Key Points
This represents the largest data set for any chimeric antigen receptor T-cell therapy in pediatric and young adult patients with B-ALL.
Outcomes in the real-world setting continue to demonstrate high RFS and favorable short- and long-term safety.
Visual Abstract
Since the first approval of tisagenlecleucel in 2017, pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) may receive this CD19-directed chimeric antigen receptor T-cell therapy. We report real-world data from the Center for International Blood and Marrow Transplant Research (>2.5 years of follow-up). As of 4 May 2022, 768 patients with B-ALL had received tisagenlecleucel. Patients aged ≥18 and <18 years of age (including those <3 years) were treated during first relapse (26.6% and 26.7% [<3 years, 44.8%], respectively) or primary refractory disease (12.4% and 12.1% [<3 years, 15.5%], respectively) with 17.6% and 11.6% (<3 years, 13.8%), respectively, having high disease burden (≥50% bone marrow [BM] blasts) and 20.2% and 20.2% (<3 years, 13.8%), respectively, having low disease burden (>0 to <5% BM blasts). Among patients with ≥12 months postinfusion follow-up (n = 578; median follow-up, 32.1 months), the best overall response of complete remission/complete remission with incomplete blood count recovery was 86.0%. The 12-month relapse-free survival (RFS) and overall survival (OS) were 61.8% and 79.4%, respectively, whereas the 24-month RFS and OS were 50.3% and 63.8%, respectively. Age (<18 years) and disease burden (<50% BM blasts) were associated with better outcomes. Previous inotuzumab therapy and KMT2A rearrangement were associated with worse outcomes. Older patients (≥18 years) experienced a higher rate of any-grade cytokine release syndrome (CRS) associated with higher disease burden before infusion. Any-grade CRS and neurotoxicity were lower in patients aged <3 years. Extended follow-up continues to demonstrate high rates of RFS and favorable safety in this population.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common form of childhood cancer globally; B-cell ALL (B-ALL) accounts for >80% of ALL cases.1,2 Approximately 60% occur in patients younger than 20 years with a peak incidence in children aged 2 to 5 years.3 Despite the high rates of initial response to frontline treatment for ALL, relapse may occur in 15% to 35% of pediatric and young adult (Ped-YA) patients.4-8 Postrelapse outcomes for these patients are generally poor (5-year survival, 20%-58%),5-7 indicating a need for improved therapeutic options and outcomes.
CD19-directed chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment options for Ped-YA patients with relapsed or refractory (R/R) B-ALL.9-12 Tisagenlecleucel (Kymriah, Novartis) is an autologous CD19-directed T-cell immunocellular therapy in which a patient’s T cells are genetically modified to express a second-generation CAR, composed of an FMC63 scFv, 4-1BB costimulatory domain, and CD3ζ activation domain.9,13 Tisagenlecleucel is approved by the US Food and Drug Administration, European Medicines Agency, and 13 other countries for Ped-YA (aged ≤25 years) patients with R/R B-ALL based on results of the registrational phase 2 ELIANA trial (N = 79; ClinicalTrials.gov identifier: NCT02435849).9,10,14-16 The pivotal ELIANA trial included patients from 3 to ≤24 years with CD19+ R/R B-ALL.9,10 At the 3-year follow-up, the overall remission rate was 82%, the overall survival (OS) rate was 63%, and the long-term safety profile was favorable.17 Pooled data from ELIANA and the earlier phase 2 ENSIGN trial (N = 29; NCT02228096) demonstrated that the efficacy and safety of tisagenlecleucel were consistent across age groups (<18 and ≥18-25 years) and demonstrated high levels of durable response in YA patients.12,18 When compared with the current treatment options for Ped-YA patients with R/R ALL,19 tisagenlecleucel has demonstrated improved survival outcomes and offers a potentially curative option for those with R/R B-ALL, including patients with and without previous allogeneic stem cell transplantation (allo-SCT).10,12,20-25
Real-world evidence (RWE) confirmed the efficacy and tolerability of tisagenlecleucel in Ped-YA patients with R/R B-ALL and highlighted predictors of response.26-28 High remission rates and satisfactory safety were reported with tisagenlecleucel in clinical studies in patients with R/R B-ALL, including patients with Down syndrome (DS) and those with isolated central nervous system (CNS) disease.29-31 Poorer efficacy and safety outcomes have been associated with greater disease burden (DB) and previous nonresponse to blinatumomab, in part, because of diminished CD19 expression and CD19– relapse.26-28
The Center for International Blood and Marrow Transplant Research (CIBMTR) cellular therapy registry contains the largest global data set of patients treated with commercial tisagenlecleucel with a postauthorization safety study goal of enrolling 1000 patients with B-ALL for 15 years of follow-up.32,33 Early RWE for tisagenlecleucel from the CIBMTR show similar efficacy as reported for ELIANA with no new safety signals.33 Here, we report updated data for 768 Ped-YA patients with R/R B-ALL who received commercial tisagenlecleucel (including out-of-specification products) as previously described.33 We analyzed factors that affected relapse and survival in patients ≥18 and <18 years with special focus on patients who would have been excluded from ELIANA (<3 years, CNS disease before infusion [including isolated CNS relapse], <5% bone marrow blasts (BMBs), or previous blinatumomab treatment). Importantly, we also report postinfusion data, including CD19+/CD19– relapse status and subsequent neoplasms.
Methods
Data source
This noninterventional, prospective, longitudinal study used data from the CIBMTR, a collaboration between the Medical College of Wisconsin and the National Marrow Donor Program.32 Clinical data were collected through standardized forms from participating centers in the United States and Canada and reported by each treatment center to the CIBMTR, as previously described.33
All patients/guardians provided written informed consent. All study designs and amendments received institutional review board and/or ethics committee approval.
Tisagenlecleucel manufacturing
Tisagenlecleucel was manufactured at centralized facilities using cryopreserved leukapheresis material, as previously described.33,34 Tisagenlecleucel was tested to ensure product safety, purity, potency, and identity before shipment to the clinical site.33,34 Patients may have received tisagenlecleucel that was out of specification (eg, <80% cell viability), given under the expanded access protocol, but that was deemed safe for infusion based on previous studies showing comparable efficacy and safety.33,35
Patients and study design
Eligible patients were ≤25 years (at the time of order for tisagenlecleucel), had R/R B-ALL, and were reported to the CIBMTR registry as being infused with commercial tisagenlecleucel (including out-of-specification doses) between 30 August 2017 and 4 May 2022. Efficacy and safety outcomes were assessed in the overall population (OP) and in subgroups by age (≥18, <18, and <3 years) and by other key baseline characteristics, namely DS diagnosis, previous treatment in number of lines of therapy (allo-SCT, CAR T-cell therapy, blinatumomab, inotuzumab), cytogenetics (KMT2A-rearranged leukemia and Philadelphia chromosome positive), third or later relapse, minimal residual disease (MRD) status, DB, and isolated CNS disease. The <18-year-old subgroup included patients younger than 3 years. Low (<5%), intermediate (≥5 to <50%), and high (≥50%) DB were defined based on the percentage of BMBs before infusion (timing of preinfusion disease assessment was not standardized between participating sites).
Assessments
The efficacy and safety assessments were reported at day 100, months 6 and 12, and then annually. The efficacy data set included patients who had ≥12 months of follow-up (including deaths before 12 months) between infusion and the analysis cutoff date, and the safety data set included all patients who had completed the day 100 safety form (3-month follow-up; supplemental Figure 1).
Manufacturing turnaround time was defined as time from receipt of the apheresis product at the manufacturing site to shipment of the product to the treatment center. This was a factor in data analyses that were based on time to infusion.
The participating centers reported the best overall response (BOR) of complete remission (CR) or CR with incomplete hematologic recovery (CRi), MRD response (by flow cytometry), relapse-free survival (RFS), and OS, along with supporting data to analyze these outcomes. BOR of CR/CRi was recorded from the infusion until relapse or start of a new anticancer therapy (whichever came first). CR/CRi was defined as complete morphologic response (<5% BMBs). MRD was reported at the 100-day assessment and during the entire follow-up period (method of assessment not specified). RFS was defined as the time from first disease response (CR/CRi after infusion) to relapse or death from any cause. OS was defined as the date of first CAR T-cell infusion to death from any cause (RFS and OS were analyzed with and without censoring of any postinfusion allo-SCT).
The numbers of CD19+ or CD19– relapse patients (among those with BOR of CR/CRi; captured after 2020), subsequent neoplasms (including lineage switch from ALL), or pregnancies with live births were reported.
The safety outcomes included cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), prolonged neutropenia and thrombocytopenia, cerebral edema, and death. The reporting centers graded CRS and ICANS according to the American Society of Transplant and Cellular Therapy criteria36 based on the most severe event within 100 days of infusion. Neutropenia and thrombocytopenia were defined as prolonged in patients who had persistent grade 4 (by Common Terminology Criteria for Adverse Events version 4.03) neutropenia and/or thrombocytopenia at 100 days, 6 months, and 1 year of follow-up after infusion and who also had >30 days of follow-up and failed to recover their neutrophil count (absolute neutrophil count of ≥0.5 × 103/µL achieved and sustained for 3 laboratory values) or platelet count (≥20 × 109/L achieved) levels at day 30 with either no recovery or recovery after 30 days. These analyses were not adjusted for DB or other covariates.
Statistical analyses
Patients were followed from the time of first infusion to data cutoff. The manufacturing turnaround time was reported as the median number of days with interquartile range. The BOR of CR/CRi was summarized using descriptive statistics (number [percent]), along with the 2-sided standard asymptotic 95% confidence interval (CI) in the efficacy set. Time-to-event end points (RFS and OS) were estimated using the Kaplan-Meier method. The median (95% CI) duration and proportion (percent, 95% CI) of patients who remained event-free at month 12 were presented for the OP and compared with subgroups by age (≥18, <18, and <3 years); the log-rank P value was used for significance tests between the age groups of ≥18 and <18 years (unadjusted for multiplicity). P values < .05 were considered statistically significant. Univariate analyses were performed using linear regressions. Multivariate analyses were performed using a Cox proportional hazard model (for time-to-event–based end points). For the BOR analysis, a multivariate logistic regression was used to calculate the odds ratio.
Patients with CD19+ or CD19– relapse at 12 months after infusion or with subsequent neoplasms were reported as frequencies (number [percent]).
The total number of patients who presented with CRS or ICANS was recorded as frequency (number [percent]), including frequency by maximum grade. The time to onset of CRS and ICANS was recorded as the median (range) in days. Kaplan-Meier analyses were used to estimate the median duration of CRS and ICANS, measured in days with 2-sided 95% CIs.
The SAS, version 9.4 (SAS Institute), software package was used for all data analyses, which were conducted by Novartis.
Results
Baseline demographics and disease characteristics
As of 4 May 2022, 768 patients with R/R B-ALL received infusion of commercial or out-of-specification tisagenlecleucel. The median age at infusion was 13.8 years (range, 0.4-25.9; <3 years, n = 58; <18 years, n = 535; and ≥18 years, n = 233). Patients received a median of 4 (range, 1-19) previous lines of therapy. The median manufacturing turnaround time for all infused patients was 26 days (interquartile range, 24-30; Table 1).
Baseline demographics and disease characteristics by age infused set
| . | All patients (N = 768) . | ≥18 years (n = 233) . | <18 years (n = 535) . | <3 years (n = 58) . |
|---|---|---|---|---|
| Median age at infusion (range), y | 13.8 (0.4-25.9) | 21.8 (18.0-25.9) | 10.6 (0.4-17.9) | 1.6 (0.4-2.9) |
| Male sex | 459 (59.8) | 144 (61.8) | 315 (58.9) | 29 (50.0) |
| Race | ||||
| White | 542 (70.6) | 171 (73.4) | 371 (69.3) | 37 (63.8) |
| African-American | 43 (5.6) | 14 (6.0) | 29 (5.4) | 9 (15.5) |
| Asian | 26 (3.4) | 4 (1.7) | 22 (4.1) | 2 (3.4) |
| American Indian or Alaskan Native | 5 (0.7) | 1 (0.4) | 4 (0.7) | 0 |
| Other | 30 (3.9) | 6 (2.6) | 24 (4.5) | 4 (6.9) |
| Unknown | 75 (9.8) | 21 (9.0) | 54 (10.1) | 4 (6.9) |
| Not reported | 47 (6.1) | 16 (6.9) | 31 (5.8) | 2 (3.4) |
| Ethnicity | ||||
| Hispanic or Latino | 304 (39.6) | 118 (50.6) | 186 (34.8) | 12 (20.7) |
| Non-Hispanic or Latino | 383 (49.9) | 100 (42.9) | 283 (52.9) | 41 (70.7) |
| Nonresident of the United States | 55 (7.2) | 9 (3.9) | 46 (8.6) | 2 (3.4) |
| Unknown | 26 (3.4) | 6 (2.6) | 20 (3.7) | 3 (5.2) |
| Karnofsky/Lansky score of <80 | 106 (13.8) | 40 (17.2) | 66 (12.3) | 3 (5.2) |
| DS | 47 (6.1) | 7 (3.0) | 40 (7.5) | 0 |
| Cytogenetics | ||||
| Abnormal 11q23/KMT2A rearrangement | 110 (14.3) | 23 (9.9) | 87 (16.3) | 44 (75.9) |
| Ph+ | 46 (6.0) | 19 (8.2) | 27 (5.0) | 0 |
| Previous systemic therapy | ||||
| Median previous lines of therapy (range), n | 4 (1-19) | 4 (1-15) | 4 (1-19) | 3 (1-13) |
| Chemotherapy | 622 (81.0) | 182 (78.1) | 440 (82.2) | 51 (87.9) |
| Tyrosine kinase inhibitors∗ | 80 (10.4) | 32 (13.7) | 48 (9.0) | 0 |
| allo-SCT | 195 (25.4) | 61 (26.2)† | 134 (25.0) | 12 (20.7) |
| CAR T-cell therapy | 16 (2.1) | 6 (2.6) | 10 (1.9) | 0 |
| Blinatumomab | 142 (18.5) | 62 (26.6) | 80 (15.0) | 7 (12.1) |
| Inotuzumab | 71 (9.2) | 39 (16.7) | 32 (6.0) | 4 (6.9) |
| Rituximab | 22 (2.9) | 16 (6.9) | 6 (1.1) | 0 |
| Other | 172 (22.4) | 61 (26.2) | 111 (20.7) | 14 (24.1) |
| Most recent disease status before infusion | ||||
| Primary refractory‡ | 94 (12.2) | 29 (12.4) | 65 (12.1) | 9 (15.5) |
| First relapse | 205 (26.7) | 62 (26.6) | 143 (26.7) | 26 (44.8) |
| Second relapse | 139 (18.1) | 45 (19.3) | 94 (17.6) | 5 (8.6) |
| Third or later relapse | 56 (7.3) | 26 (11.2) | 30 (5.6) | 2 (3.4) |
| Morphologic CR‡ | 271 (35.3) | 71 (30.5) | 200 (37.4) | 16 (27.6) |
| MRD negative | 163 (60.1) | 44 (62.0) | 119 (59.5) | 11 (68.8) |
| MRD positive | 100 (36.9) | 25 (35.2) | 75 (37.5) | 5 (31.3) |
| Not reported or not tested | 9 (3.3) | 2 (2.8) | 7 (3.5) | 0 |
| BMB percentage before infusion | ||||
| 0 | 181 (23.6) | 48 (20.6) | 133 (24.9) | 14 (24.1) |
| >0 to <5% | 155 (20.2) | 47 (20.2) | 108 (20.2) | 8 (13.8) |
| ≥5% to <50% | 128 (16.7) | 34 (14.6) | 94 (17.6) | 12 (20.7) |
| ≥50% | 103 (13.4) | 41 (17.6) | 62 (11.6) | 8 (13.8) |
| Not reported | 201 (26.2) | 63 (27.0) | 138 (25.8) | 16 (27.6) |
| Extramedullary disease before infusion | ||||
| No | 561 (73.0) | 163 (70.0) | 398 (74.4) | 37 (63.8) |
| Yes | 114 (14.8) | 42 (18.0) | 72 (13.5) | 16 (27.6) |
| Isolated CNS disease | 71 (9.2) | 25 (10.7) | 46 (8.6) | 11 (19.0) |
| . | All patients (N = 768) . | ≥18 years (n = 233) . | <18 years (n = 535) . | <3 years (n = 58) . |
|---|---|---|---|---|
| Median age at infusion (range), y | 13.8 (0.4-25.9) | 21.8 (18.0-25.9) | 10.6 (0.4-17.9) | 1.6 (0.4-2.9) |
| Male sex | 459 (59.8) | 144 (61.8) | 315 (58.9) | 29 (50.0) |
| Race | ||||
| White | 542 (70.6) | 171 (73.4) | 371 (69.3) | 37 (63.8) |
| African-American | 43 (5.6) | 14 (6.0) | 29 (5.4) | 9 (15.5) |
| Asian | 26 (3.4) | 4 (1.7) | 22 (4.1) | 2 (3.4) |
| American Indian or Alaskan Native | 5 (0.7) | 1 (0.4) | 4 (0.7) | 0 |
| Other | 30 (3.9) | 6 (2.6) | 24 (4.5) | 4 (6.9) |
| Unknown | 75 (9.8) | 21 (9.0) | 54 (10.1) | 4 (6.9) |
| Not reported | 47 (6.1) | 16 (6.9) | 31 (5.8) | 2 (3.4) |
| Ethnicity | ||||
| Hispanic or Latino | 304 (39.6) | 118 (50.6) | 186 (34.8) | 12 (20.7) |
| Non-Hispanic or Latino | 383 (49.9) | 100 (42.9) | 283 (52.9) | 41 (70.7) |
| Nonresident of the United States | 55 (7.2) | 9 (3.9) | 46 (8.6) | 2 (3.4) |
| Unknown | 26 (3.4) | 6 (2.6) | 20 (3.7) | 3 (5.2) |
| Karnofsky/Lansky score of <80 | 106 (13.8) | 40 (17.2) | 66 (12.3) | 3 (5.2) |
| DS | 47 (6.1) | 7 (3.0) | 40 (7.5) | 0 |
| Cytogenetics | ||||
| Abnormal 11q23/KMT2A rearrangement | 110 (14.3) | 23 (9.9) | 87 (16.3) | 44 (75.9) |
| Ph+ | 46 (6.0) | 19 (8.2) | 27 (5.0) | 0 |
| Previous systemic therapy | ||||
| Median previous lines of therapy (range), n | 4 (1-19) | 4 (1-15) | 4 (1-19) | 3 (1-13) |
| Chemotherapy | 622 (81.0) | 182 (78.1) | 440 (82.2) | 51 (87.9) |
| Tyrosine kinase inhibitors∗ | 80 (10.4) | 32 (13.7) | 48 (9.0) | 0 |
| allo-SCT | 195 (25.4) | 61 (26.2)† | 134 (25.0) | 12 (20.7) |
| CAR T-cell therapy | 16 (2.1) | 6 (2.6) | 10 (1.9) | 0 |
| Blinatumomab | 142 (18.5) | 62 (26.6) | 80 (15.0) | 7 (12.1) |
| Inotuzumab | 71 (9.2) | 39 (16.7) | 32 (6.0) | 4 (6.9) |
| Rituximab | 22 (2.9) | 16 (6.9) | 6 (1.1) | 0 |
| Other | 172 (22.4) | 61 (26.2) | 111 (20.7) | 14 (24.1) |
| Most recent disease status before infusion | ||||
| Primary refractory‡ | 94 (12.2) | 29 (12.4) | 65 (12.1) | 9 (15.5) |
| First relapse | 205 (26.7) | 62 (26.6) | 143 (26.7) | 26 (44.8) |
| Second relapse | 139 (18.1) | 45 (19.3) | 94 (17.6) | 5 (8.6) |
| Third or later relapse | 56 (7.3) | 26 (11.2) | 30 (5.6) | 2 (3.4) |
| Morphologic CR‡ | 271 (35.3) | 71 (30.5) | 200 (37.4) | 16 (27.6) |
| MRD negative | 163 (60.1) | 44 (62.0) | 119 (59.5) | 11 (68.8) |
| MRD positive | 100 (36.9) | 25 (35.2) | 75 (37.5) | 5 (31.3) |
| Not reported or not tested | 9 (3.3) | 2 (2.8) | 7 (3.5) | 0 |
| BMB percentage before infusion | ||||
| 0 | 181 (23.6) | 48 (20.6) | 133 (24.9) | 14 (24.1) |
| >0 to <5% | 155 (20.2) | 47 (20.2) | 108 (20.2) | 8 (13.8) |
| ≥5% to <50% | 128 (16.7) | 34 (14.6) | 94 (17.6) | 12 (20.7) |
| ≥50% | 103 (13.4) | 41 (17.6) | 62 (11.6) | 8 (13.8) |
| Not reported | 201 (26.2) | 63 (27.0) | 138 (25.8) | 16 (27.6) |
| Extramedullary disease before infusion | ||||
| No | 561 (73.0) | 163 (70.0) | 398 (74.4) | 37 (63.8) |
| Yes | 114 (14.8) | 42 (18.0) | 72 (13.5) | 16 (27.6) |
| Isolated CNS disease | 71 (9.2) | 25 (10.7) | 46 (8.6) | 11 (19.0) |
Data are reported as n (%), unless otherwise specified.
Ph+, Philadelphia chromosome positive.
Includes dasatinib, imatinib, ponatinib, and nilotinib.
One patient aged >18 years received an autologous SCT.
Disease status was defined by response to CIBMTR Comprehensive Report Form 2402 R3.0 Question 147: primary refractory was classified as primary induction failure, and morphologic CR included first, second, or third or later CR.
In patients with available baseline bone marrow assessments (without a defined time point for the assessment), 181 patients (23.6%) had 0% BMB by morphology, 155 (20.2%) had >0% to <5% (low DB), 128 (16.7%) had ≥5% to <50% (intermediate DB), and 103 (13.4%) had ≥50% (high DB). Of the 271 patients (35.3%) in morphologic CR before infusion, 163 (60.1%) were MRD negative (Table 1). Patients ≥18 years had a higher DB and more were in their third or later relapse or received previous blinatumomab or inotuzumab when compared with patients aged <18 years (Table 1). When comparing patients <18 year and those ≥18 years, KMT2A rearrangement was seen in 16.3% and 9.9%, respectively, extramedullary disease in 13.5% and 18.0%, isolated CNS disease in 8.6% and 10.7%, 12.1% and 12.4% had primary refractory disease (PRD), respectively, and 26.7% and 26.6% had their first relapse. Within the <3-year-old subgroup, there were more patients who had the following risk factors: KMT2A rearrangement (75.9%), extramedullary disease (27.6%), isolated CNS disease (19.0%), PRD (15.5%), and first relapse (44.8%). Overall, the proportion of patients who received allo-SCT was similar in the <18- and ≥18-year age groups (25.0% and 26.2%, respectively) but was lower (20.7%) in the <3-year group.
Across all patients, the incidence of high DB, third or later relapse, and previous allo-SCT at the time of infusion decreased over time since tisagenlecleucel approval (2018-2022; supplemental Figure 1).
Efficacy
Response to treatment
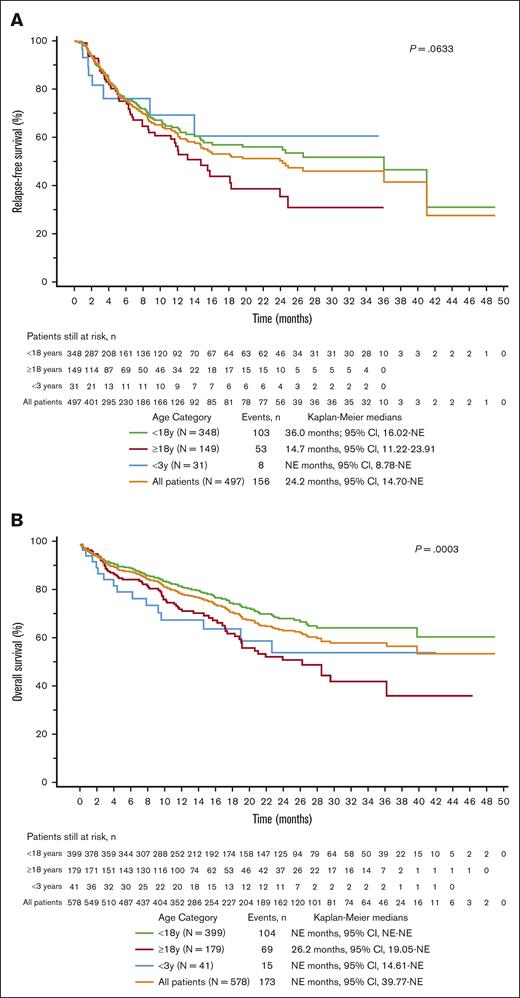
The baseline characteristics (efficacy set, n = 578; median follow-up of 32.1 months [range, 12.0-55.3]) are summarized in supplemental Table 1. The BOR of CR/CRi across all patients was 86.0%. Among patients in CR with available MRD status (n = 267), 98.9% were MRD negative. The BOR of CR/CRi was higher in the ≥18-year and <18-year subgroups (83.2% and 87.2%, respectively) than in the <3-year subgroup (75.6%); however, this difference was not significant. The 12-month RFS (censoring for allo-SCT) and OS (no censoring) rates across all patients were 61.8% and 79.4%, respectively, and the 24-month RFS and OS were 50.3% and 63.8%, respectively (Figure 1).
RFS and OS Kaplan-Meier curves across the age categories. (A) RFS∗. (B) OS. ∗Censored for allo-SCT. P values represent comparisons between patients aged <18 and ≥18 years. NE, not estimable.
RFS and OS Kaplan-Meier curves across the age categories. (A) RFS∗. (B) OS. ∗Censored for allo-SCT. P values represent comparisons between patients aged <18 and ≥18 years. NE, not estimable.
Predictors of response
When compared with the OP, patients ≥18 years had numerically lower RFS and a significantly lower OS (Figure 1). In the univariate analyses, patients with DS, previous allo-SCT or CAR T-cell therapy, and isolated CNS disease had improved RFS and OS. Patients with high DB, previous inotuzumab treatment, KMT2A rearrangement, bone marrow disease or non-CNS extramedullary disease, and those aged ≥18 years demonstrated inferior RFS and OS (supplemental Figure 2). The multivariate analyses further demonstrated that low and intermediate DB were associated with significantly improved outcomes (when compared with high DB) across all 12-month time-to-event efficacy end points (low DB: RFS hazard ratio [HR], 0.24; 95% CI, 0.13-0.45; and OS HR, 0.26; 95% CI, 0.15-0.48; intermediate DB: RFS HR, 0.36; 95% CI, 0.22-0.60; and OS HR, 0.37; 95% CI, 0.23-0.60; Figure 2; supplemental Figure 3). In addition, the multivariate analysis showed that KMT2A rearrangement and previous inotuzumab treatment were further predictive of lower RFS (HR, 1.87; 95% CI, 1.13-3.11; and HR, 2.27; 95% CI, 1.33-3.89, respectively) and OS (HR, 2.49; 95% CI, 1.58-3.94; and HR, 2.43; 95% CI, 1.46-4.05, respectively; Figure 2).
Forest plots for OS and RFS by multivariate analysis without censoring for allo-SCT. Covariates with multivariate P value of < .05 are shown in red (dotted line). HCT, hematopoietic stem cell transplant; Ph+, Philadelphia chromosome positive.
Forest plots for OS and RFS by multivariate analysis without censoring for allo-SCT. Covariates with multivariate P value of < .05 are shown in red (dotted line). HCT, hematopoietic stem cell transplant; Ph+, Philadelphia chromosome positive.
Postinfusion outcomes
allo-SCT
Among patients in the <18-year-old group, the median RFS was 36.02 months with and without censoring for postinfusion allo-SCT, and the median OS was not estimable. Among patients in the ≥18-year-old group, the median RFS was numerically longer without censoring for allo-SCT (15.76 months) vs with censoring for allo-SCT (14.70 months), suggesting that allo-SCT had a minimal effect on RFS. However, the median OS was ∼3 months longer with censoring for allo-SCT (29.54 months) than without censoring (26.22 months).
CD19+ and CD19– relapse
Among patients with BOR of CR/CRi who experienced disease relapse (n = 155), 47 (30.3%) had CD19+ disease and 39 (25.2%) had CD19– disease (69 [44.5%] had unknown status). Patients <3 years had more CD19– relapses (62.5%) and fewer CD19+ relapses (12.5%) than patients ≥18 years (20.5% and 40.9%, respectively; Table 2). Previous exposure to blinatumomab did not seem to influence the rate of CD19– relapses; however, a substantial number of patients had unknown CD19 status at relapse; therefore, no definitive conclusions can be made.
Relapse status in patients with BOR of CR/CRi
| . | All patients (N = 497) . | ≥18 years (n = 149) . | <18 years (n = 348) . | <3 years (n = 31) . |
|---|---|---|---|---|
| Ongoing remission∗ | 189 (38.0) | 56 (37.6) | 133 (38.2) | 9 (29.0) |
| Patients censored by therapy | ||||
| allo-SCT | 96 (19.3) | 28 (18.8) | 68 (19.5) | 11 (35.5) |
| Tisagenlecleucel reinfusion | 47 (9.5) | 9 (6.0) | 38 (10.9) | 3 (9.7) |
| Other CAR T-cell therapy | 6 (1.2) | 1 (0.7) | 5 (1.4) | 0 |
| Other cell therapy | 3 (0.6) | 2 (1.3) | 1 (0.3) | 0 |
| Death | 13 (2.6) | 9 (6.0) | 4 (1.1) | 0 |
| Relapse | 143 (28.8) | 44 (29.5) | 99 (28.4) | 8 (25.8) |
| CD19+ | 46 (32.2) | 18 (40.9) | 28 (28.3) | 1 (12.5) |
| Previous blinatumomab | 14 (30.4) | 9 (50.0) | 5 (17.9) | 0 |
| CD19– | 37 (25.9) | 9 (20.5) | 28 (28.3) | 5 (62.5) |
| Previous blinatumomab | 7 (18.9) | 4 (44.4) | 3 (10.7) | 0 |
| CD19 unknown | 60 (42.0) | 17 (38.6) | 43 (43.4) | 2 (25.0) |
| Previous blinatumomab | 15 (25.0) | 4 (23.5) | 11 (25.6) | 1 (50.0) |
| . | All patients (N = 497) . | ≥18 years (n = 149) . | <18 years (n = 348) . | <3 years (n = 31) . |
|---|---|---|---|---|
| Ongoing remission∗ | 189 (38.0) | 56 (37.6) | 133 (38.2) | 9 (29.0) |
| Patients censored by therapy | ||||
| allo-SCT | 96 (19.3) | 28 (18.8) | 68 (19.5) | 11 (35.5) |
| Tisagenlecleucel reinfusion | 47 (9.5) | 9 (6.0) | 38 (10.9) | 3 (9.7) |
| Other CAR T-cell therapy | 6 (1.2) | 1 (0.7) | 5 (1.4) | 0 |
| Other cell therapy | 3 (0.6) | 2 (1.3) | 1 (0.3) | 0 |
| Death | 13 (2.6) | 9 (6.0) | 4 (1.1) | 0 |
| Relapse | 143 (28.8) | 44 (29.5) | 99 (28.4) | 8 (25.8) |
| CD19+ | 46 (32.2) | 18 (40.9) | 28 (28.3) | 1 (12.5) |
| Previous blinatumomab | 14 (30.4) | 9 (50.0) | 5 (17.9) | 0 |
| CD19– | 37 (25.9) | 9 (20.5) | 28 (28.3) | 5 (62.5) |
| Previous blinatumomab | 7 (18.9) | 4 (44.4) | 3 (10.7) | 0 |
| CD19 unknown | 60 (42.0) | 17 (38.6) | 43 (43.4) | 2 (25.0) |
| Previous blinatumomab | 15 (25.0) | 4 (23.5) | 11 (25.6) | 1 (50.0) |
Efficacy set. Data are presented as n (%).
With censoring for allo-SCT.
Subsequent neoplasm
Eight patients (1.1%) developed subsequent neoplasms (Table 3). The rate of subsequent neoplasms was low and similar across the age groups. Most of these subsequent malignancies (5/8) represented a lineage switch from KMT2A rearrangement–positive ALL to acute myeloid leukemia (AML). Patients developed these subsequent neoplasms within a median of 83 days (range, 24-728) from the time of infusion.
Subsequent neoplasms
| . | All patients (N = 703) . | ≥18 years (n = 209) . | <18 years (n = 494) . | <3 years (n = 54) . |
|---|---|---|---|---|
| Subsequent neoplasm | 8 (1.1) | 3 (1.4) | 5 (1.0) | 1 (1.9) |
| ALL to AML lineage switch | 5 (62.5) | 3 (100) | 2 (40) | 1 (100) |
| Myelodysplasia (MDS) | 2 (25) | 0 | 2 (40) | 0 |
| Other new malignancy∗ | 1 (12.5) | 0 | 1 (20) | 0 |
| Time from infusion to subsequent neoplasm,† median (range), d | 83 (24-728) | 73 (35-93) | 155 (24-728) | 42 (42-42) |
| . | All patients (N = 703) . | ≥18 years (n = 209) . | <18 years (n = 494) . | <3 years (n = 54) . |
|---|---|---|---|---|
| Subsequent neoplasm | 8 (1.1) | 3 (1.4) | 5 (1.0) | 1 (1.9) |
| ALL to AML lineage switch | 5 (62.5) | 3 (100) | 2 (40) | 1 (100) |
| Myelodysplasia (MDS) | 2 (25) | 0 | 2 (40) | 0 |
| Other new malignancy∗ | 1 (12.5) | 0 | 1 (20) | 0 |
| Time from infusion to subsequent neoplasm,† median (range), d | 83 (24-728) | 73 (35-93) | 155 (24-728) | 42 (42-42) |
Safety-evaluable set. Data are presented as n (%).
MDS, myelodysplastic syndrome.
The type of new malignancy was not reported.
Inclusive of lineage switch.
Safety
Among the safety set (n = 703; median follow-up, 28.0 months [range, 3.5-55.3]), a higher proportion of patients ≥18 years experienced any-grade CRS (Table 4) than younger patients. In patients <3 years, grade ≥3 CRS and ICANS rates were lower than in the OP and other age subgroups, whereas any-grade CRS and ICANS rates were lower in patients <18 years. The grade ≥3 CRS and ICANS rates were higher among patients with a higher DB when compared with the OP (supplemental Table 2). Patients with DS and isolated CNS disease had lower grade ≥3 CRS rates than the OP, and patients with isolated CNS disease had higher rates of grade ≥3 ICANS (supplemental Table 2). Deaths caused by CRS or ICANS were very rare (<1% for both).
Safety outcomes across age groups
| . | All patients (N = 703) . | ≥18 years (n = 209) . | <18 years (n = 494) . | <3 years (n = 54) . |
|---|---|---|---|---|
| CRS∗ | ||||
| All grade | 404 (57.5) | 131 (62.7) | 273 (55.3) | 33 (61.1) |
| Grade ≥3 | 115 (16.4) | 36 (17.2) | 79 (16.0) | 4 (7.4) |
| Median time to onset (range), d | 5 (1-38) | 5 (1-38) | 5 (1-24) | 6 (1-11) |
| Median duration (95% CI), d | 6 (6-7) | 6 (5-8) | 6 (6-7) | 5 (3-7) |
| Therapies | 245 (60.6) | 86 (65.6) | 159 (58.2) | 19 (57.6) |
| Corticosteroids | 62 (15.3) | 26 (19.8) | 36 (13.2) | 3 (9.1) |
| Siltuximab | 21 (5.2) | 7 (5.3) | 14 (5.1) | 2 (6.1) |
| Tocilizumab | 214 (53.0) | 78 (59.5) | 136 (49.8) | 14 (42.4) |
| Resolution | 381 (94.3) | 128 (97.7) | 253 (92.7) | 30 (90.9) |
| Death† | 3 (0.4) | 0 | 3 (0.6) | 0 |
| ICANS∗ | ||||
| All grade | 156 (22.2) | 47 (22.5) | 109 (22.1) | 7 (13.0) |
| Grade ≥3 | 63 (9.0) | 18 (8.6) | 45 (9.1) | 5 (9.3) |
| Median time to onset (range), d | 7 (1-93) | 8 (1-33) | 7 (2-93) | 6 (3-12) |
| Median duration (95% CI), d | 7 (6-8) | 7 (4-9) | 7 (5-8) | 7 (3-8) |
| Therapies | 106 (67.9) | 38 (80.9) | 68 (62.4) | 4 (57.1) |
| Antiepileptics | 53 (34.0) | 18 (38.3) | 35 (32.1) | 3 (42.9) |
| Corticosteroids | 47 (30.1) | 20 (42.6) | 27 (24.8) | 2 (28.6) |
| Siltuximab | 3 (1.9) | 1 (2.1) | 2 (1.8) | 0 |
| Tocilizumab | 24 (15.4) | 10 (21.3) | 14 (12.8) | 1 (14.3) |
| Resolution | 142 (91.0) | 44 (93.6) | 98 (89.9) | 7 (16.3) |
| Death† | 1 (0.1) | 1 (0.5) | 0 | 0 |
| Other outcomes of interest | ||||
| Prolonged neutropenia‡ | 152 (21.6) | 60 (28.7) | 92 (18.6) | 12 (22.2) |
| Duration, median (95% CI), d | 55 (48-60) | 57 (45-61) | 53 (44-60) | 63 (33 to NE) |
| Recovery§ | 102 (67.1) | 40 (66.7) | 62 (67.4) | 7 (13.0) |
| Prolonged thrombocytopenia‡ | 143 (20.3) | 57 (27.3) | 86 (17.4) | 10 (18.5) |
| Duration, median (95% CI), d | 61 (57-74) | 62 (53-81) | 61 (55-78) | NE (36 to NE) |
| Recovery|| | 87 (60.8) | 36 (63.2) | 51 (83.6) | 4 (7.4) |
| Death any time after infusion¶ | 188 (26.7) | 74 (35.4) | 114 (23.1) | 16 (29.6) |
| Death due to recurrence, persistence, or progression of primary disease | 96 (13.7) | 37 (17.7) | 59 (11.9) | 10 (18.5) |
| Nonrelapse mortality# | 90 (12.8) | 35 (16.7) | 55 (11.1) | 6 (11.1) |
| . | All patients (N = 703) . | ≥18 years (n = 209) . | <18 years (n = 494) . | <3 years (n = 54) . |
|---|---|---|---|---|
| CRS∗ | ||||
| All grade | 404 (57.5) | 131 (62.7) | 273 (55.3) | 33 (61.1) |
| Grade ≥3 | 115 (16.4) | 36 (17.2) | 79 (16.0) | 4 (7.4) |
| Median time to onset (range), d | 5 (1-38) | 5 (1-38) | 5 (1-24) | 6 (1-11) |
| Median duration (95% CI), d | 6 (6-7) | 6 (5-8) | 6 (6-7) | 5 (3-7) |
| Therapies | 245 (60.6) | 86 (65.6) | 159 (58.2) | 19 (57.6) |
| Corticosteroids | 62 (15.3) | 26 (19.8) | 36 (13.2) | 3 (9.1) |
| Siltuximab | 21 (5.2) | 7 (5.3) | 14 (5.1) | 2 (6.1) |
| Tocilizumab | 214 (53.0) | 78 (59.5) | 136 (49.8) | 14 (42.4) |
| Resolution | 381 (94.3) | 128 (97.7) | 253 (92.7) | 30 (90.9) |
| Death† | 3 (0.4) | 0 | 3 (0.6) | 0 |
| ICANS∗ | ||||
| All grade | 156 (22.2) | 47 (22.5) | 109 (22.1) | 7 (13.0) |
| Grade ≥3 | 63 (9.0) | 18 (8.6) | 45 (9.1) | 5 (9.3) |
| Median time to onset (range), d | 7 (1-93) | 8 (1-33) | 7 (2-93) | 6 (3-12) |
| Median duration (95% CI), d | 7 (6-8) | 7 (4-9) | 7 (5-8) | 7 (3-8) |
| Therapies | 106 (67.9) | 38 (80.9) | 68 (62.4) | 4 (57.1) |
| Antiepileptics | 53 (34.0) | 18 (38.3) | 35 (32.1) | 3 (42.9) |
| Corticosteroids | 47 (30.1) | 20 (42.6) | 27 (24.8) | 2 (28.6) |
| Siltuximab | 3 (1.9) | 1 (2.1) | 2 (1.8) | 0 |
| Tocilizumab | 24 (15.4) | 10 (21.3) | 14 (12.8) | 1 (14.3) |
| Resolution | 142 (91.0) | 44 (93.6) | 98 (89.9) | 7 (16.3) |
| Death† | 1 (0.1) | 1 (0.5) | 0 | 0 |
| Other outcomes of interest | ||||
| Prolonged neutropenia‡ | 152 (21.6) | 60 (28.7) | 92 (18.6) | 12 (22.2) |
| Duration, median (95% CI), d | 55 (48-60) | 57 (45-61) | 53 (44-60) | 63 (33 to NE) |
| Recovery§ | 102 (67.1) | 40 (66.7) | 62 (67.4) | 7 (13.0) |
| Prolonged thrombocytopenia‡ | 143 (20.3) | 57 (27.3) | 86 (17.4) | 10 (18.5) |
| Duration, median (95% CI), d | 61 (57-74) | 62 (53-81) | 61 (55-78) | NE (36 to NE) |
| Recovery|| | 87 (60.8) | 36 (63.2) | 51 (83.6) | 4 (7.4) |
| Death any time after infusion¶ | 188 (26.7) | 74 (35.4) | 114 (23.1) | 16 (29.6) |
| Death due to recurrence, persistence, or progression of primary disease | 96 (13.7) | 37 (17.7) | 59 (11.9) | 10 (18.5) |
| Nonrelapse mortality# | 90 (12.8) | 35 (16.7) | 55 (11.1) | 6 (11.1) |
Efficacy-evaluable set. Data are presented as n (%).
NE, not estimable.
Onset within 100 days after infusion.
Primary cause of death within 30 days.
Assessments conducted at day 100, month 6, and month 12.
Absolute neutrophil count ≥0.5 × 103/µL achieved and sustained for 3 laboratory values.
Platelet count of ≥20 × 109/L.
Treatment-related mortality was not captured separately.
Causes of nonrelapse mortality are listed in supplemental Table 3.
Prolonged (>30 days) neutropenia and thrombocytopenia occurred in 152 (21.6%) and 143 (20.3%) patients, respectively, of whom 102 (67.1%) and 87 (60.8%) subsequently recovered. Patients recovered from prolonged neutropenia by 55 days (median; 95% CI, 48-60) and from prolonged thrombocytopenia by 61 days (median; 95% CI, 57-74) after infusion (Table 4). The most common cause of death in the overall study population was disease recurrence, persistence, or progression (n = 96 [13.7% of all patients]), whereas 92 (13.1%) experienced nonrelapse mortality (Table 4). Importantly, 3 female patients treated with tisagenlecleucel were able to conceive following CAR T-cell therapy with 3 live births reported.
Discussion
Numerically lower efficacy and safety outcomes were observed in patients ≥18 years when compared with younger subgroups. This may be a consequence of more resistant leukemia known to occur in adults, as seen by the greater DB and higher proportions of patients in their third or later relapse, or a consequence of previous exposure to blinatumomab and inotuzumab, which is consistent with the risk factors for poorer outcomes described across recent studies of CAR T-cell therapy.26,28,37,38 Interestingly, the efficacy and safety outcomes from the subgroup of patients aged ≥18 years (age range, 18-25 years) in this CIBMTR analysis seem to be more favorable than outcomes reported for brexucabtagene autoleucel in the ZUMA-3 trial among the OP of adult patients with R/R B-ALL (age range, 18-77 years).11,39 Although the ≥18-year-old patient populations in the registry and ZUMA-3 trial are not directly comparable and crosstrial comparisons should be evaluated with caution, the young adults who received tisagenlecleucel in the CIBMTR registry had a numerically longer median OS (26.2 months vs 18.2 months) and lower rates of grade ≥3 CRS (17% vs 24%) and ICANS (9% vs 25%) than the adult patients in ZUMA-3.11,39 It is important to note that in ZUMA-3, 62% of patients had >25% BMBs after bridging therapy, which may have contributed to the high rates of CRS and ICANS.11,39 When compared with the 18- to 39-year-old subgroup in the ZUMA-3 trial, an age range closer to the young adults in our CIBMTR analysis (aged 18-25 years), the remission rate (CR/CRi) was higher for patients in CIBMTR than for those in ZUMA-3 (83% vs 62%, respectively), but the 12-month OS rates were similar between the studies (73% vs 68%, respectively). A recent analysis of real-world use of brexucabtagene autoleucel in a broader population of adults with R/R B-ALL (age range, 18-84 years) reported efficacy outcomes that were consistent with those in the ZUMA-3 trial.40 The rates of grade ≥3 ICANS were higher with real-world brexucabtagene autoleucel (24% vs 9%), whereas the grade ≥3 CRS rates were similar (13% vs 17%) when compared with the findings in our CIBMTR analysis of tisagenlecleucel.40 Overall, these data suggest that tisagenlecleucel may be considered for the YA patient population, especially those who may have a higher risk for ICANS.
In the multivariate analysis, higher relapse rates and worse survival were observed following tisagenlecleucel in patients with high DB, previous treatment with inotuzumab, or KMT2A rearrangement. Interestingly, in the <3 years age subgroup in which 75.9% of patients also had KMT2A rearrangements, numerically improved RFS was seen when compared with the overall patient population. Multicenter retrospective cohort studies using RWE previously demonstrated that BMB ≥5% was associated with inferior event-free survival and OS following commercial tisagenlecleucel or another CD19-directed CAR T-cell therapy.26-28 However, the definitions of high DB vary across studies,27,28,41 and we have observed high rates of durable response in patients with up to 50% of blasts at baseline. A current challenge that physicians are facing is how to reduce DB to improve long-term CAR T-cell outcomes while avoiding the potential negative consequences of targeted immunotherapy and cytotoxic chemotherapy.
In contrast with our findings, an international, retrospective analysis (N = 39) found that previous inotuzumab treatment did not negatively affect the 12-month event-free survival and OS (when compared with published data) when used as bridging therapy before CAR T-cell therapy.42,43 However, a greater proportion of those patients had previously received blinatumomab (38%) or SCT (46%).42,43 Another study (N = 8) demonstrated that inotuzumab bridging therapy had no negative effect on CAR T-cell therapy; however, fewer patients had previously received SCT (12%).44 Interestingly, the 12-month OS (85.7%) was comparable with that of the current study (79.4%).44 However, inotuzumab use may negatively impact CAR T-cell proliferation by limiting CD19+ targets (both blasts and normal B cells) or may indicate aggressive disease characteristics rather than directly influencing CAR T-cell therapy efficacy, thus contributing to the observed negative effect on the outcomes here. Because of the recent approval of inotuzumab in patients ≥1 year of age,45 we may see changes in the reported outcomes for Ped-YA patients treated with inotuzumab and CAR T-cell therapies.
The real-world efficacy outcomes with tisagenlecleucel were similar to those of the phase 2 ELIANA trial, whereas the safety outcomes were generally more favorable.10 This is consistent with previous real-world data on the use of tisagenlecleucel for treating R/R B-ALL.12,17,18,33 However, patients in ELIANA were required to have ≥5% BMBs at screening and patients <3 years, those with isolated extramedullary disease, or previous CAR T-cell therapy were excluded from ELIANA but were included in this real-world data set.10 In this study, fewer patients had previous allo-SCT when compared with ELIANA (25.4% vs 61%) and more had PRD (12.2% vs 8%). Furthermore, 27% of patients in this study were treated at first relapse, whereas ELIANA required ≥2 relapses for inclusion,10 suggesting patients were treated at earlier disease stages in the real-world setting.
The efficacy and safety data were favorable for patients in this study who would have been excluded from ELIANA, especially patients <3 years. Recent data highlighted the feasibility of leukapheresis and tisagenlecleucel manufacturing in patients <3 years at screening.46-51 In this study, patients <3 years demonstrated numerically improved RFS but inferior OS and BOR of CR/CRi when compared with the OP. The BOR (CR/CRi) in patients <3 years (n = 31 [76%]) was also lower than in a recent real-world study in this age group (n = 28 [80%]), in which more patients had received previous allo-SCT (66% vs 21% in this study).50 Interestingly, although a high percentage of patients <3 years had KMT2A rearrangements, the efficacy was comparable with the OP, consistent with a previous study.52 Previously reported data from the CIBMTR showed that 43.9% of patients aged <3 years who received infusions underwent allo-SCT after treatment, and only 19.1% had received previous allo-SCT,47 which was a significant difference when compared with the treatments available before tisagenlecleucel access for these patients.10,53
This RWE provides information on the outcomes of other patients who would have been excluded from ELIANA, including those with isolated extramedullary disease or those previously treated with blinatumomab.26,27,30,47,50,51 Previous blinatumomab treatment was not found to significantly affect the outcomes in the multivariate analyses in contrast with retrospective studies that demonstrated inferior outcomes with previous blinatumomab treatment (∼4 months before CAR T-cell therapy), especially in nonresponders who may be resistant to immunotherapy.37,38 Efficacy outcomes were favorable in patients with isolated CNS disease, consistent with reports from the Children’s Hospital of Philadelphia and Pediatric Real World CAR Consortium (BOR of CR/CRi: 97% from Children’s Hospital of Philadelphia; 88% from Pediatric Real World CAR Consortium; 82.5% in this study).30,31
Favorable outcomes were observed in the univariate analyses of patients with DS, isolated CNS relapse, and patients who received allo-SCT or previous CAR T-cell therapy before infusion; however, additional analyses are needed with a larger cohort to confirm this observation. This was consistent with favorable outcomes observed for patients with DS in the pooled analysis, including in the ELIANA and ENSIGN trials (BOR of CR/CRi of 88%, n = 16; vs 94% in this study, n = 33).29 Although patients with prievious allo-SCT had positive outcomes, similar to the OP in this study, the number of those patients has decreased over time. This aligns with a recent retrospective cohort study in patients <3 years who received tisagenlecleucel therapy for R/R B-ALL, which showed postinfusion efficacy outcomes comparable with our study, despite the former having a higher proportion of patients who received previous allo-SCT (66% vs 21%).50 For pediatric patients, especially young patients (<3 years), avoiding CNS radiation and allo-SCT when possible is an important treatment goal because of the significant transplant-related toxicities, including infertility, endocrine dysfunction, cognitive and developmental issues, growth effects, graft-versus-host disease, secondary malignancies, and other late effects.54-57 Avoiding allo-SCT may also benefit patients with DS who have an increased risk for transplant-related mortality.58-61 In this study, we report high BOR of CR/CRi (93.9%) and high rates of RFS (80.3%) and OS (100%) at month 12 among the subgroup of patients with DS, suggesting that these patients can achieve excellent outcomes while also avoiding allo-SCT.
A higher proportion of patients <3 years developed CD19– relapse, which was potentially driven by the high number of patients with KMT2A rearrangements and an increased probability for lineage switch.62,63 Interestingly, in this study, we found that in patients ≥18 years, all 3 cases of reported subsequent neoplasms were attributed to lineage switch; however, they also had a higher proportion of CD19+ relapse after tisagenlecleucel. Patients <18 years had equal proportions of CD19+ and CD19– relapse. Previous blinatumomab treatment did not influence the relapse type; however, nonresponse to blinatumomab is a predictor for inferior outcomes, possibly because of the high DB or diminished CD19 expression in these patients.28 In a previous study, patients who were treated with blinatumomab had an increased risk of post–CAR T-cell therapy relapse with CD19– disease.28 Although fewer pediatric patients previously received blinatumomab than patients ≥18 years, a higher proportion of patients <3 years developed CD19– relapse. It remains unclear whether previous immunotherapies have a direct impact on the incidence of CD19– relapse.
The subsequent neoplasm rates were very low (∼1%) in the current follow-up and were primarily a consequence of AML lineage switch. Importantly, there were no T-cell malignancies reported in this study. Previous studies showed that conditioning regimens associated with allo-SCT may increase the risk for subsequent neoplasms.55-57,64 Although the follow-up in this study was relatively short (median, 32.1 months), the rate of subsequent neoplasms among patients who received tisagenlecleucel was well below the rate previously reported among patients who received allo-SCT alone (up to 11% incidence over 10-20 years).64 It is worth noting that the ALL to AML lineage switch was reported in CIBMTR as a subsequent neoplasm, although most clinicians do not consider it to be caused by CAR T-cell therapy.
Consistent with a previous CIBMTR report,65 we also report successful pregnancies and live births years after tisagenlecleucel therapy. These results are promising because there is a risk of fetal toxicity, including B-cell lymphopenia, if tisagenlecleucel crosses the placenta.14,15
Inherent limitations of data collection in registry studies include bias introduced by patients lost to follow-up if the reason was related to the outcome of interest. In addition, important data on the long-term outcomes, including B-cell aplasia and B-cell recovery were underreported in the registry, making interpretation of these data a challenge. Furthermore, CD19 status was only assessed among patients who experienced relapse and was not fully captured in the CIBMTR registry. The differences in data collection for real-world studies vs clinical trials (eg, timing of assessments) can cause challenges for comparisons. The treatment and assessment protocols were based on physician decisions or institutional guidelines at each treatment center and were not mandated by the CIBMTR; therefore, the data obtained from each treatment center may differ. Except for the multivariate analysis, no formal statistical modeling was performed (ie, no adjustment of potential confounding factors) when trends were assessed for the baseline characteristics and outcomes. Assessment of disease and tumor burden was performed at the last disease evaluation before infusion, which was not standardized across sites; therefore, comparisons of tumor burden should be interpreted with caution.
This report contains a large data set of Ped-YA patients with R/R B-ALL who were treated with tisagenlecleucel. Favorable response and survival were observed, consistent with recent updates of the pivotal ELIANA trial; however, the safety profile of this CIBMTR data set was improved when compared with that of ELIANA.17,66 Importantly, improved outcomes when compared with the pivotal trial were observed among patients with isolated CNS relapse and DS. We reported postinfusion outcomes for patients <3 years who were treated with tisagenlecleucel and who were previously excluded from the pivotal studies. These patients showed favorable efficacy outcomes, although ∼80% had KMT2A mutations, and demonstrated successful manufacturing feasibility.46-49,51 Significant predictors of worse response were higher DB, previous treatment with inotuzumab, and KMT2A rearrangement (when all age groups were combined). Patients ≥18 years showed inferior outcomes, potentially because of higher risk disease, a higher proportion of patients with high DB, previous exposure to inotuzumab, and third or later relapse. Ongoing accrual of patient data in the CIBMTR registry, along with important work being done in RWE CAR T-cell consortia, will provide further insights into the real-world use of tisagenlecleucel in Ped-YA patients, including outcomes in patient subgroups not included in ELIANA, and long-term toxicities after CAR T-cell therapy.
Acknowledgments
The authors thank the patients who participated in the Center for International Blood and Marrow Transplant Research (CIBMTR) registry and their families, all participating treatment centers, physicians, and support staff. The authors also thank Michael Heim for his contribution to previous analyses of these data. Medical writing support was provided by Meghan Mooring of HCG, and was funded by Novartis Pharmaceuticals Corporation.
The CIBMTR was supported primarily by Public Health Service grant U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); NHLBI grants OT3HL147741 and U24HL138660; NCI grants U24CA233032 and U24HL157560; Health Resources and Services Administration (HRSA) grants HHSH250201700006C, 75R60222C00008, 75R60222C00009, and 75R60222C00011; and Office of Naval Research grants N00014-21-1-2954 and N00014-23-1-2057. Additional federal support was provided by NCI grants UM1CA121947, P01CA111412, R01CA100019, R01CA218285, R01CA231141, R01CA231838, and R01CA262899; NIAID grants U01AI126612, U01AI069197, R01AI128775, R01AI150999, R01AI158861, and U01AI069197; and NHLBI grants U01H L128568, R01HL155741, and UG1HL069254. Support was also provided by the Be The Match Foundation, Boston Children’s Hospital, Dana-Farber Cancer Center, Fred Hutchinson Cancer Center, Gateway for Cancer Research, Inc, Jeff Gordon Children’s Foundation, Pediatric Blood and Marrow Transplantation Foundation, Stanford University, Medical College of Wisconsin, National Marrow Donor Program, University of Pittsburgh, and the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; AlloVir, Inc; Amgen, Inc; Angiocrine; Astellas Pharma US; Atara Biotherapeutics; BeiGene; bluebird bio, Inc; Bristol Myers Squibb Co; CareDx, Inc; CSL Behring; CytoSen Therapeutics, Inc; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida Cell, Ltd; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Merck & Co; Mesoblast; Millennium, Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; Pharmaceutical Product Development, LP; REGiMMUNE; Sanofi; Sarah Cannon; Sobi, Inc; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Takeda Oncology Co; Vertex Pharmaceuticals; Vor Biopharma, Inc; and Xenikos BV. The ELIANA clinical trial was sponsored by Novartis Pharmaceuticals Corporation.
All analyses reported in the study were conducted by Novartis Pharmaceuticals Corporation. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, the HRSA, or any other agency of the US government. The National Comprehensive Cancer Network makes no warranties of any kind whatsoever regarding their content, use or application, and disclaims any responsibility for their application or use in any way.
Authorship
Contribution: All authors conceived or designed the study, acquired, analyzed, or interpreted data, reviewed the manuscript, provided final approval of the manuscript, and were accountable for all aspects of the work.
Conflict of interest disclosure: K.J.C. reports serving as a consultant for Novartis; participating as board member and shareholder for Turn Bio and PromiCell; and research funding from Novartis, Cellectis, and Atara Bio. E.M.H.’s spouse reports participating in a speaker's bureau for Boehringer Ingelheim; honoraria from Vida Diagnostics; and participating on a scientific advisory committee for Polarean. S.H.C.B.’s spouse reports former employment for Alexion and Takeda Pharmaceuticals and holds Takeda and AstraZeneca stock and stock options. S.N. reports participating in ad hoc advisory boards for A2 Bio, Legend Biotech, Kite/Gilead, Iovance, SmartImmune, and GlaxoSmithKline. C.L.P. reports serving as a member on the board of directors or advisory committees for Novartis. M.A.P. reports participating in scientific advisory boards for Medexus, Equillium, Mesoblast, and Novartis; participating in sponsored invited educational talks for Novartis and Miltenyi Biotec; and study support from Miltenyi Biotec and Adaptive Biotechnologies. R.H.R. reports honoraria from Novartis; research funding from Tessa Therapeutics; and serving as a consultant for Pfizer. M.C.P. reports research funding from Bristol Myers Squibb, Novartis, Kite Pharma, and GlaxoSmithKline; and serving as a consultant for Bristol Myers Squibb. R.T., J.W., and R.R. report current employment with Novartis. J.K. reports serving as a consultant for Kite/Gilead and Novartis. E.G. reports participating in scientific advisory boards for Syndax and Jazz Pharmaceuticals. S.A.G. reports research and/or clinical trial support from Novartis, Servier, Vertex, and Kite Pharma; and participating in study steering committees or serving as a consultant or on scientific advisory boards for Novartis, Allogene, Cellectis, CRISPR/Vertex, Jazz Pharmaceuticals, TCR2, Allogene, BioLineRx, Mckesson, and Cabaletta. The remaining authors declare no competing financial interests.
Correspondence: Samuel John, Department of Pediatrics, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390; email: samuel.john@utsouthwestern.edu.
References
Author notes
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health data-sharing policy and the National Cancer Institute Cancer Moonshot public access and data-sharing policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.