Key Points
Tisa-cel is associated with inferior survival, whereas liso-cel and axi-cel show similar survival outcomes.
Liso-cel exhibits the most favorable toxicity profile and requires fewer resources for the management of postinfusion toxicities.
Visual Abstract
Although 3 commercial CD19-targeted chimeric antigen receptor (CAR) T-cell therapies are available for large B-cell lymphomas (LBCLs), no randomized clinical trials have compared their efficacy and safety. In this retrospective multicenter cohort study, we evaluated real-world clinical outcomes of patients with relapsed/refractory LBCL treated with axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), or lisocabtagene maraleucel (liso-cel). Between April 2016 and July 2024, 624 patients received CD19-targeted CAR T-cell therapies (344 axi-cel, 142 tisa-cel, and 138 liso-cel). At a median follow-up of 20.9 months, the respective estimated 2-year progression-free survival (PFS) and overall survival (OS) rates were 46% and 63% for axi-cel, 30% and 45% for tisa-cel, and 45% and 58% for liso-cel. After adjusting for potential confounders in multivariate analyses, tisa-cel was associated with inferior PFS and OS compared to axi-cel. No significant survival differences were found between liso-cel and axi-cel. Propensity score and subanalyses of patients treated in the second-line vs third-line or later settings yielded similar outcomes. Compared to axi-cel, the objective response rate at 100 days was higher for liso-cel and lower for tisa-cel. Rates of cytokine release syndrome, immune effector cell–associated neurotoxicity syndrome, and immune effector cell–associated hematotoxicity, and febrile neutropenia were significantly higher with axi-cel. However, no significant differences in the cumulative incidence of infections or nonrelapse mortality were found. Axi-cel was associated with faster vein-to-vein time (axi-cel, 35 days; tisa-cel, 43 days; liso-cel, 41 days) and fewer out-of-specification products (axi-cel, 2%; tisa-cel, 4%; liso-cel, 11%). These results provide insights into potential differential outcomes depending on product selection.
Introduction
The approval of axicabtagene ciloleucel (axi-cel) by the US Food and Drug Administration in 2017 marked a transformative milestone in the treatment of relapsed/refractory large B-cell lymphoma (LBCL).1 Since then, 2 additional CD19-directed chimeric antigen receptor (CAR) T-cell therapies, tisagenlecleucel (tisa-cel) and lisocabtagene maraleucel (liso-cel), have been approved for use in third-line or later settings.2,3 In 2022, axi-cel and liso-cel also gained approval for second-line treatment after pivotal trials demonstrating superiority over the previous standard of care.4,5 In contrast, the BELINDA trial showed no superiority for tisa-cel over the standard of care in this setting.6,7
Currently, no randomized clinical trials have directly compared these CAR T-cell therapies. Moreover, cross-trial comparisons are challenging due to differences in trial design, patient eligibility criteria, patient populations, and primary end point definitions. In this context, real-world retrospective studies comparing these therapies provide valuable insights to guide product selection.
Recent studies demonstrated increased toxicity but greater efficacy with axi-cel compared to tisa-cel.8-15 These findings also supported the cost-effectiveness of axi-cel vs tisa-cel for patients who have received ≥2 lines of systemic therapy.16,17 However, comparisons of axi-cel with liso-cel are limited. A recent retrospective study, restricted to patients treated in the third-line or later setting, suggested superior progression-free survival (PFS) but higher toxicity with axi-cel.18 Therefore, additional data comparing liso-cel with other CAR T-cell therapies for LBCL, particularly in the second-line setting, are needed.
In this study, we leveraged a large international cohort to retrospectively compare real-world clinical outcomes of patients with relapsed/refractory LBCL treated with the 3 commercially available CAR T-cell products. Comparative subanalyses were conducted to specifically evaluate the efficacy and toxicities of these therapies in the second-line vs third-line or later settings.
Methods
Study design and patients
This retrospective multicenter study included 624 patients with relapsed/refractory LBCL who received CAR T-cell therapies between April 2016 and July 2024 across 4 centers: 2 in the United States (Memorial Sloan Kettering Cancer Center [MSKCC] and Hackensack University Medical Center) and 2 in Israel (Rambam Health Care Campus and Chaim Sheba Medical Center). Patient selection, postinfusion monitoring, and toxicity management were conducted according to institutional guidelines. Patients in clinical trials with these products alone were included (ClinicalTrials.gov identifiers: NCT03391466 and NCT02631044), whereas those in trials with additional therapies were excluded.
The medical records of all patients who completed CAR T-cell infusion, were retrospectively reviewed. Clinical data, including demographics, disease and treatment characteristics, and outcomes, were recorded in REDCap. This information was continuously updated, with data export set for 17 December 2024.
This study was approved by the local institutional review boards of all participating centers and was led by MSKCC.
Clinical outcomes
The primary efficacy outcome was PFS, defined as the time from CAR T-cell infusion to confirmed disease recurrence/progression, initiation of the next treatment, death from any cause, or last follow-up, whichever occurred first. Secondary efficacy outcomes included overall survival (OS), objective response rate (ORR), complete response rate (CRR), and duration of response (DOR). OS was defined as the time from CAR T-cell infusion to death or last follow-up. Clinical response was assessed according to the Center for International Blood and Marrow Transplant Research lymphoma response criteria, adapted from the Lugano 2014 criteria, approximately at 1, 3, 6, and 12 months after infusion.19 DOR was defined as the time from the first documented partial or complete response to the date of confirmed disease recurrence/progression, initiation of the next treatment, death from any cause, or last follow-up, whichever occurred first.
The primary safety outcomes were the incidence and severity of cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), and immune effector cell–associated hematotoxicity (ICAHT). CRS and ICANS were graded using the consensus criteria from the American Society for Transplantation and Cellular Therapy.20 ICAHT was graded according to the European Hematology Association and European Society for Blood and Marrow Transplantation consensus grading system.21 Data for ICAHT grading were only available for patients from MSK, Chaim Sheba Medical Center, and Rambam Health Care Campus. Secondary safety outcomes included the incidence of neutropenic fever, infections, anemia, thrombocytopenia, and nonrelapse mortality (NRM). Anemia and thrombocytopenia were graded according to the Common Terminology Criteria for Adverse Events, version 5.0. Infections (bacterial, viral, or fungal) were captured from the start of lymphodepletion until 100 days after CAR T-cell infusion. NRM was defined as death in the absence of relapse, with any relapse considered a competing event. Patients who began subsequent treatment for consolidation or maintenance therapy in the absence of relapse were censored for all time to event analyses except DOR.
An exploratory outcome was resource utilization, which included the administration of steroids, tocilizumab, and anakinra, as well as intensive care unit admissions within 30 days of CAR T-cell infusion.
Statistical analysis
Categorical variables were compared using Pearson χ2 test or Fisher exact test, whereas continuous variables were compared with the Kruskal-Wallis rank sum test. Response rates were compared using logistic regression and odds ratios (ORs) were estimated. The cumulative incidence of infections, anemia, thrombocytopenia, and NRM was compared using Gray test. PFS/OS and DOR curves were generated using the Kaplan-Meier method and compared with the log-rank test. Survival probabilities at 1 and 2 years were calculated.
Univariable Cox proportional hazards (PH) regression was used to analyze the associations of treatment groups and clinical covariates with survival outcomes. Multivariable Cox PH models were used to estimate hazard ratios (HRs) for OS and PFS, adjusting for the following covariates: CAR T-cell products, age, sex, performance status (Karnofsky Performance Scale [KPS]), LBCL subtype, primary refractory disease, stage at apheresis, bulky disease (any mass ≥10 cm or more than one-third of mediastinum by tomodensitometry or positron emission tomography scan), lactate dehydrogenase (LDH) level, line of treatment, bridging therapy, disease response before infusion, and treatment era (2016-2020 vs 2021-2024). Multivariable analyses (MVAs) were conducted for patients with complete data for all covariates.
A propensity score matching weights (PSMW) analysis was performed to compare survival between axi-cel and liso-cel and account for differences in product selection. Propensity scores were calculated using a multivariable logistic regression model, adjusted for the following covariates: age, performance status, LBCL subtype, primary refractory disease, stage at apheresis, bulky disease, LDH level, line of treatment, and response to the last line of treatment. Inverse probability of treatment weights were calculated using the resulting propensity scores and used to estimate inverse probability of treatment–weighted Cox PH models for survival outcomes.
All analyses were performed using R version 4.4.1 with the tidyverse, gtsummary, Hmisc, survival, survminer, and tidycmprsk packages. For descriptive statistics, percentages and means or medians with interquartile range (IQR) were used. Statistical significance was set at a 2-sided P value < .05, and binomial 95% confidence intervals (CIs) were calculated.
Subgroup analyses
Subgroup analyses were conducted to compare the CAR T-cell outcomes by product when used in second-line vs third-line or later treatment settings. The statistical methods were applied as described above, but the line of treatment was excluded from the MVAs. There were only 5 patients treated with tisa-cel in the second-line setting; these patients were excluded from this subgroup analysis.
To mitigate potential biases arising from patients being treated in different countries, another subgroup analysis was conducted including only patients treated in the United States. All statistical analyses were performed as previously described.
Results
Patient characteristics
We included 624 patients with relapsed/refractory LBCL who were treated with axi-cel, tisa-cel, or liso-cel across the following centers: 299 at MSKCC, 130 at Chaim Sheba Medical Center, 126 at Hackensack University Medical Center, and 69 at Rambam Health Care Campus.
Patient characteristics are summarized in Table 1. The median age was 65 years (IQR, 56-73). Patients who received liso-cel or tisa-cel were significantly older than those treated with axi-cel (median ages, 71, 71, and 62 years, respectively; P < .001). Male patients were overrepresented (61%), and more females were treated with tisa-cel compared to liso-cel and axi-cel (50%, 36%, and 36%, respectively; P = .018). Most patients had a KPS of <90 (62%), but patients with a lower performance status were overrepresented in the liso-cel group compared to the tisa-cel and axi-cel groups (75%, 57%, and 58%, respectively; P = .001).
Patient and disease characteristics
| Characteristics . | Overall, N = 624 . | Axi-cel, n = 344 . | Tisa-cel, n = 142 . | Liso-cel, n = 138 . | P value∗ . |
|---|---|---|---|---|---|
| Sex | .018 | ||||
| Male | 377 (61) | 219 (64) | 71 (50) | 87 (64) | |
| Female | 245 (39) | 125 (36) | 70 (50) | 50 (36) | |
| Age, y | <.001 | ||||
| ≤65 | 320 (51) | 228 (66) | 50 (35) | 42 (30) | |
| >65 | 304 (49) | 116 (34) | 92 (65) | 96 (70) | |
| Median (IQR) | 65 (56-73) | 62 (52-68) | 71 (62-77) | 71 (64-78) | <.001 |
| Performance status (KPS) | .001 | ||||
| ≥90 | 234 (38) | 140 (42) | 60 (43) | 34 (25) | |
| <90 | 380 (62) | 197 (58) | 79 (57) | 104 (75) | |
| LBCL subtype | .022 | ||||
| DLBCL, NOS | 515 (83) | 271 (79) | 124 (87) | 120 (87) | |
| HGBL | 64 (10) | 38 (11) | 14 (9.9) | 12 (8.7) | |
| Other† | 45 (7.2) | 35 (10) | 4 (2.8) | 6 (4.3) | |
| Double or triple hit | 81 (19) | 53 (23) | 13 (15) | 15 (13) | .036 |
| Cell of origin | .035 | ||||
| GCB | 281 (50) | 159 (53) | 53 (40) | 69 (53) | |
| Non-GCB | 281 (50) | 140 (47) | 79 (60) | 62 (47) | |
| Transformation status | .31 | ||||
| De novo | 427 (69) | 234 (69) | 98 (69) | 95 (69) | |
| Transformed FL | 121 (20) | 69 (20) | 22 (15) | 30 (22) | |
| Transformed from other | 69 (11) | 34 (10) | 22 (15) | 13 (9.4) | |
| Primary refractory | 265 (43) | 170 (50) | 48 (34) | 47 (35) | <.001 |
| Previous treatment lines‡ | <.001 | ||||
| <2 | 103 (17) | 67 (20) | 5 (3.5) | 31 (23) | |
| ≥2 | 509 (83) | 268 (80) | 137 (96) | 104 (77) | |
| Previous auto-HCT | 114 (18) | 60 (18) | 31 (22) | 23 (17) | .47 |
| Previous allo-HCT | 15 (2.4) | 7 (2) | 6 (4.2) | 2 (1.5) | .30 |
| Previous CAR T-cell therapy | 5 (0.8) | 3 (0.9) | 2 (1.4) | 0 (0) | .41 |
| CAR T-cell indication | <.001 | ||||
| Relapsed/refractory after ≥2 lines | 498 (80) | 265 (77) | 137 (96) | 96 (71) | |
| Relapsed >12 months after first line | 2 (0.3) | 0 (0) | 0 (0) | 2 (1.5) | |
| Relapsed ≤12 months after first line | 47 (7.6) | 28 (8.2) | 2 (1.4) | 17 (13) | |
| Refractory to first line | 67 (11) | 46 (13) | 3 (2.1) | 18 (13) | |
| Other | 6 (1.0) | 3 (0.9) | 0 (0) | 3 (2.2) | |
| Stage at apheresis | .14 | ||||
| ≤II | 137 (26) | 66 (23) | 32 (25) | 39 (32) | |
| >II | 400 (74) | 221 (77) | 97 (75) | 82 (68) | |
| Bulky disease at apheresis§ | 99 (16) | 71 (21) | 13 (9.6) | 15 (11) | .002 |
| CNS disease at apheresis | 47 (8) | 21 (6.6) | 9 (6.5) | 17 (13) | .043 |
| Bridging therapy|| | .027 | ||||
| No bridging | 210 (34) | 132 (38) | 40 (28) | 38 (28) | |
| Systemic therapy | 332 (53) | 166 (48) | 88 (62) | 78 (57) | |
| Nonsystemic therapy | 81 (13) | 45 (13) | 14 (9.9) | 22 (16) | |
| Response prelymphodepletion | .002 | ||||
| CR | 58 (9.5) | 23 (6.9) | 15 (11) | 20 (15) | |
| PR | 174 (29) | 103 (31) | 26 (19) | 45 (33) | |
| SD/PD | 377 (62) | 206 (62) | 99 (71) | 72 (53) | |
| LDH prelymphodepletion | .93 | ||||
| Normal (≤250 U/L) | 308 (54) | 163 (54) | 72 (53) | 73 (55) | |
| Elevated (>250 U/L) | 266 (46) | 141 (46) | 65 (47) | 60 (45) | |
| Lymphodepletion | .042 | ||||
| FluCy | 551 (88) | 297 (86) | 133 (94) | 121 (88) | |
| Bendamustine | 72 (12) | 47 (14) | 8 (5.7) | 17 (12) | |
| Apheresis to infusion (IQR), d | 39 (33-47) | 35 (28-43) | 43 (37-52) | 41 (37-51) | <.001 |
| OOS products | 20 (4) | 6 (2) | 4 (4) | 10 (11) | .004 |
| Treatment era | <.001 | ||||
| 2016-2020 | 222 (36) | 111 (32) | 83 (58) | 28 (20) | |
| 2021-2024 | 402 (64) | 233 (68) | 59 (42) | 110 (80) |
| Characteristics . | Overall, N = 624 . | Axi-cel, n = 344 . | Tisa-cel, n = 142 . | Liso-cel, n = 138 . | P value∗ . |
|---|---|---|---|---|---|
| Sex | .018 | ||||
| Male | 377 (61) | 219 (64) | 71 (50) | 87 (64) | |
| Female | 245 (39) | 125 (36) | 70 (50) | 50 (36) | |
| Age, y | <.001 | ||||
| ≤65 | 320 (51) | 228 (66) | 50 (35) | 42 (30) | |
| >65 | 304 (49) | 116 (34) | 92 (65) | 96 (70) | |
| Median (IQR) | 65 (56-73) | 62 (52-68) | 71 (62-77) | 71 (64-78) | <.001 |
| Performance status (KPS) | .001 | ||||
| ≥90 | 234 (38) | 140 (42) | 60 (43) | 34 (25) | |
| <90 | 380 (62) | 197 (58) | 79 (57) | 104 (75) | |
| LBCL subtype | .022 | ||||
| DLBCL, NOS | 515 (83) | 271 (79) | 124 (87) | 120 (87) | |
| HGBL | 64 (10) | 38 (11) | 14 (9.9) | 12 (8.7) | |
| Other† | 45 (7.2) | 35 (10) | 4 (2.8) | 6 (4.3) | |
| Double or triple hit | 81 (19) | 53 (23) | 13 (15) | 15 (13) | .036 |
| Cell of origin | .035 | ||||
| GCB | 281 (50) | 159 (53) | 53 (40) | 69 (53) | |
| Non-GCB | 281 (50) | 140 (47) | 79 (60) | 62 (47) | |
| Transformation status | .31 | ||||
| De novo | 427 (69) | 234 (69) | 98 (69) | 95 (69) | |
| Transformed FL | 121 (20) | 69 (20) | 22 (15) | 30 (22) | |
| Transformed from other | 69 (11) | 34 (10) | 22 (15) | 13 (9.4) | |
| Primary refractory | 265 (43) | 170 (50) | 48 (34) | 47 (35) | <.001 |
| Previous treatment lines‡ | <.001 | ||||
| <2 | 103 (17) | 67 (20) | 5 (3.5) | 31 (23) | |
| ≥2 | 509 (83) | 268 (80) | 137 (96) | 104 (77) | |
| Previous auto-HCT | 114 (18) | 60 (18) | 31 (22) | 23 (17) | .47 |
| Previous allo-HCT | 15 (2.4) | 7 (2) | 6 (4.2) | 2 (1.5) | .30 |
| Previous CAR T-cell therapy | 5 (0.8) | 3 (0.9) | 2 (1.4) | 0 (0) | .41 |
| CAR T-cell indication | <.001 | ||||
| Relapsed/refractory after ≥2 lines | 498 (80) | 265 (77) | 137 (96) | 96 (71) | |
| Relapsed >12 months after first line | 2 (0.3) | 0 (0) | 0 (0) | 2 (1.5) | |
| Relapsed ≤12 months after first line | 47 (7.6) | 28 (8.2) | 2 (1.4) | 17 (13) | |
| Refractory to first line | 67 (11) | 46 (13) | 3 (2.1) | 18 (13) | |
| Other | 6 (1.0) | 3 (0.9) | 0 (0) | 3 (2.2) | |
| Stage at apheresis | .14 | ||||
| ≤II | 137 (26) | 66 (23) | 32 (25) | 39 (32) | |
| >II | 400 (74) | 221 (77) | 97 (75) | 82 (68) | |
| Bulky disease at apheresis§ | 99 (16) | 71 (21) | 13 (9.6) | 15 (11) | .002 |
| CNS disease at apheresis | 47 (8) | 21 (6.6) | 9 (6.5) | 17 (13) | .043 |
| Bridging therapy|| | .027 | ||||
| No bridging | 210 (34) | 132 (38) | 40 (28) | 38 (28) | |
| Systemic therapy | 332 (53) | 166 (48) | 88 (62) | 78 (57) | |
| Nonsystemic therapy | 81 (13) | 45 (13) | 14 (9.9) | 22 (16) | |
| Response prelymphodepletion | .002 | ||||
| CR | 58 (9.5) | 23 (6.9) | 15 (11) | 20 (15) | |
| PR | 174 (29) | 103 (31) | 26 (19) | 45 (33) | |
| SD/PD | 377 (62) | 206 (62) | 99 (71) | 72 (53) | |
| LDH prelymphodepletion | .93 | ||||
| Normal (≤250 U/L) | 308 (54) | 163 (54) | 72 (53) | 73 (55) | |
| Elevated (>250 U/L) | 266 (46) | 141 (46) | 65 (47) | 60 (45) | |
| Lymphodepletion | .042 | ||||
| FluCy | 551 (88) | 297 (86) | 133 (94) | 121 (88) | |
| Bendamustine | 72 (12) | 47 (14) | 8 (5.7) | 17 (12) | |
| Apheresis to infusion (IQR), d | 39 (33-47) | 35 (28-43) | 43 (37-52) | 41 (37-51) | <.001 |
| OOS products | 20 (4) | 6 (2) | 4 (4) | 10 (11) | .004 |
| Treatment era | <.001 | ||||
| 2016-2020 | 222 (36) | 111 (32) | 83 (58) | 28 (20) | |
| 2021-2024 | 402 (64) | 233 (68) | 59 (42) | 110 (80) |
Data are presented as n (%) unless otherwise specified. Values in bold are statistically significant.
allo-HCT, allogeneic hematopoietic cell transplantation; auto-HCT, autologous hematopoietic cell transplantation; CR, complete response; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; FluCy, fludarabine + cyclophosphamide; HGBL, high-grade B-cell lymphoma; NOS, not otherwise specified; PD, progressive disease; PR, partial response; SD, stable disease.
Categorical variables were compared using Pearson χ2 test or Fisher exact test; continuous variables, with the Kruskal-Wallis rank sum test.
Includes 28 patients with primary mediastinal B-cell lymphoma, 7 with T-cell/histiocyte-rich LBCL, 5 with Epstein-Barr virus–positive DLBCL, 3 with leg type of primary cutaneous DLBCL, 1 with intravascular LBCL, and 1 with anaplastic lymphoma kinase–positive large B-cell lymphoma.
Includes all therapies administered before apheresis, including disease-holding therapy.
Bulky disease is defined as any mass ≥10 cm or more than one-third of mediastinum by tomodensitometry or positron emission tomography scan.
Systemic therapy includes all chemotherapies and course of steroids. Nonsystemic therapy includes radiation therapy and surgical debulking.
The most common histology was diffuse LBCL not otherwise specified (83%). A slightly higher proportion of patients were treated for other histologies in the axi-cel group compared to the tisa-cel and liso-cel groups (10%, 2.8%, and 4.3%, respectively; P = .022). Additionally, a slightly higher proportion of patients exhibited double or triple-hit disease in the axi-cel group compared to the tisa-cel and liso-cel groups (23%, 15%, and 13%, respectively; P = .036). Although the proportion of patients with lymphoma from a nongerminal center cell (non-GCB) origin was higher with tisa-cel, it was similar between axi-cel and liso-cel (60%, 47%, and 47%, respectively; P = .035).
Patients treated with axi-cel more often had primary refractory disease compared to those treated with tisa-cel and liso-cel (50%, 34%, and 35%, respectively; P < .001). As expected, significantly fewer patients received tisa-cel in the second-line setting compared to axi-cel and liso-cel (3.5%, 21.2%, and 27.5%, respectively; P < .001).
At leukapheresis, most patients had advanced disease (74% with stage >II), with a similar distribution across the products. However, bulky disease was more common in patients treated with axi-cel compared to those treated with tisa-cel and liso-cel (21%, 9.6%, and 11%, respectively; P = .002). Conversely, central nervous system (CNS) involvement was more frequent in patients treated with liso-cel compared to axi-cel and tisa-cel (13%, 6.6%, and 6.5%, respectively; P = .043).
Bridging therapy was more commonly used before liso-cel and tisa-cel than before axi-cel (72%, 72%, and 62%, respectively; P = .027). At lymphodepletion, a higher proportion of patients had stable or progressive disease before tisa-cel compared to axi-cel and liso-cel (71%, 62%, and 53%, respectively; P = .002). The most common lymphodepletion regimen was fludarabine and cyclophosphamide (FluCy, 88%), and fewer patients received bendamustine before tisa-cel compared to axi-cel and liso-cel (5.7%, 14%, and 12%, respectively; P < .001).
The median time from leukapheresis to CAR T-cell infusion was shortest for axi-cel (35 days; IQR, 28-43) compared to tisa-cel (43 days; IQR, 37-52) and liso-cel (41 days; IQR, 37-51; P < .001). Finally, the infusion of out-of-specification (OOS) products was less frequent with axi-cel and tisa-cel compared to liso-cel (2%, 4%, and 11%, respectively; P = .004).
Patients treated in the recent era (2021-2024), compared to those treated in the earlier era (2016-2020), were less frequently treated with tisa-cel (15% vs 37%) and more frequently with axi-cel (58% vs 50%) and liso-cel (27% vs 13%; P < .001; Table 1; supplemental Table 1). Significant differences between eras included LDH levels prior to lymphodepletion, response to lymphodepletion, use of bridging therapy (especially nonsystemic), and the number of previous lines of therapy.
Subgroup analyses
In the subgroup of patients treated in the third-line or later setting, the main differences between CAR T-cell products mirrored those observed in the overall cohort (supplemental Table 2). Exceptions included the lack of significant differences in the proportions of patients with non-GCB lymphoma, CNS involvement, and disease response before lymphodepletion.
For patients treated in the second-line setting, a few differences were observed compared to the whole cohort (supplemental Table 3). There were no significant differences in the proportions of females, lymphoma subtypes, double- or triple-hit lymphomas, non-GCB lymphomas, primary refractory disease, bulky disease, use of bridging therapy, disease response to the last treatment before lymphodepletion, or the type of lymphodepletion. However, the liso-cel group had a higher proportion of patients with transformed lymphoma compared to the axi-cel group (45%, 19%, respectively; P = .032).
Countries may differ in product approval dates and availability, preferences, patient selection, and management approaches. To mitigate potential biases arising from these differences between Israel and the United States, a subgroup analysis was conducted using only patients treated in the United States (supplemental Table 4). While most patient characteristics were comparable to the whole cohort, a notable difference was the similar proportion of patients with a KPS <90 across the products (axi-cel, 80%; tisa-cel, 76%; liso-cel, 75%; P = .58). Additionally, there were no statistically significant differences in the proportions of females, lymphoma subtypes, double- or triple-hit lymphomas, CNS involvement, or patients with a disease response to the last treatment before lymphodepletion.
Survival outcomes
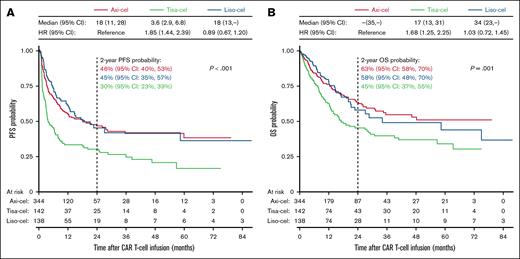
After a median follow-up of 20.9 months (IQR, 10.1-40.4), the median PFS was 18 months for axi-cel (95% CI, 11-28) and liso-cel (95% CI, 13 to not reached), compared to 3.6 months for tisa-cel (95% CI, 2.9-6.8; P < .001; Figure 1A). The estimated 2-year PFS was 46% (95% CI, 40-53) for axi-cel, 45% (95% CI, 35-57) for liso-cel, and 30% (95% CI, 23-39) for tisa-cel. In univariable analyses, tisa-cel was associated with inferior PFS compared to axi-cel (HR, 1.85; 95% CI, 1.44-2.39; P < .001), whereas no significant difference was observed between liso-cel and axi-cel.
Comparison of survival outcomes. Kaplan-Meier estimates of PFS (A) and OS (B) for patients treated with axi-cel, tisa-cel, or liso-cel. Differences in survival were assessed using the log-rank test. Median OS and PFS in months, HRs from the univariable Cox regression model, and 2-year survival probabilities are indicated on the graph.
Comparison of survival outcomes. Kaplan-Meier estimates of PFS (A) and OS (B) for patients treated with axi-cel, tisa-cel, or liso-cel. Differences in survival were assessed using the log-rank test. Median OS and PFS in months, HRs from the univariable Cox regression model, and 2-year survival probabilities are indicated on the graph.
The median OS was not reached for axi-cel, 34 months for liso-cel (95% CI, 23 to not reached), and 17 months for tisa-cel (95% CI, 13-31; P < .001; Figure 1B). The estimated 2-year OS was 63% (95% CI, 58-70) for axi-cel, 58% (95% CI, 48-70) for liso-cel, and 45% (95% CI, 37-55) for tisa-cel. The difference in OS between tisa-cel and axi-cel in the univariable analyses was significant (HR, 1.68; 95% CI, 1.25-2.25; P < .001), whereas there was no significant difference between liso-cel and axi-cel.
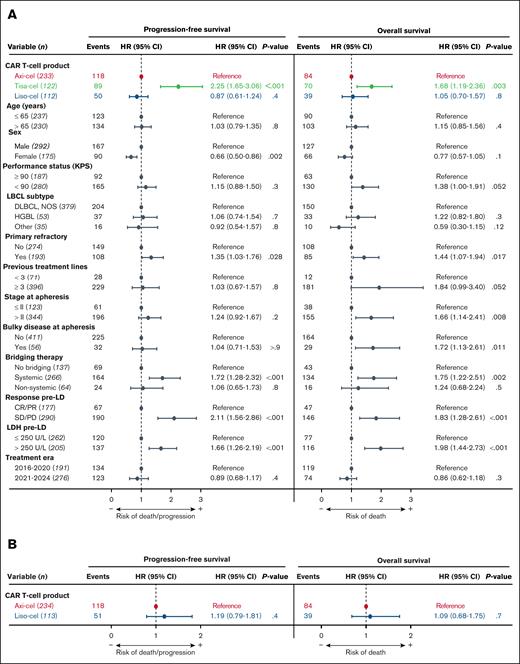
Univariable prognostic analyses identified several patient characteristics significantly associated with survival, highlighting potential confounders (supplemental Figure 1). MVAs were conducted to account for unbalanced patient characteristics and significant potential confounders (Figure 2A). In the MVA model, treatment with tisa-cel compared to axi-cel emerged as the strongest predictor of relapse/progression (HR, 2.25; 95% CI, 1.65-3.06; P < .001). No significant PFS difference was observed between liso-cel and axi-cel. Tisa-cel was also associated with worse OS compared to axi-cel (HR, 1.68; 95% CI, 1.19-2.36; P = .003), whereas no OS difference was observed between liso-cel and axi-cel.
Comparison of survival outcomes after adjustment for confounders. (A) MVAs of factors potentially impacting survival. (B) PSMW sensitivity analysis for patients treated with axi-cel and liso-cel, adjusting for factors that may have influenced product selection. CR, complete response; DLBCL, diffuse large B-cell lymphoma; HGBL, high-grade B-cell lymphoma; LD, lymphodepletion; NOS, not otherwise specified; PD, progressive disease; PR, partial response; SD, stable disease.
Comparison of survival outcomes after adjustment for confounders. (A) MVAs of factors potentially impacting survival. (B) PSMW sensitivity analysis for patients treated with axi-cel and liso-cel, adjusting for factors that may have influenced product selection. CR, complete response; DLBCL, diffuse large B-cell lymphoma; HGBL, high-grade B-cell lymphoma; LD, lymphodepletion; NOS, not otherwise specified; PD, progressive disease; PR, partial response; SD, stable disease.
To further assess any survival differences between liso-cel and axi-cel and account for differences in product selection, a PSMW analysis was conducted (Figure 2B). In this model, no significant differences were observed in PFS or OS.
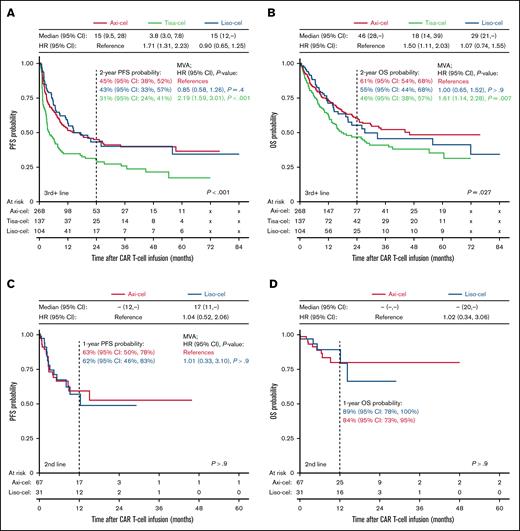
Tisa-cel is not approved for second-line use, which may represent a significant confounder. To account for treatment setting, subgroup analyses were conducted for patients treated in the second-line and third-line or later settings (Figure 3).
Survival outcomes for patients treated in the second-line vs third-line or later settings. (A-B) Kaplan-Meier estimates of PFS (A) and OS (B) for patients treated with axi-cel, tisa-cel, or liso-cel in the third-line or later setting. (C-D) Kaplan-Meier estimates of PFS (C) and OS (D) for patients treated in the second-line setting. Differences in survival were assessed using the log-rank test. Median OS and PFS in months and HRs of the univariable Cox regression model are superimposed. HRs after adjusting for confounders in MVAs and 1- or 2-year survival probabilities are shown on the graph. MVAs could not be performed for OS in the second-line setting due to insufficient events.
Survival outcomes for patients treated in the second-line vs third-line or later settings. (A-B) Kaplan-Meier estimates of PFS (A) and OS (B) for patients treated with axi-cel, tisa-cel, or liso-cel in the third-line or later setting. (C-D) Kaplan-Meier estimates of PFS (C) and OS (D) for patients treated in the second-line setting. Differences in survival were assessed using the log-rank test. Median OS and PFS in months and HRs of the univariable Cox regression model are superimposed. HRs after adjusting for confounders in MVAs and 1- or 2-year survival probabilities are shown on the graph. MVAs could not be performed for OS in the second-line setting due to insufficient events.
In the subgroup of patients treated after ≥2 lines of prior therapy, the results were similar to the overall cohort, with a median PFS of 15 months for axi-cel (95% CI, 9.5-28) and liso-cel (95% CI, 12 to not reached), compared to 3.8 months for tisa-cel (95% CI, 3.0-7.8; P < .001; Figure 3A). The median OS was 46 months for axi-cel (95% CI, 28 to not reached), 29 months for liso-cel (95% CI, 21 to not reached), and 18 months for tisa-cel (95% CI, 14-39; P = .027; Figure 3B). After adjusting for potential confounders with MVAs, tisa-cel was associated with worse PFS (HR, 2.19; 95% CI, 1.59-3.01; P < .001) and OS (HR, 1.61; 95% CI, 1.14-2.28; P = .007) compared to axi-cel, but no statistically significant differences in PFS or OS were observed between liso-cel and axi-cel (Figure 3A-B; supplemental Figure 2).
In the second-line setting, the median PFS was not reached for axi-cel, and was 17 months for liso-cel (95% CI, 11 to not reached; P > .9). The estimated 1-year PFS for axi-cel was 63% (95% CI, 50-78), and 62% (95% CI, 46-83) for liso-cel (Figure 3C). The median OS was not reached for either axi-cel or liso-cel, and the estimated 1-year OS was 84% (95% CI, 73-95) for axi-cel and 89% (95% CI, 78-100) for liso-cel (Figure 3D). The MVAs showed no statistically significant differences in PFS (Figure 3C; supplemental Figure 3). There were only 14 OS events and MVA for OS was not performed.
Finally, survival analyses were performed on the subgroup of patients treated in the United States (supplemental Figure 4A-B). The results were consistent with those seen for the whole cohort, and tisa-cel, compared with axi-cel, was associated with inferior PFS (HR, 2.23; 95% CI, 1.50-3.33; P < .001) and OS (HR, 1.96; 95% CI, 1.25-3.07; P = .003) in the MVA model (supplemental Figure 5A). There were no statistically significant differences in PFS and OS for liso-cel compared to axi-cel (supplemental Figure 5A-B).
Efficacy outcomes
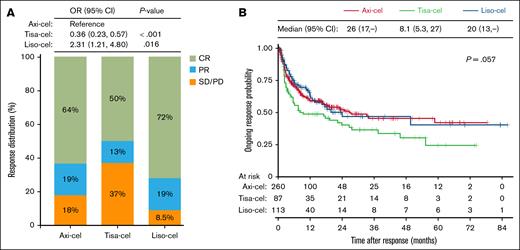
Compared to axi-cel, which had an ORR at 100 days of 82%, the ORR was higher with liso-cel at 92% (OR, 2.31; 95% CI, 1.21-4.80; P = .016) and lower with tisa-cel at 63% (OR, 0.36; 95% CI, 0.23-0.57; P < .001). The CRRs were 72%, 64%, and 50% for liso-cel, axi-cel, and tisa-cel, respectively (Figure 4A). Odds of achieving CRR within 100 days were also favorable with axi-cel compared to tisa-cel (OR, 0.56; 95% CI, 0.37-0.85; P = .006); the difference in CRR between axi-cel and liso-cel was not significant. The median DOR was 26 months for axi-cel (95% CI, 17 to not reached), 20 months for liso-cel (95% CI, 13 to not reached), and 8.1 months for tisa-cel (95% CI, 5.3-27; P = .057; Figure 4B).
Comparison of treatment response. (A) Distribution of best response at 100 days after CAR T-cell infusion. ORs from logistic regression comparing ORRs in tisa-cel and liso-cel to axi-cel are indicated. (B) Kaplan-Meier estimates of the DOR. Differences in DOR were evaluated using the log-rank test, and median DOR (in months) is shown. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Comparison of treatment response. (A) Distribution of best response at 100 days after CAR T-cell infusion. ORs from logistic regression comparing ORRs in tisa-cel and liso-cel to axi-cel are indicated. (B) Kaplan-Meier estimates of the DOR. Differences in DOR were evaluated using the log-rank test, and median DOR (in months) is shown. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Patients treated after ≥2 lines showed response rates similar to those observed in the whole cohort, although differences in the odds of ORR and CRR for liso-cel compared to axi-cel did not reach statistical significance (supplemental Figure 6). In the second-line setting, the ORR and CRR were 97% and 76% for liso-cel, and 85% and 67% for axi-cel, respectively, but the differences were not statistically significant.
For patients treated in the United States, the response rates and DOR were also consistent with those observed in the whole cohort (supplemental Figure 4C-D).
Safety outcomes and resources
Rates of any-grade CRS were significantly higher with axi-cel compared to tisa-cel and liso-cel, at 88%, 72%, and 51%, respectively (P < .001; Figure 5A). Rates of grade ≥2 CRS were also significantly higher for axi-cel (42%) compared to tisa-cel (35%) and liso-cel (16%; P < .001). The most notable difference was observed in the rates of grade ≥3 CRS between axi-cel and liso-cel: 13% for axi-cel, 13% for tisa-cel, and only 1.4% for liso-cel (P < .001). The median time to onset of CRS was slightly shorter for tisa-cel (2 days; IQR, 1-3) compared to axi-cel (3 days; IQR, 1-5) and liso-cel (3 days; IQR, 2-6; P < .005), whereas the median duration of CRS was longer after axi-cel (5 days; IQR, 3-7) compared to tisa-cel (4 days; IQR, 3-7) and liso-cel (2.5 days; IQR, 1-5; P < .001).
Comparison of toxicities. (A-C) Grades of CRS (A), ICANS (B), and ICAHT (C). (D) Proportion of patients who had neutropenic fever (214 patients had missing data for neutropenic fever). Comparisons in panels A-D were performed using Pearson χ2 test. (E) Cumulative incidence of infections (viral, bacterial, or fungal) from lymphodepletion to 30 days and 1 year after infusion. (F) Cumulative incidence of NRM, with incidence at 3 months and 2 years indicated. Comparisons in panels E-F were performed using Gray test.
Comparison of toxicities. (A-C) Grades of CRS (A), ICANS (B), and ICAHT (C). (D) Proportion of patients who had neutropenic fever (214 patients had missing data for neutropenic fever). Comparisons in panels A-D were performed using Pearson χ2 test. (E) Cumulative incidence of infections (viral, bacterial, or fungal) from lymphodepletion to 30 days and 1 year after infusion. (F) Cumulative incidence of NRM, with incidence at 3 months and 2 years indicated. Comparisons in panels E-F were performed using Gray test.
Patients treated with axi-cel developed more any-grade ICANS (37%) compared to tisa-cel (18%) and liso-cel (14%; P < .001). They also had more grade ≥2 ICANS (axi-cel, 24%; tisa-cel, 9.9%; liso-cel, 8.7%; P < .001) and grade ≥3 ICANS (axi-cel, 16%; tisa-cel, 5.0%; liso-cel, 5.8%; P < .001; Figure 5B). The median time to ICANS onset was shorter for tisa-cel (4 days; IQR, 2-7) compared to axi-cel (6 days; IQR, 5-8) and liso-cel (6.5 days; IQR, 2-8; P = .016). There were no significant differences in the duration, with an overall median duration of 4 days (IQR, 2-8).
When patients were stratified by treatment era, we observed a slight decrease in both all-grade and high-grade ICANS after axi-cel, and a higher incidence of CRS with tisa-cel in the recent era. Otherwise, CRS and ICANS rates remained largely similar across eras (supplemental Figure 7).
Rates of ICAHT grade 1 to 4 was higher after axi-cel (90%) compared to liso-cel (84%) and tisa-cel (70%; P < .001; Figure 5C). However, there were no significant differences in the cumulative incidence of grade ≥3 anemia or thrombocytopenia among the 3 products (supplemental Figure 8).
Consistent with the rates of CRS, neutropenic fever was more frequent with axi-cel (73%) compared to tisa-cel (57%) and liso-cel (36%; P < .001; Figure 5D). However, the 3 products were associated with a similar cumulative incidence of infections (Figure 5E).
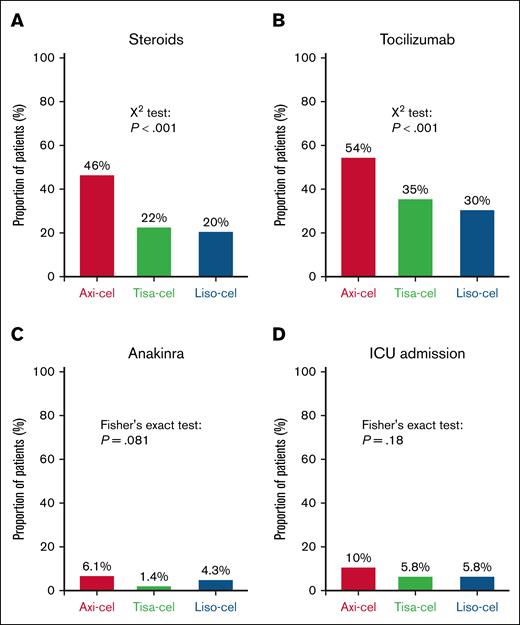
No significant difference in the cumulative incidence of NRM was found between the 3 products (Figure 5F). However, consistent with the higher rates of CRS and ICANS, patients treated with axi-cel required more treatments for toxicities (Figure 6). Steroids were administered to 46% of patients after axi-cel, compared to 22% after tisa-cel and 20% after liso-cel (P < .001). Tocilizumab was given to 54% of patients after axi-cel, 35% after tisa-cel, and 30% after liso-cel (P < .001). Despite a trend toward a higher proportion of patients after treatment with axi-cel, there was no statistically significant difference in the rates of anakinra administration or intensive care unit admissions.
Resources for managing toxicities. Proportion of patients requiring steroids (A), tocilizumab (B), anakinra (C), or admission to ICU (D). Of note, data on ICU admission were missing for 20 patients. Comparisons were performed using Pearson χ2 test. ICU, intensive care unit.
Resources for managing toxicities. Proportion of patients requiring steroids (A), tocilizumab (B), anakinra (C), or admission to ICU (D). Of note, data on ICU admission were missing for 20 patients. Comparisons were performed using Pearson χ2 test. ICU, intensive care unit.
Discussion
In this cohort of patients with relapsed/refractory LBCL, tisa-cel was associated with worse survival, whereas axi-cel and liso-cel showed similar outcomes. Liso-cel was associated with lower rates of CRS and ICANS, though ICAHT and infection rates were similar to axi-cel.
Real-world studies have confirmed the comparable efficacy of commercial CAR T-cell therapies to clinical trial results.1-5,11,22-33 Although our findings generally align with these studies, survival following axi-cel infusion appears slightly improved in our cohort, and liso-cel showed a higher response rate than previously reported. This may reflect better disease control prior to CAR T-cell infusion, as response to bridging therapy is a significant survival predictor.14 In our cohort, 66% of patients received bridging therapy, including 62% of those treated with axi-cel and 72% with liso-cel. Although most patients in the JULIET study received bridging for tisa-cel, bridging was restricted in the ZUMA-1 and ZUMA-7 trials.1,2,4 As a result, only 22% to 53% of patients in previously published real-world studies on axi-cel received bridging therapy.11,27-29 Meanwhile, 59% of patients in the TRANSCEND NHL-001 study received bridging, similar to what was reported in a recent real-world study, and the choice of regimens was restricted in the TRANSFORM trial.3,5,33 Finally, greater experience with CAR T-cell therapy over time may have improved patient selection, the choice of bridging regimens, and toxicity management.
Consistent with previous reports, tisa-cel showed inferior survival and response rates.8-14 Our MVA model identified tisa-cel infusion, rather than axi-cel, as the strongest predictor of relapse/progression. In contrast to a previous matching-adjusted indirect comparison of JULIET and TRANSCEND NHL-001, we also observed worse survival with tisa-cel compared to liso-cel.34 Comparisons between liso-cel and axi-cel are limited and reflect patients treated in the third-line setting.18,35 In the study by Looka et al, a propensity score–matching analysis was performed on a cohort of 87 patients, which demonstrated inferior PFS and ORR for liso-cel.18 Conversely, and consistent with Portuguese et al, we did not find any significant differences in survival between these 2 products, even after adjusting for potential confounders using MVAs and PSMW analyses.35 These findings were consistently observed in subanalyses stratifying patients by the indication for CAR T-cell therapy and the country in which the therapy was provided.
The patient characteristics in our study and the Looka et al18 study were overall similar. Although the axi-cel group in our cohort included more patients with double- or triple-hit disease, primary refractory disease, and bulky disease, these covariates were well balanced in the subgroup of patients treated in the second-line setting. Also, in our cohort, slightly more patients were in CR after their last treatment before liso-cel infusion, though the differences were not significant in the third-line setting. A notable difference in our study was that patients treated with liso-cel had significantly worse performance status, though this was accounted for in our analyses. Furthermore, despite the worse performance status in our liso-cel cohort, this group had overall favorable response rates and survival. A key difference between the studies was the proportion of patients receiving OOS products. In the study by Looka et al,18 27% of liso-cel patients received OOS products, compared to 11% in our cohort, though not all centers reported OOS products. Other factors, such as practice heterogeneity across centers or unmeasured confounders, may also explain the discrepancies. However, our study was much larger (624 patients) and multicenter, which may better account for these potential confounders. Finally, methodological differences may have introduced distinct biases. Although Looka et al18 compared 2 separate cohorts treated preferentially with axi-cel or liso-cel during different time periods, we instead reviewed all patients treated with CAR T cells within a single cohort.
Regarding toxicities, our results confirm higher CRS and ICANS rates with axi-cel compared to tisa-cel and liso-cel.8-14,18,36,37 We also observed higher rates of hematotoxicity and neutropenic fever within 30 days of axi-cel infusion.38 Interestingly, the cumulative incidence of infection and NRM was similar across all products, suggesting similar immunosuppression.39 This is consistent with a recent report identifying infection as the leading cause of NRM after CAR T-cell therapy.40 However, the higher overall toxicity associated with axi-cel in our cohort translated into greater use of resources to manage complications, including increased administration of steroids and tocilizumab. This has implications for the cost of these therapies. While axi-cel has demonstrated cost-effectiveness over tisa-cel due to its greater efficacy, a recent study suggested that liso-cel is the most cost-effective option in the third-line setting.16,17,41 Additionally, the lower toxicity profile of liso-cel makes it more suitable for outpatient administration, an important advantage given the growing number of patients eligible for CAR T-cell therapies and limited resource capacities for hospitalization.
Beyond the retrospective design of the study, we acknowledge other limitations. Because of missing data and heterogeneity in laboratory measurements, inflammation was not included in our analyses. Although more frequent in patients with high tumor burden, elevated systemic inflammation has been shown to be an independent risk factor for CAR T-cell treatment failure.42 Whether patients with systemic inflammation were balanced across products is unknown and may represent a potential bias. Additionally, although we performed a subanalysis of patients treated in different countries, it was not possible to capture heterogeneity in patient management across centers, and follow-up for patients returning to local health centers was sometimes incomplete.
In conclusion, our real-world analysis confirmed tisa-cel's inferior efficacy and showed comparable efficacy between axi-cel and liso-cel. Liso-cel exhibited a slightly higher ORR than axi-cel, but similar PFS and OS, along with a more favorable toxicity profile. These findings provide valuable insights into product selection, suggesting that liso-cel, often chosen for older or frail patients, may also be considered for younger, fitter individuals. This also provides perspectives on the suitability of these products for outpatient administration and the cost of postinfusion patient management. However, product selection should also account for factors such as longer manufacturing times and higher rates of OOS products with liso-cel, particularly in settings where expanded access protocols are unavailable. Further large-scale real-world studies with longer follow-up, particularly in the second-line setting, and cost-effectiveness analyses are needed to confirm our findings and provide more guidance on product selection.
Acknowledgments
X.D.-S. was supported by a University of Montreal Hospital Centre Foundation fellowship, a Perras, Cholette & Cholette Clinical Subspecialty Scholarship, and a Detweiler Travelling fellowship from the Royal College of Physicians and Surgeons of Canada. This work was supported in part by National Cancer Institute core grant P30 CA008748 and National Institutes of Health award P01 CA23766.
Authorship
Contribution: X.D.-S., M.S., and P.B.D. conceived and designed the study; X.D.-S., O.B.-K., A.I., R.M., A.A., A.B., E.R., M.A., A.R.D., M.C.D.L., and A.L.D.A. collected data; M.B., S.M.D., M.G., X.D.-S., and R.S. interpreted data and performed statistical analyses; X.D.-S. wrote the initial manuscript; M.S., P.B.D., R.S., and M.B. edited the manuscript; O.B.-K., A.I., R.M., A.A., M.L.P., G.L.S., R.L., A.P.B., L.F., J.L., G.S., and M.-A.P. revised the manuscript; and all authors read and approved the final version for publication.
Conflict-of-interest disclosure: X.D.-S. has served on advisory boards for Kite/Gilead; and has received speaker honoraria from Bristol Myers Squibb and Hoffmann-La Roche. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience Inc, and Omeros Corporation; reports research funding from Angiocrine Bioscience Inc, Omeros Corporation, Amgen Inc, Bristol Myers Squibb, and Sanofi; served on ad hoc advisory boards for Kite (a Gilead company) and Miltenyi Biotec; and reports honoraria from i3 Health, Medscape, CancerNetwork, Intellisphere LLC, Curio Science LLC, and IDEOlogy. M.-A.P. reports honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, ExeVir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, Orca Bio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; serves on data and safety monitoring boards for Cidara Therapeutics and Sellas Life Sciences; participates on the scientific advisory board of NexImmune; reports ownership interests in NexImmune, Omeros, and Orca Bio; and institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. A.P.B. reports consultancy for Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Xavier Deschênes-Simard, Centre Hospitalier de l’Université de Montréal, 1000 Rue St-Denis, Montreal, QC, Canada, H2X 0C1; email: xavier.deschenes-simard@umontreal.ca; and Michael Scordo, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: scordom@mskcc.org.
References
Author notes
Data are available upon reasonable request from the corresponding authors, Xavier Deschênes-Simard (xavier.deschenes-simard@umontreal.ca) and Michael Scordo (scordom@mskcc.org).
The full-text version of this article contains a data supplement.