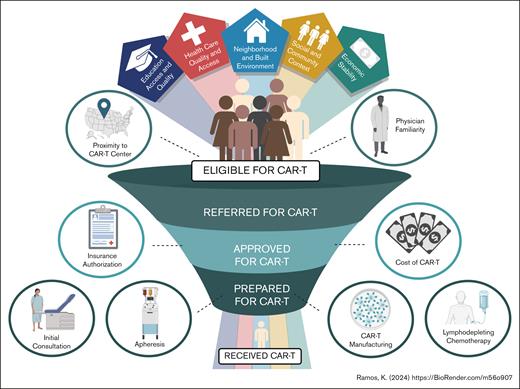
In this issue of Blood Advances, Battiwalla et al1 analyzed access barriers to anti-CD19 chimeric antigen receptor (CAR) T cells for non-Hodgkin lymphoma (NHL) within the Sarah Cannon Transplant and Cellular Therapy Network (SCTCTN). Although median time from consultation to CAR T-cell infusion declined from 207 to 108 days during the study period, 41% of community-referred patients were unable to access CAR T-cell therapy due to disease progression or poor health. The current study highlights the multifactorial complexities and barriers that persist in accessing CAR T-cell therapy, many of which are magnified for racial and ethnic minorities and individuals of lower socioeconomic stratum (SES) (see figure).
Factors affecting whether patients receive CAR T-cell therapy. Access barriers to CAR T-cell therapy exist at all levels of the referral-to-infusion continuum and social determinants of health affect patient access and outcomes. Access barriers translate into a smaller percentage of referred eligible individuals receiving CAR T-cell therapy, with racial and ethnic minority patients often discordantly hindered relative to NHW patients.
Factors affecting whether patients receive CAR T-cell therapy. Access barriers to CAR T-cell therapy exist at all levels of the referral-to-infusion continuum and social determinants of health affect patient access and outcomes. Access barriers translate into a smaller percentage of referred eligible individuals receiving CAR T-cell therapy, with racial and ethnic minority patients often discordantly hindered relative to NHW patients.
CD19-directed CAR T-cell therapy has significantly altered the treatment landscape for adult patients with relapsed/refractory NHL and use has recently advanced to earlier stages of disease after the ZUMA-7 trial.2 Despite the ever-growing demand across an increasing number of CAR T-cell–eligible patients, access to CAR T-cell therapy remains limited. Thoughtful analysis of the disparities related to CAR T-cell therapy, particularly because they affect minority and marginalized populations, poses a unique opportunity to ensure that equitable access to these novel therapies is achieved for all patients in need.
Battiwalla et al described the complexity of CAR T-cell therapy, which includes logistical challenges extending across the referral, evaluation, authorization, apheresis and manufacturing, and infusion continuum (see figure). In their analysis, the investigators observed a mean duration of 3 to 4 months between the time of initial referral and CAR T-cell infusion. Among referred patients within the SCTCTN, the primary reason for CAR T-cell ineligibility was disease progression (34%) or “poor general health” (15%) defined as general disability and death, with demographic, financial, and social determinants of health found to be nonsignificant contributors to CAR T-cell ineligibility. Although this community-based study was not designed to comprehensively evaluate the impact of race and ethnicity superimposed upon SES, these are important actionable items in the ongoing effort to extend CAR T-cell therapy to all eligible patients.
The financial burden of CAR T-cell therapy alone remains a significant limitation. The real-world cost of CAR T-cell therapy is estimated at $700 000 to $1 million dollars,3 an insurmountable cost for patients who are among the millions of Americans living below the poverty line or not having health insurance.4 Battiwalla et al note that state and national area deprivation index of infused patients in the SCTCTN was substantially more affluent than the state and national area deprivation index averages, supporting disparity between individuals who receive CAR T-cell therapy and those who do not. Furthermore, studies have observed that Black or African American (BAA),5 Hispanic (HIS),6 uninsured,5 Medicare-receiving,5,6 and lower SES5,7 patients are less likely to receive CAR T-cell therapy, illuminating the vast discrepancies for whom CAR T-cell therapy is realistically available in the United States.
Barriers associated with patient location are important, given that cellular therapy remains available in <4% of US health centers and is primarily restricted to large academic institutions.3 Battiwalla et al did not observe distance to a CAR T-cell–accredited center as a significant contributor to patient ineligibility. However, other studies have found that up to one-third of patients reside >2 hours away from a CAR T-cell center and cite distance as an important deterrent to both referral and management.5,8 Similarly, the location barrier for CAR T-cell therapy is magnified among racial and ethnic minority groups, given that only one-third of BAA persons reside in a county with an available CAR T-cell therapy or bispecific trial,9 emphasizing the need for hybrid and collaborative care models to improve accessibility to CD19-directed CAR T-cell therapy to patients.
Real-world analyses have found that racial and ethnic minorities experience inferior CAR T-cell outcomes; therefore, addressing access barriers and potential disease biology differences within these at-risk populations is critical. For example, BAA patients have inferior overall response and complete response rates10 and shorter progression-free survival10,11 than their non-HIS White (NHW) counterparts. Furthermore, HIS patients have a higher likelihood of experiencing severe cytokine release syndrome than NHW patients.12 Therefore, efforts to mitigate access barriers and increase representation of minority groups in clinical trials are needed to improve CAR T-cell outcomes in diverse patient populations.
Racial and ethnic minorities comprise a small percentage of patients enrolled in CAR T-cell clinical trials.2,5,13 In Ahmed et al’s recent analysis of 1077 clinical trial participants, 68.7% of CAR T-cell recipients were NHW, with BAA (5.9%), HIS (6.1%), and Asian individuals (4.5%) accounting for very small proportions of these study cohorts.5 These discrepancies continue in contemporary trials, as BAA persons included only 5% of patients worldwide and 6.4% of US participants in the ZUMA-7 trial.2 Given that BAA and HIS persons represent 18.7% and 12.4% of the US population, respectively,4 ensuring their representation in upcoming clinical trials, particularly in the context of their inferior CAR T-cell outcomes, is important.
In summary, the study by Battiwalla et al highlights the continued need to reduce multifactorial complexities and access barriers associated with CAR T-cell therapy. Next steps for ensuring equal outcomes for all patients needing CAR T-cell therapy include reducing logistical challenges from referral to infusion, mitigating the impact of financial and geographical burdens, enhancing inclusion of racial and ethnic minorities in upcoming clinical trials, and identifying impact metrics relevant to these patients. Only through such deliberate intent to improve access to and outcomes after CAR T-cell therapy can all patients benefit from this transformational therapy.
Conflict-of-interest disclosure: J.J.A. reports serving on an advisory board for AscellaHealth. K.N.R. declares no competing financial interests.