstraZeneca Rare Disease; and received consultancy fees from Kyowa Kirin, Novartis, and Sanofi. C.J.P. has received consultancy fees from Alexion, AstraZeneca Rare Disease, BioCryst, Novartis, Roche, Sanofi, Sobi, and Takeda; and received speaker’s bureau fees from Alexion, AstraZeneca Rare Disease, Amgen, Novartis, and Sobi. C.I.P. has received consulting fees from Alexion, AstraZeneca Rare Disease/AstraZeneca, and Rigel; received advisory board fees from Alexion, Annexon Biosciences, AstraZeneca Rare Disease/AstraZeneca, Apellis, Sanofi, Sobi/Dova, Novartis, and Rigel; received research support from Alexion, Apellis, Argenx, AstraZeneca Rare Disease/AstraZeneca, Celgene, Incyte, Oscotec, Rigel, and Sanofi; and received speaker’s bureau fees from Apellis and Sobi/Dova. A.R. has received research funding from Roche; received travel support from AbbVie, Alexion, AstraZeneca Rare Disease, and Sobi; received lecture honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Grifols, Novartis, Roche, Sanofi, and Sobi; and received consultancy fees from Alexion, AstraZeneca Rare Disease, Apellis, BioCryst, Bioverativ, Kira, Novartis, Pfizer, and Sanofi. R.A.B. has received research funding and consultancy fees from Alexion, AstraZeneca Rare Disease; and received honoraria from Alexion, AstraZeneca Rare Disease, American Society of Hematology, Indy Hematology Review, International Society on Thrombosis and Haemostasis Congress, and UpToDate. J.-i.N. has a membership on the board of directors or advisory committees for Alexion, AstraZeneca Rare Disease, Chugai Pharmaceutical Co, and Roche; and has received research funding from Alexion, AstraZeneca Rare Disease. J.Y. is a current employee of and shareholder in Alexion, AstraZeneca Rare Disease. M.M. is a current employee of and shareholder in Alexion, AstraZeneca Rare Disease. R.P.d.L. has served as an advisory board member for and has received consultancy fees, research funding, and speaker’s bureau fees from Alexion Pharmaceuticals Inc. The remaining authors declare no competing financial interests.
Correspondence: Regis Peffault De Latour, Saint-Louis Hospital, 1 Ave Claude Vellefaux, 75010 Paris, France; email: regis.peffaultdelatour@aphp.fr.
References
Author notes
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data, provided that participant privacy is assured through methods such as data deidentification, pseudonymization, or anonymization (as required by applicable law) and if such disclosure was included in the relevant study, informed consent form, or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://www.alexionclinicaltrialtransparency.com/data-requests/. Link to data request form: https://alexion.com/contact-alexion/medical-information.
The full-text version of this article contains a data supplement.