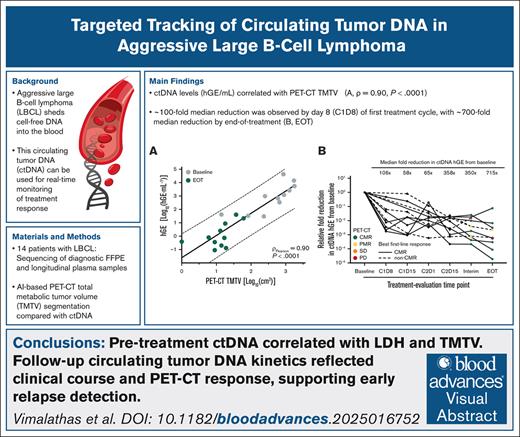
Key Points
ctDNA levels correlated with PET-CT–based TMTV and may enable earlier relapse detection than imaging alone.
A rapid ∼100-fold median reduction in ctDNA was observed by day 8 of the first treatment cycle and mirrored clinical response dynamics.
Visual Abstract
Positron emission tomography–computed tomography (PET-CT) is recommended for response evaluation in aggressive large B-cell lymphoma (LBCL) but cannot detect minimal residual disease (MRD). Circulating tumor DNA (ctDNA) has emerged as a promising biomarker for real-time disease monitoring. This study evaluated longitudinal ctDNA monitoring as an MRD marker in LBCL. In this prospective, single-center study, 14 newly diagnosed patients with LBCL receiving first-line immunochemotherapy underwent frequent longitudinal blood sampling. A 53-gene targeted sequencing panel quantified ctDNA and evaluated its kinetics, correlating it with clinical parameters and PET-CT, including total metabolic tumor volume (TMTV) calculated using artificial intelligence–based analysis via RECOMIA. Baseline ctDNA was detected in 11 of 14 patients (79%) with a median variant allele frequency of 6.88% (interquartile range, 1.19%-10.20%). ctDNA levels correlated significantly with TMTV (ρ = 0.90; P < .0001) and lactate dehydrogenase. ctDNA kinetics, including after 1 treatment cycle, mirrored PET-CT metabolic changes and identified relapsing or refractory patients. This study demonstrates ctDNA-based MRD monitoring in LBCL using a fixed targeted assay with an analytical sensitivity of at least 10−3. The kinetics of ctDNA reflects the clinical course and PET-CT findings, underscoring its complementary potential to PET-CT.
Introduction
Treatment of aggressive large B-cell lymphoma (LBCL), herein defined as diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma (HGBL), and primary mediastinal large B-cell lymphoma (PMBCL), reached a major milestone in 2001 with the addition of rituximab to the standard cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy regimen (R-CHOP), achieving unprecedented improvements in survival.1,2 Since then, progress in first-line treatment has stagnated, with the addition of precision therapies, apart from polatuzumab vedotin,3 to the R-CHOP backbone failing to surpass R-CHOP in achieving significant survival improvements.4 Modern immunotherapy, including chimeric antigen receptor T-cell therapy and bispecific antibodies, is transforming relapsed/refractory (R/R) LBCL treatment and moving into earlier lines via biomarker-driven, adaptive approaches,5-10 substituting R-CHOP in patients with an early unfavorable response.
After standard first-line immunochemotherapy, up to 40% of patients with LBCL develop R/R disease, emphasizing the highly heterogeneous therapeutic response in LBCL.11 Current prognostic tools, such as the International Prognostic Index (IPI), fail to fully account for this heterogeneity in outcomes and lack the ability to identify individual high-risk patients.12 The current recommended diagnostic tool for response assessment is positron emission tomography–computed tomography (PET-CT) imaging.13 Despite the prognostic value of interim PET-CT, treatment intensification based on interim PET-CT is not recommended due to ambiguous results, and posttreatment surveillance PET-CT is no longer recommended.14-18 Notably, 15% of PET-CT–negative patients with DLBCL in complete remission will relapse.19 Most relapses (∼80%) are not captured by PET-CT but are detected through overt clinical symptoms between follow-ups.16,17 The main limitations of PET-CT include low specificity, restrictions on serial monitoring due to radiation exposure, and the inability to detect disease at the molecular level, thus impeding its use for monitoring minimal residual disease (MRD). However, quantitative PET-CT metrics, such as total metabolic tumor volume (TMTV), have emerged as new biomarkers showing potential for tumor burden assessment and prognostication in lymphomas.20-22
In parallel with therapeutic advances, liquid biopsy and circulating tumor DNA (ctDNA) have gained traction in several cancers, including B-cell lymphomas, with research accelerating at immense speed, particularly regarding MRD monitoring.23-28 ctDNA has sparked interest in MRD-based early identification of R/R disease, because conventional MRD monitoring targeting circulating tumor cells via polymerase chain reaction and flow cytometry is inapplicable in LBCL due to the rare leukemic involvement.25
The minimally invasive access by simple blood sampling and the short half-life of ctDNA make it an appealing biomarker for real-time dynamic monitoring,27,29 uniquely poised to overcome limitations imposed by tissue biopsies and imaging. ctDNA levels and molecular responses have demonstrated independent prognostic value in DLBCL, with residual ctDNA predicting adverse outcomes even in PET-CT–negative patients.30-38 A challenge of ctDNA detection is its often low abundance in blood among nonmalignant cell-free DNA (cfDNA), particularly in low–tumor burden settings inherent to MRD monitoring.39,40 Consequently, ultrasensitive assays have emerged, including cancer personalized profiling by deep sequencing39 and, more recently, targeted sequencing of phased variants (PhasED-seq), conceptualized by Kurtz et al.41 PhasED-seq enhances sensitivity by leveraging phased variants, mutations that occur in close genomic proximity, allowing for more confident ctDNA detection in low-abundance settings. Although highly sensitive, PhasED-seq requires careful selection of co-occurring variants and specialized analytical pipelines.
In this prospective study, we aimed to investigate the clinical performance of ctDNA as a potential MRD marker for longitudinal monitoring in patients with LBCL undergoing R-CHOP/R-CHOP–like first-line treatment within a clinically feasible framework, an approach designed to balance sensitivity with practicality, ensuring accessibility and scalability for routine clinical use. Unlike highly specialized assays requiring extensive computational integration, our method is aimed at broader applicability across standard laboratories. Our objectives included assessing ctDNA kinetics and correlating ctDNA levels longitudinally with PET-CT response evaluation, incorporating artificial intelligence (AI)–generated PET-CT metrics and clinical parameters.
Materials and methods
In this prospective, observational, noninterventional, single-center study, we consecutively enrolled patients with newly diagnosed LBCL undergoing first-line treatment outside a clinical trial with R-CHOP/R-CHOP–like therapy at the Department of Haematology, Odense University Hospital, Denmark. Eligible patients were considered using the following inclusion criteria: newly diagnosed, histologically verified DLBCL, PMBCL, or HGBL according to the 2016 World Health Organization Classification; previously untreated; aged ≥18 years; received first-line curative-intent rituximab-based immunochemotherapy; and provided written informed consent. Patients were recruited for the study from May 2022 to August 2023, with a total of 14 patients included in the final study cohort (supplemental Methods).
The study was approved by the Regional Committees on Health Research Ethics for Southern Denmark (S-20210106) and the Danish Data Protection Agency (21/48591). All patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Sample collection and processing
Plasma samples were collected at prespecified time points: at baseline, before initiation of treatment; on day 1 of each treatment cycle; at interim; and at the end of treatment (EOT). To study ctDNA kinetics early in the treatment course, sampling was also performed on day 15 of cycle 1 (C1D15) and cycle 2 (C2D15), as well as on C1D8 (supplemental Figure 1). Longitudinal peripheral blood samples (60 mL each) were collected in K2EDTA tubes. Additionally, paired diagnostic formalin-fixed, paraffin-embedded (FFPE) tumor tissue was obtained, with histological diagnosis confirmed by an experienced hematopathologist and tumor infiltration assessed via immunohistochemistry. Matched peripheral blood mononuclear cells (PBMCs) were collected as germ line controls, with no morphological or flow cytometric evidence of leukemic involvement. Blood sample cfDNA from 10 healthy donors was included as a reference panel of normals (PON).
Plasma was isolated within 2 hours of collection using double centrifugation and cryopreserved (–80 °C) until DNA extraction. cfDNA was extracted using the Quick-cfDNA kit (Zymo Research Corporation, Irvine, CA). Genomic DNA from 10 μm FFPE tissue sections was purified using the QIAamp DNA FFPE Advanced Kit (QIAGEN, Hilden, Germany). PBMC DNA was extracted with the MagNA Pure 24 system (Roche Diagnostics, Mannheim, Germany) or the Qiagen AllPrep DNA/RNA Mini Kit (QIAGEN; supplemental Methods).
Targeted panel sequencing
Sequencing was performed on a NovaSeq 6000 (Illumina, San Diego, CA) using a custom capture-based panel (Twist Bioscience, South San Francisco, CA) targeting coding exons of 53 genes and immunoglobulin (Ig) loci recurrently mutated in LBCL (supplemental Table 1). Unique Molecular Identifiers (UMIs) were implemented for error-corrected variant calling.
As detailed previously,42 Burrows-Wheeler Aligner43 and Samtools44 were used for alignment and sequence processing, with fgbio (v2; Fulcrum Genomics, Somerville, MA) implemented for UMI processing. Quality assessment and adapter trimming were performed with fastp and FastQC. The variant pipeline implemented GATK 4.345 and MuTect246 using patient PBMCs as germ line controls and annotation via SnpEff,47 SnpSift,48 gnomAD,49 ClinVar,50 1KGP,51 and COSMIC.52 Common single-nucleotide polymorphisms were removed, and pipeline artifacts were filtered using the established PON. Statistical analyses were conducted using GraphPad Prism (v. 10.2.2; GraphPad Software, Boston, MA), Wolfram (Mathematica v. 13.3; Wolfram Research, Champaign, IL), and R (v. 4.3.1).
Sequencing yielded a median number of raw paired-end reads of 68 million (interquartile range [IQR], 65 million to 78 million) per sample. The collapsed UMI-based ctDNA variant-specific coverage was 1865× (IQR, 1080× to 2545×), in contrast to a raw median noncollapsed depth of coverage of 55 943× per sample (IQR: 45 357× to 64 144×; supplemental Figure 2). Valid ctDNA variant candidates eligible as follow-up targets were evaluated using diagnostic FFPE DNA or pretreatment cfDNA samples and restricted to variants observed in at least 1 longitudinal cfDNA follow-up sample. The threshold separating signal from noise for cfDNA samples was established using the PBMC controls, patient interim and EOT variant allele frequencies (VAFs), and the Tukey outlier method. All variants in the PBMC control samples or PON were discarded. In patients in whom diagnostic FFPE tissue was unavailable (n = 2), cfDNA served as a surrogate.
Quantitative ctDNA levels were expressed in tumor haploid genome equivalents (hGEs) per milliliter of blood plasma, determined as the product of the median VAF of the somatic mutations and the total cfDNA concentration in plasma (nanograms per milliliter).
PET-CT imaging and analysis
Baseline, interim (performed after 3 treatment cycles), and EOT [18F]fluorodeoxyglucose (FDG) PET-CT scans were performed for all patients, with additional scans conducted when clinically indicated. All scans followed the Lugano criteria and European Association of Nuclear Medicine guidelines53 and were interpreted by board-certified nuclear medicine physicians and radiologists.
FDG was administered IV at 4 MBq/kg before 1 September 2022 and 3 MBq/kg thereafter, with negligible impact on results. Imaging was conducted using GE Discovery MI (4-ring), 710, and DMI5 PET-CT scanners. CT attenuation correction used 120 kV, Smart-MA dose modulation, and standard reconstruction protocols (GE Q.AC). PET acquisition parameters varied slightly by scanner, with 95 to 140 seconds per bed position and 32% overlap. Images were reconstructed using the Q.Clear algorithm (time-of-flight; β = 500).
TMTV and maximum standardized uptake value (SUVmax) were calculated using an AI approach (Lymphoma FDG-PET/CT v2.1) on the RECOMIA platform,54 using 3-dimensional U-Net convolutional neural networks trained to segment nodal and extranodal lesions, with SUV thresholds applied for organ segmentation. Postprocessing excluded small low-uptake lesions.55 In total, 58 scans were analyzed; 5 patients underwent 3 scans each, with no further imaging after achieving complete metabolic response (CMR).
Results
The cohort consisted of 9 patients with DLBCL, 2 with HGBL, and 2 with PMBCL (Table 1). One additional patient was initially diagnosed with grade 3 follicular lymphoma but was judged to have clinically transformed disease and treated accordingly. After being refractory to first-line therapy, a subsequent biopsy histologically confirmed transformation to DLBCL. The median cohort age was 72 years (range, 38-81). Most patients (11/14) presented with advanced Ann-Arbor stage (III/IV) disease, and approximately half (6/14) had high-risk IPI (IPI ≥ 4).
Patient characteristics for the study cohort
| Characteristics . | Number . | Percentage (%) . |
|---|---|---|
| Women/men | 4/10 | 29/71 |
| Median age (range), y | 72 (38-81) | |
| Histological diagnosis | ||
| DLBCL | 9 | 64 |
| HGBL | 2 | 14 |
| PMBCL | 2 | 14 |
| FL, grade 3∗ | 1 | 7 |
| COO | ||
| GCB | 2 | 14 |
| Non-GCB | 6 | 43 |
| NR/UD | 6 | 43 |
| Extranodal involvement | ||
| 0-1 | 9 | 64 |
| ≥2 | 5 | 36 |
| IPI ≥4 | 6 | 43 |
| Ann Arbor stage III-IV | 11 | 79 |
| ECOG PS ≥2 | 4 | 29 |
| Elevated LDH | 9 | 64 |
| B-symptoms | 9 | 64 |
| First-line treatment | ||
| R-CHOP | 10 | 71 |
| R-CHOP–like regimens | 4 | 29 |
| Baseline PET-CT metrics | ||
| Median TMTV, cm3 | 450.5 | |
| Median SUVmax | 27.02 | |
| PET-CT response on first-line therapy | ||
| Interim | ||
| CMR | 8 | 57 |
| PMR | 4 | 29 |
| SD | 1 | 7 |
| PD | 1 | 7 |
| EOT (those completing first-line therapy) | ||
| CMR | 9 | 69 |
| PMR | 2 | 15 |
| PD | 2 | 15 |
| Status at study termination | ||
| Alive | 11 | 79 |
| Observation in CR | 10 | 71 |
| Relapse treatment ongoing | 1 | 7 |
| Death from progression | 3 | 21 |
| Characteristics . | Number . | Percentage (%) . |
|---|---|---|
| Women/men | 4/10 | 29/71 |
| Median age (range), y | 72 (38-81) | |
| Histological diagnosis | ||
| DLBCL | 9 | 64 |
| HGBL | 2 | 14 |
| PMBCL | 2 | 14 |
| FL, grade 3∗ | 1 | 7 |
| COO | ||
| GCB | 2 | 14 |
| Non-GCB | 6 | 43 |
| NR/UD | 6 | 43 |
| Extranodal involvement | ||
| 0-1 | 9 | 64 |
| ≥2 | 5 | 36 |
| IPI ≥4 | 6 | 43 |
| Ann Arbor stage III-IV | 11 | 79 |
| ECOG PS ≥2 | 4 | 29 |
| Elevated LDH | 9 | 64 |
| B-symptoms | 9 | 64 |
| First-line treatment | ||
| R-CHOP | 10 | 71 |
| R-CHOP–like regimens | 4 | 29 |
| Baseline PET-CT metrics | ||
| Median TMTV, cm3 | 450.5 | |
| Median SUVmax | 27.02 | |
| PET-CT response on first-line therapy | ||
| Interim | ||
| CMR | 8 | 57 |
| PMR | 4 | 29 |
| SD | 1 | 7 |
| PD | 1 | 7 |
| EOT (those completing first-line therapy) | ||
| CMR | 9 | 69 |
| PMR | 2 | 15 |
| PD | 2 | 15 |
| Status at study termination | ||
| Alive | 11 | 79 |
| Observation in CR | 10 | 71 |
| Relapse treatment ongoing | 1 | 7 |
| Death from progression | 3 | 21 |
COO, cell of origin; CR, complete remission; ECOG, Eastern Cooperative Oncology Group; FL, follicular lymphoma; GCB, germinal-center B cell; LDH, lactate dehydrogenase; NR, not reported; PD, progressive disease; PS, performance status; SD, stable disease; UD, undetermined.
This patient later transformed to DLBCL.
All patients received first-line curative-intent immunochemotherapy. The majority of patients (n = 10) received R-CHOP. The 2 patients with PMBCL received R-CHOEP (combination rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone), followed by DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), whereas the 2 patients with HGBL were treated according to the Nordic BioCHIC regimen,56 including the DA-EPOCH-R backbone.
Eight of the 14 patients reached CMR at interim PET-CT, 4 obtained partial metabolic response (PMR), 1 had stable disease and 1 had progressive disease. At EOT, 9 patients were in CMR (8 with CMR and 1 with PMR at interim), 2 patients were in PMR (both achieved PMR at interim), and 2 had progressive disease (1 with PMR and 1 with SD at interim). Among those who attained CMR on EOT PET-CT after first-line therapy (n = 9), 2 later relapsed at 4 months and 13 months. Cumulatively, during the study period, 5 patients experienced R/R disease: 3 patients had refractory disease (patients 1199, 1303, and 1201), 1 had an early relapse (<1 year; patient 1063), and 1 had a late relapse (≥1 year; patient 1174). Four of these R/R patients with LBCL received second-line therapy or beyond (patients 1174, 1199, 1303, and 1201). Three of the 5 R/R patients died due to lymphoma progression. As of the last follow-up in February 2025, all surviving patients (n = 11) remained in complete remission.
Identification of baseline variants for longitudinal disease monitoring
In total, 151 samples were sequenced, comprising 115 cfDNA samples, 12 paired diagnostic FFPE tissue samples, 14 patient-matched PBMC samples, and cfDNA samples from 10 healthy controls for the reference PON. The median plasma cfDNA concentration in pretreatment cfDNA samples was 18 ng/mL (range, 3-809).
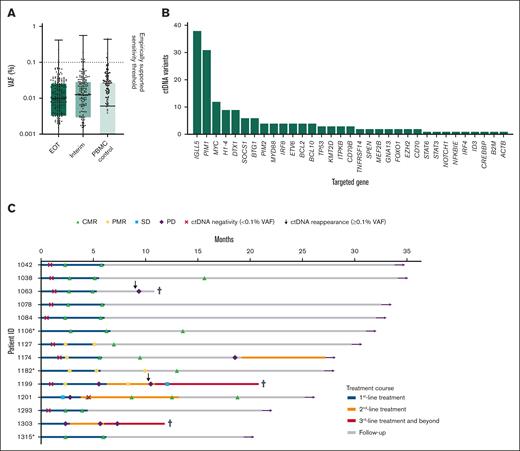
By estimating VAF background levels in cfDNA at interim, EOT, and matched PBMCs, the overall ctDNA assay sensitivity was cautiously established to be 10−3, regardless of UMI implementation, with 75% of the background noise falling below 0.03% for both sample types (Figure 1A). In addition, UMIs had limited impact on noise-reduction in FFPE tissue variant calling.
Sensitivity of the cfDNA assay, distribution of targeted gene variants, and clinical patient courses. (A) cfDNA VAFs at the EOT and midtreatment (interim) time points compared with matched PBMCs controls, establishing the empirically supported sensitivity threshold of 10−3. Null values are omitted due to log-scale representation. (B) Distribution of ctDNA follow-up variants, with the 3 most frequently mutated genes collectively accounting for 47% (81/170) of all non-Ig ctDNA variants, each detected in multiple samples. (C) Patient-specific treatment courses and ctDNA dynamics are shown horizontally. Colored segments indicate first- to third-line treatments and beyond (blue, yellow, and red, respectively) and follow-up (gray). PET-CT responses are marked with symbols: CMR, PMR, SD, and progressive disease (PD). ctDNA negativity (<0.1% VAF, red cross) and ctDNA reappearance (≥0.1% VAF, vertical arrow) are indicated. Additional markers include undetectable ctDNA (asterisk), death (black cross), and being alive at study termination (horizontal arrow). SD, stable disease.
Sensitivity of the cfDNA assay, distribution of targeted gene variants, and clinical patient courses. (A) cfDNA VAFs at the EOT and midtreatment (interim) time points compared with matched PBMCs controls, establishing the empirically supported sensitivity threshold of 10−3. Null values are omitted due to log-scale representation. (B) Distribution of ctDNA follow-up variants, with the 3 most frequently mutated genes collectively accounting for 47% (81/170) of all non-Ig ctDNA variants, each detected in multiple samples. (C) Patient-specific treatment courses and ctDNA dynamics are shown horizontally. Colored segments indicate first- to third-line treatments and beyond (blue, yellow, and red, respectively) and follow-up (gray). PET-CT responses are marked with symbols: CMR, PMR, SD, and progressive disease (PD). ctDNA negativity (<0.1% VAF, red cross) and ctDNA reappearance (≥0.1% VAF, vertical arrow) are indicated. Additional markers include undetectable ctDNA (asterisk), death (black cross), and being alive at study termination (horizontal arrow). SD, stable disease.
Genotyping of diagnostic FFPE tumor tissue and pretreatment cfDNA resulted in a total of 170 non-Ig somatic variants for longitudinal follow-up. ctDNA-specific variants, that is, eligible follow-up variants, were detected in 11 of 14 patients (79%) at baseline, with a median of 6 variants (range, 0-43; IQR, 1-13) and a median VAF of 6.88% (IQR, 1.19%-10.20%), in agreement with previous findings in DLBCL.42IGLL5, PIM1, and MYC were the 3 most frequently mutated genes (Figure 1B), observed with multiple hits per sample, comprising nearly half of all combined non-Ig ctDNA variants (81/170 [47%]). All 3 patients with undetectable ctDNA at baseline (patients 1106, 1182, and 1315) achieved CMR after first-line therapy and remained in clinical remission at study termination, while maintaining undetectable ctDNA levels. One patient (patient 1182) had persistent PMR on both interim and EOT PET-CT but ultimately transitioned to CMR after consolidative radiation. The other 2 patients achieved and maintained CMR at both time points. Treatment and longitudinal response timeline for the cohort is outlined in Figure 1C, and VAF topography is provided in supplemental Figure 3.
The median overall VAF of the tumor-specific variants in FFPE tissue was 34% (range, 7.70%-43.60%; IQR, 22.45%-40.45%). In comparison, the allelic ctDNA burden was 8.71% (range, 0.33%-18.91%; IQR, 1.47%-16.37%) at baseline, corresponding to ∼1% to 38% of total cfDNA per sample, assuming a heterozygous origin. By interim analysis, the ctDNA burden had generally fallen below the 10-3 consensus sensitivity threshold, averaging 0.09% VAF (range, 0.01%-0.61%).
Using immunoglobulin loci for longitudinal monitoring
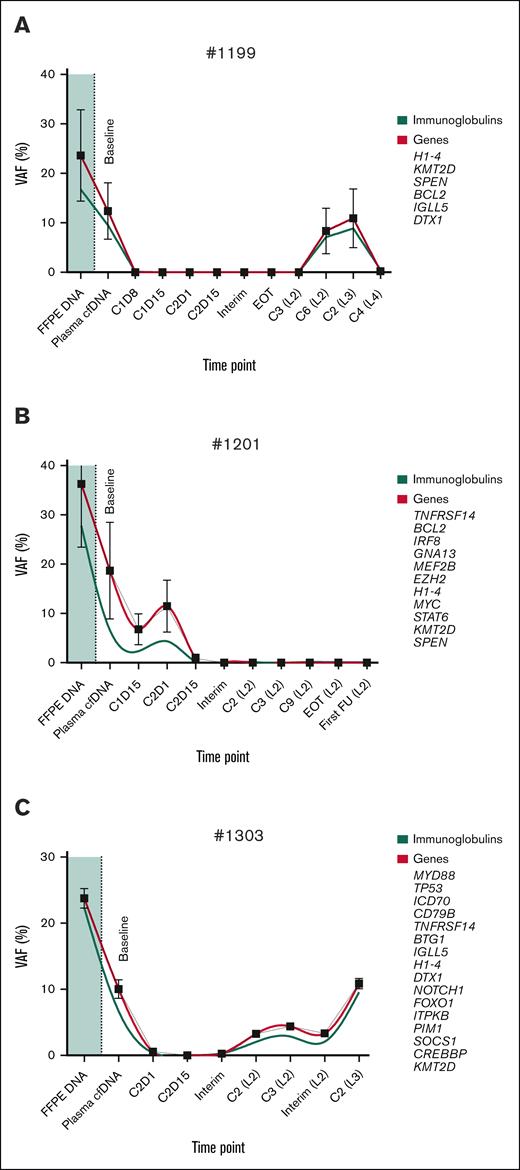
Next, we investigated whether Ig gene variants, comprising the heavy chain, lambda, and kappa light chain loci, could be implemented for longitudinal monitoring without sequence-specific clonotyping. Variant calling in these loci was reproducible in the same manner as non-Ig genes, albeit with a lower signal-to-noise ratio, showing median overall VAF decreases of 16% in FFPE tissue and 5% in cfDNA baseline samples. All 11 ctDNA-positive patients, who already demonstrated valid follow-up variants, harbored 3 to 48 Ig variant candidates and followed the established clonal trajectory, as exemplified by patients 1199, 1201, and 1303 (Figure 2).
Longitudinal tracking of Ig and non-Ig VAFs in FFPE tissue DNA and cfDNA. (A-C) Representative patient cases showing VAF dynamics for both Ig and non-Ig somatic variants in FFPE tumor tissue (shaded region) and corresponding cfDNA at baseline, during treatment, including interim, and EOT time points: patient 1199 (A); patient 1201 (B); and patient 1303 (C). Ig-related variants (green) complement non-Ig markers (red), such as BCL2, MYC, MYD88, or TP53, at generally lower VAFs and reflect the clinical treatment response. Locally weighted smoothing (LOWESS) was used for nonparametric curve regression. For simplicity, a 95% confidence interval at each time point is shown for non-Ig markers only. C, cycle; FU, follow-up; L, treatment line.
Longitudinal tracking of Ig and non-Ig VAFs in FFPE tissue DNA and cfDNA. (A-C) Representative patient cases showing VAF dynamics for both Ig and non-Ig somatic variants in FFPE tumor tissue (shaded region) and corresponding cfDNA at baseline, during treatment, including interim, and EOT time points: patient 1199 (A); patient 1201 (B); and patient 1303 (C). Ig-related variants (green) complement non-Ig markers (red), such as BCL2, MYC, MYD88, or TP53, at generally lower VAFs and reflect the clinical treatment response. Locally weighted smoothing (LOWESS) was used for nonparametric curve regression. For simplicity, a 95% confidence interval at each time point is shown for non-Ig markers only. C, cycle; FU, follow-up; L, treatment line.
ctDNA kinetics and longitudinal correlation to PET-CT quantitative metrics
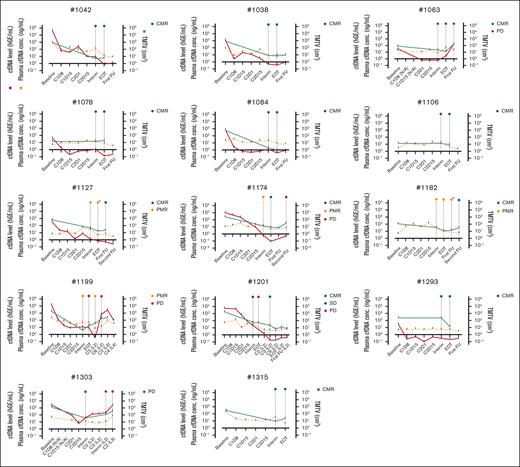
Of the 115 longitudinal cfDNA plasma samples, 6 to 12 samples were analyzed for each patient to track longitudinal ctDNA kinetics alongside metabolic response on PET-CT imaging. Generally, ctDNA, quantified in hGEs per milliliter of plasma, was concordant with TMTV, albeit with a larger dynamic range (Figure 3). Despite evident fluctuations in the treatment dynamics, ctDNA levels generally demonstrated rapid reductions from baseline to interim, regardless of the patient’s best PET-CT response during first-line therapy (Figure 4A). The decline was particularly pronounced toward C1D8, with an inverse median fold change of 106 at C1D8 and 358 at C2D15. A 350- and 715-fold reduction relative to baseline levels was observed at first-line interim and EOT assessments, respectively (Figure 4A).
Longitudinal monitoring of ctDNA levels, cfDNA concentration, and PET-CT TMTV. The panels represent each of the 14 patients, tracking ctDNA levels (hGEs per milliliter plasma; red), cfDNA concentration (nanograms per milliliter plasma; yellow; dashed), and TMTV (cubic centimeter; green) across multiple time points, including baseline, early treatment cycles for blood sampling (C1D8, C1D15, C2D1, and C2D15), interim, EOT, or later time points. Treatment beyond the first-line is indicated in parentheses after the time point, whereas time points during first-line treatment are presented without parentheses. Short curve bars indicate individual samplings. Three patients (patients 1106, 1182, and 1315) lacked ctDNA variants, meaning they had no measurable hGE. First-line interim and EOT PET-CT response, if available, and selected additional scans are depicted with color-coded circles at the top of each panel. Asterisk (∗) in patient 1127 (PMBCL) represents Deauville score 4 but clinically assessed to be in complete remission; double asterisks (∗∗) in patient 1182, Deauville score 4 after consolidative radiation. C, cycle; FU, follow-up; L, treatment line; N/A, not applicable.
Longitudinal monitoring of ctDNA levels, cfDNA concentration, and PET-CT TMTV. The panels represent each of the 14 patients, tracking ctDNA levels (hGEs per milliliter plasma; red), cfDNA concentration (nanograms per milliliter plasma; yellow; dashed), and TMTV (cubic centimeter; green) across multiple time points, including baseline, early treatment cycles for blood sampling (C1D8, C1D15, C2D1, and C2D15), interim, EOT, or later time points. Treatment beyond the first-line is indicated in parentheses after the time point, whereas time points during first-line treatment are presented without parentheses. Short curve bars indicate individual samplings. Three patients (patients 1106, 1182, and 1315) lacked ctDNA variants, meaning they had no measurable hGE. First-line interim and EOT PET-CT response, if available, and selected additional scans are depicted with color-coded circles at the top of each panel. Asterisk (∗) in patient 1127 (PMBCL) represents Deauville score 4 but clinically assessed to be in complete remission; double asterisks (∗∗) in patient 1182, Deauville score 4 after consolidative radiation. C, cycle; FU, follow-up; L, treatment line; N/A, not applicable.
Longitudinal changes in ctDNA levels during treatment evaluation. (A) Fold change in ctDNA genome equivalents (hGEs) is shown at multiple treatment-evaluation time points, including baseline, C1D8, C1D15, C2D1, C2D15, interim, and EOT. Solid lines represent patients achieving CMR on PET-CT, whereas dashed lines represent non-CMR patients. PET-CT responses are color coded: CMR (green), PMR (yellow), SD (orange) and PD (red). (B) Correlation between PET-CT TMTV and ctDNA levels (hGEs per milliliter) at baseline (grey) and EOT (green) time points (Pearson correlation coefficient, ρ = 0.90; P < .0001). (C) Comparison of PET-CT TMTV and ctDNA VAF at baseline and interim time points. Significant reductions in both TMTV (Δ = 445 cm3; median of differences) and VAF (Δ = 9%; Psigned rank test < .001). These reductions correspond to a 96.78% and 99.86% decrease, respectively, or a 31- vs 691-fold reduction. Zero values are not shown due to log scaling. Significant associations are indicated by an asterisk. PD, progressive disease; SD, stable disease.
Longitudinal changes in ctDNA levels during treatment evaluation. (A) Fold change in ctDNA genome equivalents (hGEs) is shown at multiple treatment-evaluation time points, including baseline, C1D8, C1D15, C2D1, C2D15, interim, and EOT. Solid lines represent patients achieving CMR on PET-CT, whereas dashed lines represent non-CMR patients. PET-CT responses are color coded: CMR (green), PMR (yellow), SD (orange) and PD (red). (B) Correlation between PET-CT TMTV and ctDNA levels (hGEs per milliliter) at baseline (grey) and EOT (green) time points (Pearson correlation coefficient, ρ = 0.90; P < .0001). (C) Comparison of PET-CT TMTV and ctDNA VAF at baseline and interim time points. Significant reductions in both TMTV (Δ = 445 cm3; median of differences) and VAF (Δ = 9%; Psigned rank test < .001). These reductions correspond to a 96.78% and 99.86% decrease, respectively, or a 31- vs 691-fold reduction. Zero values are not shown due to log scaling. Significant associations are indicated by an asterisk. PD, progressive disease; SD, stable disease.
Correlation analysis revealed a significant correlation between ctDNA levels and PET-CT–based TMTV at both interim (ρPearson = 0.88; P < .0001) and EOT (ρPearson = 0.90, P < .0001; Figure 4B) and, to a lesser extent, SUVmax (ρPearson = 0.71; P < .0002). In terms of allelic burden, ctDNA VAF levels showed a significant median paired reduction (ΔctDNA) of 9% from baseline to interim (Psigned-rank test = .001), compared with a median TMTV decrease (ΔTMTV) of 445 cm3 (Figure 4C).
Association between ctDNA and clinical characteristics
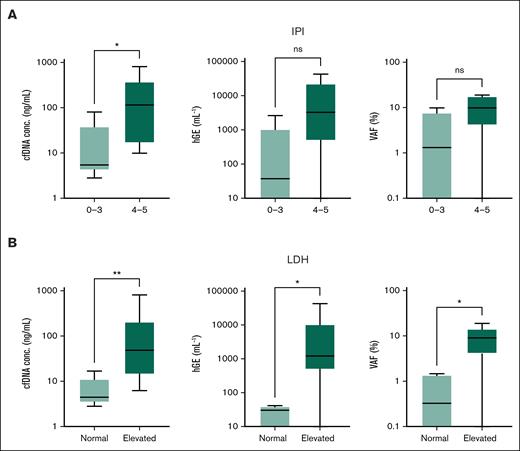
High IPI was positively associated with baseline cfDNA concentration (PU test = .02) and displayed numerically higher baseline ctDNA levels and VAF, although these were not significant (Figure 5A). Elevated lactate dehydrogenase (LDH) was associated with higher baseline cfDNA concentration (PU test < .01), ctDNA levels (hGEs per milliliter; PU test = .01), and ctDNA VAF (PU test = .01; Figure 5B). Similarly, LDH was significantly associated with TMTV (data not shown). No significant association was observed with age, performance status, extranodal sites, or Ann Arbor stage.
ctDNA metrics stratified by IPI score and LDH levels. Box plots depict the distribution of cfDNA concentration (nanograms per milliliter), ctDNA genomic equivalents (hGEs per milliliter), and ctDNA VAF (%) across the 2 clinical stratifications: IPI scores 0 to 3 vs 4 to 5 (A) and normal vs elevated LDH levels (B). Significant associations are indicated by asterisk. ∗PU test < 0.05, ∗∗PU test < 0.01. ns, not significant.
ctDNA metrics stratified by IPI score and LDH levels. Box plots depict the distribution of cfDNA concentration (nanograms per milliliter), ctDNA genomic equivalents (hGEs per milliliter), and ctDNA VAF (%) across the 2 clinical stratifications: IPI scores 0 to 3 vs 4 to 5 (A) and normal vs elevated LDH levels (B). Significant associations are indicated by asterisk. ∗PU test < 0.05, ∗∗PU test < 0.01. ns, not significant.
Qualitative description of ctDNA kinetics and longitudinal PET-CT treatment response
Most patients displayed a decline in ctDNA levels from baseline and midtreatment to EOT (Figure 4A). However, in 3 of 5 patients who experienced R/R disease during or after first-line therapy, ctDNA levels either increased (2 patients) or remained stable (1 patient) from interim to EOT or an earlier time point if progression occurred before EOT. Overall, ctDNA kinetics closely aligned with the clinical disease course of patients, with levels dropping below 10−3 in patients achieving CMR and continuing at low levels during follow-up, whereas patients with refractory disease or early relapse were unable to reach the same low levels. Interestingly, the patient with a late relapse (patient 1174) reached the sensitivity threshold level, as did those in sustained remission.
Notably, patient 1303 had an aggressive multirefractory disease course. Progression was first detected on interim PET-CT during first-line therapy, which was reflected in concurrently rising ctDNA levels with TMTV. Despite initiating second-line treatment, ctDNA levels continued to rise during cycle 2 and cycle 3 of second-line therapy, which preceded second-line interim PET-CT confirmation of disease progression 2 months later. Another example of rising ctDNA levels before PET-CT–verified relapse was seen in patient 1063. The patient achieved CMR on both interim and EOT PET-CT during first-line therapy but relapsed 4 months after treatment cessation. ctDNA levels initially declined from baseline to C2D15 but then began rising again toward interim and EOT, suggestive of residual disease, which preceded PET-CT–detected relapse and TMTV increase by several months.
Discussion
The advent of plasma cfDNA–based ctDNA screening has created a window of opportunity for improving LBCL early response assessment and monitoring, enabling real-time monitoring throughout the disease course. In this prospective study, we assessed the potential of longitudinal ctDNA variants as follow-up markers for treatment response evaluation and MRD assessment using targeted panel sequencing with a small custom panel. In brief, our findings demonstrate that ctDNA kinetics closely reflect underlying tumor biology, the clinical course, and PET-CT, underscoring the validity of the assay and supporting the feasibility of a small gene panel for longitudinal ctDNA monitoring. The lowest empirically supported detection of individual ctDNA marker sets was between 10–3 and 10–4. Therefore, the sensitivity threshold for confident MRD assessment was rationally kept at 10–3. As reviewed by Chin et al,29 such a detection level is required to track mutant allele fractions after treatment. Although sensitivities of 10–5 levels have been extrapolated in silico using integrated digital error suppression cancer personalized profiling by deep sequencing,40 the empirically supported sensitivity threshold, even with this ultrasensitive method, is in the range of 10–4(39) and is increasingly challenged by artifacts <10–3.40 Such background noise is challenging even with personalized cancer profiling or the extremely high specificity of IgH clonotyping due to fluctuations in sample quality, amplification efficiency, coverage, and the stochasticity governing low-level markers, as we have shown and elaborated upon previously.57,58
The multiple early-phase sampling time points revealed interesting kinetics, with ctDNA levels rapidly declining in the early treatment phase, particularly within the first week of cycle 1, with a 2-log fold reduction, regardless of eventual disease outcome, akin to the early molecular response, defined as a 2-log fold reduction in ctDNA levels after the first treatment cycle, reported by Kurtz et al31 and similar studies.33 By C1D8, the effects of treatment had already led to diminished baseline-detected variants in half of the patients. Thus, despite our small cohort, we could qualitatively segregate patients into rapid responders, slow responders, R/R patients, and patients with undetectable ctDNA.
Both imaging- and sequencing-based modalities showed a significant reduction from baseline to interim assessment, with a strong correlation despite differences in dynamic range. Furthermore, ctDNA allele frequencies were direct proxies for longitudinal tracking without conversion to genomic equivalents. Notably, the combined evaluation of TMTV with cfDNA concentration and ctDNA VAF to hGEs provided a stronger emphasis on R/R disease identification than with imaging alone, which was appreciable even well below 10–3. In addition to the strong correlation with TMTV and SUVmax, ctDNA levels were associated with elevated LDH, all of which are markers of disease burden, further supporting the value of quantified ctDNA as a surrogate tumor burden marker, consistent with previous studies.31,33,34 The lack of association with advanced Ann Arbor stage (III/IV) could be attributed to the small cohort size and the fact that most patients in the cohort had advanced-stage disease.
In 4 of 5 patients who experienced R/R disease during the study period, rising ctDNA levels, indicative of residual disease, were seen concurrently with or before PET-CT–detected relapse. Retrospective and prospective studies have demonstrated that ctDNA detection can precede PET-CT–confirmed relapse by several months.30,32,59 Patients who developed R/R disease generally displayed numerically higher ctDNA levels throughout first-line therapy. However, statistical significance was not reached, likely due to the small cohort size. Of note, an inconspicuous longitudinal PET-CT trajectory was observed in patients with no valid follow-up ctDNA variants or ctDNA follow-up levels below the sensitivity threshold.
Generally, the most significant ctDNA kinetics occurred in the early phase during the first treatment cycles. All patients demonstrated a rapid decline in ctDNA during first-line treatment, regardless of their final PET-CT response after first-line treatment, which partly reflects an initial treatment effect. However, some of these patients did not achieve CMR on EOT PET-CT, suggesting the presence of undetected residual molecular disease in these patients. To detect true low-frequency ctDNA signals in the early treatment phase may, therefore, require enhanced sensitivity <10–3 to reliably discriminate optimal responders from those who develop R/R disease.
The immunoglobulin heavy chain, lambda, and kappa light chain gene loci were included in the custom gene panel. Despite a lower signal-to-noise ratio, Ig variants enhanced observational confidence in the patients harboring only 1 to 4 non-Ig variants. Thus, non-Ig markers can be readily supplemented by mapping the Ig loci and may even be informative in their complete absence. Intriguingly, this suggests a potential for longitudinal ctDNA monitoring without requiring full-sequence clonotyping of rearranged immunoglobulins or determination of somatic vs germ line configuration. The robustness and broader applicability of these findings warrant further investigation.
To minimize sequencing errors, we incorporated UMIs but found their omission to have minimal impact on variant detection in longitudinal ctDNA analysis and FFPE DNA samples. Although UMIs enhance cfDNA analysis due to low input DNA and low-VAF variants, their contribution may be negligible in tracking predefined mutations over time. Together with the high specificity of longitudinally detected variants, the high depth of coverage and stringent bioinformatics likely compensate for a lack of UMIs, potentially streamlining the workflow and reducing costs. In a continued follow-up, it may be argued that either FFPE DNA or baseline cfDNA is superfluous. However, as we have demonstrated previously,42 the sequencing of FFPE DNA is error prone, and cfDNA allele frequencies can be notoriously low, with highly skewed VAFs.60
Diagnostic laboratories face growing demands for molecular analyses in personalized medicine. Our study emphasizes the use of ctDNA in clinical settings, using small-panel targeted sequencing with universal primers and manageable bioinformatics. The landmark studies by Kurtz et al,31,41 Roschewski et al,30 and Rossi et al34 demonstrate ultrasensitive and personalized detection methods. However, such approaches may confer a labor-intensive laboratory workflow and high bioinformatic complexity. The risk is that the many advanced techniques may not gain widespread implementation in routine clinical practice in the near future. Furthermore, the risk of overinterpreting biologically nonsignificant findings may also raise concerns on whether ultrahigh sensitivities will gain acceptance in the clinic. Here, it is demonstrated that a streamlined, cost-effective, and simple assay, with a turnaround time within 1 to 2 weeks can be compatible with clinical decision-making before subsequent treatment cycle initiation, potentially allowing for ctDNA-based early response assessment and monitoring. However, establishing thresholds for such ctDNA-driven early risk-adapted therapy stands to be investigated further.
This study presents some limitations. The small patient cohort limited our ability to perform meaningful survival analyses and detect significant associations. However, a total of 151 samples were sequenced, providing a solid foundation for comprehensive analysis. The proportion of patients without detectable ctDNA (∼20%) may also pose a limitation. However, it aligns with the biological variability in ctDNA detection observed in other studies.33,34 This suggests that it could be an inherent characteristic of ctDNA detection variability rather than a technical limitation.
Although targeted panel sequencing enhances sequencing depth and sensitivity compared to more comprehensive sequencing such as whole-exome sequencing, genomic breadth is compromised, thereby limiting the detection of emerging new mutations and clonal evolution. Consequently, the assay could not inform structural variants, such as copy number alterations. Importantly, the potential of ctDNA does not rely on replacing existing methods, and conventional techniques can still provide valuable information on structural variants, including copy number alterations. However, in targeted assays, such as the one presented here, the flexibility of adding genes or balancing chromosomal reads is an inherent feature. Although not as sensitive as tracking rearranged immunoglobulin heavy chain gene segments, another potential is the detection of clonal progression alongside therapeutic intervention.
AI was implemented to avoid bias in manual or semiautomated radiological assessment, but we also acknowledge that this field, although promising, remains in its early stages. AI-based TMTV assessment using RECOMIA has previously been validated55; however, AI-calculated TMTV was not compared to manual segmentation in this study. Although PET-CT protocols were adjusted slightly during the study, we consider the impact negligible.
In conclusion, this study supports the value of longitudinal ctDNA monitoring in patients with LBCL using a streamlined, targeted assay with a sensitivity of 10–3. ctDNA kinetics closely reflect disease response assessed by PET-CT and tumor biology, reinforcing assay validity. Pretreatment ctDNA levels correlated with LDH and AI-based TMTV, positioning it as a surrogate tumor burden marker with the potential to complement PET-CT. Future efforts should focus on further validating simple and cost-effective assays to ensure broad clinical adoption. Establishing operational thresholds in clinical trials will also be essential for integrating ctDNA into routine practice.
Acknowledgments
The authors are grateful for the patients' participation and essential contributions. The authors also thank Catharina Strange for her excellent and dedicated assistance in organizing and collecting sequential blood samples. Furthermore, the authors acknowledge the work provided by Louise Borup and Dorte Jensen at the Clinical Genome Center, Odense University Hospital (OUH), and the Molecular Pathology Laboratory at the Department of Pathology, OUH. Sequencing data processing was performed using the UCloud interactive high-performance computing platform, provided by the eScience Center at the University of Southern Denmark.
This study was supported by the Danish Cancer Society (R302-A17408), Vissing Foundation (532937 AHO/LAX), Dagmar Marshall’s Foundation, Arvid Nilsson’s Foundation, Eva og Henry Frænkels Mindefond, OUH Internationalisation Fund, and Region of Southern Denmark (A846).
G.V. is a PhD candidate at the University of Southern Denmark, and this work is submitted in partial fulfillment of the requirements for the PhD.
Authorship
Contribution: G.V., T.S.L., C.G.N., M.B.M., M.H.H., O.M.L.C., and M.T. contributed to conception and design; G.V., M.T., M.N.-B., A.L.N., M.G.H., O.M.L.C., M.L.G.K., S.K.D., L.E., and M.H.H. collected and assembled data; G.V., M.H.H., M.N.-B., and L.E. analyzed data; and all authors wrote the manuscript and read and approved the final version.
Conflict-of-interest disclosure: T.S.L. reports research support from Genentech; and is involved in consultancy/advisory boards for Roche, Gilead, and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Gayaththri Vimalathas, Department of Pathology, Odense University Hospital, J.B. Winsløws Vej 15, 3rd Floor, 5000 Odense, Denmark; email: Gayaththri.Vimalathas2@rsyd.dk.
References
Author notes
Raw sequencing files are publicly available in the European Nucleotide Archive (European Molecular Biology Laboratory – European Bioinformatics Institute [EMBL-EBI], accession number PRJEB89850).
Processed variant data can be downloaded at Harvard Dataverse: https://doi.org/10.7910/DVN/QL5J3W. The targeted panel design and annotation strategy are detailed at https://github.com/marcus-hoy-hansen/Vimalathas2025-cfDNApanel (GitHub).
Questions concerning shared data can be directed to the author, Marcus Høy Hansen (Marcus.Hoy.Hansen@rsyd.dk).
The full-text version of this article contains a data supplement.