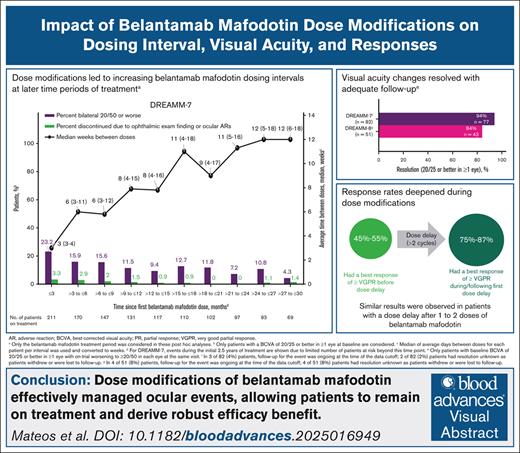
Key Points
Dose modifications in DREAMM-7 and DREAMM-8 led to less frequent belamaf dosing in later periods of treatment.
Ocular events were managed by belamaf dose modifications, which allowed for continuation of treatment and efficacy benefits.
Visual Abstract
Belantamab mafodotin (belamaf) combined with standard therapies demonstrated significant progression-free survival (PFS) and overall survival benefits in DREAMM-7 and PFS benefit in DREAMM-8 in relapsed/refractory multiple myeloma. Belamaf dose modifications managed adverse events, including belamaf-related ocular events. Ocular events included ocular adverse reactions (eg, dry eyes, photophobia, eye irritation) and protocol-mandated ophthalmic examination findings. Protocol-recommended dose modifications for ocular events were driven by ophthalmic examination findings and included belamaf dose delays until resolution and reductions. We used descriptive analyses to evaluate the impact of dose modifications on managing ocular events and treatment efficacy. In patients with normal baseline vision who were receiving treatment, dose modifications extended belamaf dosing intervals to a median of 8 to 12 weeks by 9 months; the prevalences of reduced vision to bilateral 20/50 or worse and ocular adverse reactions were highest in the first 3 months and remained low at later time points. The median time to resolution after grade ≥2 ophthalmic examination findings was 12 weeks. Rates of belamaf discontinuations due to ocular events were low. Almost all responders (partial response or better) required dose modifications. Most patients achieved a response before an extended (>2 cycles) dose delay; most who had not, subsequently achieved or deepened their response. In DREAMM-7 and DREAMM-8, the median PFS in patients with ≥1 dose delay of ≥12 weeks was 36.6 months and not reached, respectively. Ocular events were common but effectively managed with dose modifications, allowing for patients to remain on treatment and derive robust efficacy benefit. The trials were registered at www.clinicaltrials.gov as #NCT04246047 (DREAMM-7) and #NCT04484623 (DREAMM-8).
Introduction
In the DREAMM-7 and DREAMM-8 phase 3 trials, belantamab mafodotin (belamaf) in combination with standard backbone therapies was compared with standard-of-care regimens and demonstrated statistically significant and clinically meaningful progression-free survival (PFS) benefit in patients with relapsed/refractory multiple myeloma after ≥1 prior line of therapy.1,2 In DREAMM-7, PFS favored belamaf, bortezomib, and dexamethasone (BVd) vs daratumumab, bortezomib, and dexamethasone (DVd; hazard ratio [HR], 0.41; 95% confidence interval [CI], 0.31-0.53; P < .001).1 In a subsequent interim analysis for overall survival (OS), BVd demonstrated an early, sustained, and statistically significant OS benefit (HR, 0.58; 95% CI, 0.43-0.79; P = .00023).3 In DREAMM-8, PFS favored belamaf, pomalidomide, and dexamethasone (BPd) vs pomalidomide, bortezomib, and dexamethasone (PVd; HR, 0.52; 95% CI, 0.37-0.73; P < .001). BPd also demonstrated an early trend for OS benefit (HR, 0.77; 95% CI, 0.53-1.14); OS follow-up is ongoing.2
The belamaf dosing regimens in DREAMM-7 and DREAMM-8 were determined in dose-finding studies.4,5 In DREAMM-7, the recommended dose was 2.5 mg/kg every 3 weeks, whereas in DREAMM-8, the dose was 2.5 mg/kg in cycle 1, followed by 1.9 mg/kg every 4 weeks from cycle 2 onward.1,2
Belamaf is an antibody-drug conjugate (ADC) that targets B-cell maturation antigen, a protein highly expressed on most multiple myeloma cells.6 Ocular events with belamaf are due to off-target uptake of the ADC and intracellular release of the monomethyl auristatin F (MMAF) payload, which causes apoptosis of corneal epithelial cells.7 Patients who receive belamaf in clinical studies also have ophthalmic examination findings (OEFs); these are also seen with other MMAF-containing ADCs.8,9 In DREAMM-7 and DREAMM-8, protocol-recommended dose modifications were used to manage adverse events (AEs), including OEFs graded per the Keratopathy and Visual Acuity (KVA) scale.1,2,10 The objective of this manuscript is to evaluate the impact of dose modifications on the benefit-risk profile of belamaf in DREAMM-7 and DREAMM-8.
Methods
DREAMM-7 (ClinicalTrials.gov identifier: NCT04246047) and DREAMM-8 (ClinicalTrials.gov identifier: NCT04484623) are global, phase 3, open-label, randomized trials evaluating combinations of belamaf with standard backbone therapies in patients with relapsed/refractory multiple myeloma who received ≥1 prior line of therapy. The study designs and results of the primary analyses of DREAMM-7 and DREAMM-8 have been reported.1-3 DREAMM-7 compared BVd vs DVd; DREAMM-8 compared BPd vs PVd (supplemental Figure 1).
The trials were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent. The independent ethics committee or institutional review board at each site approved the protocols. The data analysis was performed by GSK, and all authors had access to the primary clinical data.
Ocular assessments
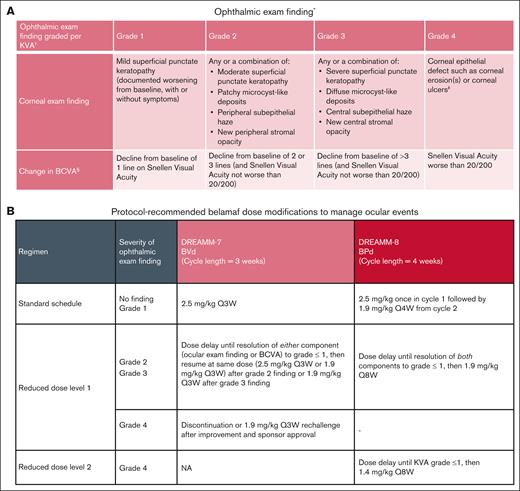
Assessment of ocular events included evaluation of ocular adverse reactions (ARs) and OEFs. OEFs, graded per KVA scale, comprised 2 components: (1) corneal examination findings by slit lamp examination and (2) changes in best-corrected visual acuity (BCVA) measured by Snellen or equivalent assessments (Figure 1A). The overall grade of the OEF was determined by the more severe grade of the 2 components in the worse affected eye; a KVA grade of ≥2 drove protocol-recommended belamaf dose modifications.1,2 See “Supplemental Methods” (Appendix, pg 2-3) for additional details including frequency of ophthalmic examinations.
Ocular event management. (A-B) Ocular ARs and OEFs were used to assess ocular events (A) and OEFs drove protocol-recommended belamaf dose modification (B). ∗Examination findings were reported as AEs before implementation of KVA scale. †Dose modification should be based on the most severe finding. If eyes differ in severity, the dose modification guideline should be applied based on the more severe eye. ‡Corneal ulcer is defined as an epithelial defect with underlying stromal infiltration. §Changes in visual acuity due to treatment-related corneal findings. CTCAE, Common Terminology Criteria for Adverse Events; NA, not applicable; Q3W, every 3 weeks.
Ocular event management. (A-B) Ocular ARs and OEFs were used to assess ocular events (A) and OEFs drove protocol-recommended belamaf dose modification (B). ∗Examination findings were reported as AEs before implementation of KVA scale. †Dose modification should be based on the most severe finding. If eyes differ in severity, the dose modification guideline should be applied based on the more severe eye. ‡Corneal ulcer is defined as an epithelial defect with underlying stromal infiltration. §Changes in visual acuity due to treatment-related corneal findings. CTCAE, Common Terminology Criteria for Adverse Events; NA, not applicable; Q3W, every 3 weeks.
Ocular ARs were ocular AEs of special interest that were reported in the electronic case report form and graded per Common Terminology Criteria for Adverse Events version 5.0. Per protocol, the recommended dose modifications were based on OEFs; however, investigators could also modify dosing due to other AEs, including ocular ARs. All AEs were collected from the start of treatment until ≥70 days after the discontinuation of study treatment. Patients reported their ability to read and drive to the eye care professional, who assessed these activities during the ocular examination conducted before each dose. Supportive care for ocular events associated with belamaf included administration of preservative-free artificial tears in each eye at least 4 to 8 times daily beginning at cycle 1 day 1 until the end of treatment.
Descriptive analysis defined a clinically meaningful change in visual acuity as a reduction in BCVA to 20/50 or worse (≥3-line change) in both eyes at the time of examination (ie, bilateral) from normal (20/25 or better in 1 or both eyes [ie, ≥1 eye]). These patients were assessed for improvement in BCVA to better than 20/50 or 20/200 (depending on the level of worsening) in ≥1 eye; resolution to normal BCVA; median time between doses; on-treatment time with bilateral 20/50 or worse; response rates; and discontinuation rates.
Dose modifications
The starting dose and dose modifications are summarized in Figure 1B. Both trials required dose delays until resolution. In DREAMM-7, resolution was defined as either OEF component grade decreasing to grade ≤1; in DREAMM-8, it was defined as both component grades decreasing to grade ≤1. In DREAMM-7, dose reduction involved reducing the dose from 2.5 mg/kg to 1.9 mg/kg; in DREAMM-8, dose reduction involved reducing the dosing frequency from every 4 weeks to every 8 weeks and/or reducing the dose from 1.9 mg/kg to 1.4 mg/kg. Therefore, in DREAMM-8, both dose delays and reductions contributed to extended dosing intervals. For comparability across studies and to better understand the impact of longer dosing intervals, dose delays were calculated based on dosing that occurred every 3 weeks (+ 3-day window) for DREAMM-7 and every 4 weeks (+ 3-day window) for DREAMM-8 (every 3 weeks [+ 3-day window] for bortezomib). Thus, unless otherwise stated, belamaf dose delays reported for DREAMM-8 in this paper include all dosing intervals that were >4 weeks (+ 3-day window), even if the patient was on the dose-reduced regimen that was administered every 8 weeks.
Dose modifications and safety outcomes
Post hoc analyses evaluated the impact of dose modifications on safety outcomes. Percentages of patients on treatment with normal baseline BCVA who experienced bilateral reduction to 20/50 or worse, ocular ARs, and discontinuations due to OEFs or ocular ARs were evaluated every 3 months after the first belamaf dose. Patients who discontinued any study treatment due to any ocular event were evaluated for the total number of belamaf infusions received per patient and the outcome of the last ocular event.
Dose delays and efficacy outcomes
Post hoc analyses investigated the impact of dose modifications on efficacy outcomes. Best responses per International Myeloma Working Group11 before and during or after the first extended dose delay, defined as lasting >2 cycles, were assessed (see “Supplemental Methods,” Appendix pg 2-3). These analyses included dose delays due to any AEs, including ocular events (ocular ARs and/or OEFs). To assess the impact of extended dose delays on PFS, a post hoc PFS analysis was conducted in patients with ≥1 extended dose delay lasting ≥12 weeks.
Statistical analyses
Results were summarized using descriptive statistics, unless otherwise specified. The median time between doses and corresponding ranges or interquartile ranges were derived from the average time between doses per patient. The Kaplan-Meier method was used to estimate the median PFS; corresponding 95% CIs were calculated with the Brookmeyer-Crowley method.12
Results
Overview of ocular events
Ocular events, which included ocular ARs and OEFs, were previously reported.1,2 Incidence of ocular ARs was similar in patients with or without ocular medical history at baseline (supplemental Table 1). Frequently occurring ocular ARs with belamaf combinations included blurred vision, dry eye, photophobia, foreign body sensation, eye irritation, and eye pain (supplemental Table 2).
Per protocol, overall grade ≥2 OEFs required a belamaf dose delay until resolution. Overall, the incidence rate of grade ≥2 OEFs was 86% and 87% with BVd and BPd, respectively (Table 1). The cumulative incidence rate of grade ≥2 OEFs after 2 doses of belamaf with BVd and BPd was 62% and 73%, respectively (supplemental Table 3). These results suggest that after ≥2 doses of belamaf, most patients would require a dose delay until resolution. The median time to onset of the first grade ≥2 findings with BVd and BPd was ∼6 weeks (range, 2-87) and ∼5 weeks (range, 3-76), respectively. The first grade ≥2 OEFs resolved in 81% of patients on BVd and 86% on BPd, and the median time to resolution was ∼12 weeks (range, 1-111) and ∼16 weeks (range, 2-107), respectively. In a pooled post hoc analysis of DREAMM-7 and DREAMM-8 (N = 392), the median time to resolution of any grade ≥2 OEF was ∼12 weeks (range, 0.3-116; Table 2). Patients could have experienced multiple events, with 53% and 57% of patients who had an event having ≥3 events with BVd and BPd, respectively (Table 1). The median duration of occurrences was generally consistent and predictable, ranging from 12 to 16 weeks (Table 1).
Grade 2 or higher OEFs in DREAMM-7 and DREAMM-8
| Grade 2 or higher OEFs . | DREAMM-7 BVd (N = 242) . | DREAMM-8 BPd (N = 150) . |
|---|---|---|
| Overall grade ≥2 OEFs, n (%) | 209 (86) | 131 (87) |
| Time to onset of first grade ≥2 event, median (range), d | 43 (15-611) | 32 (18-533) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 86 (5-774) | 109 (15-746) |
| First event resolved, n/N (%)† | 169/209 (81) | 113/131 (86) |
| Occurrences, n/N (%) | ||
| 1 | 63/209 (30) | 36/131 (27) |
| 2 | 35/209 (17) | 20/131 (15) |
| ≥3 | 111/209 (53) | 75/131 (57) |
| Grade ≥2 corneal examination finding, n (%) | 198 (82) | 120 (80) |
| Time to onset of first grade ≥2 event, median (range), d | 44 (15-967) | 47 (18-672) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 96 (8-802) | 92 (15-746) |
| First event resolved, n/N (%)† | 172/198 (87) | 108/120 (90) |
| Occurrences, n/N (%) | ||
| 1 | 63/198 (32) | 37/120 (31) |
| 2 | 33/198 (17) | 18/120 (15) |
| ≥3 | 102/198 (52) | 65/120 (54) |
| Grade ≥2 BCVA change, n (%) | 194 (80) | 124 (83) |
| Time to onset of first grade ≥2 event, median (range), d | 53 (16-627) | 59 (21-704) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 51 (4-481) | 57 (8-548) |
| First event resolved, n/N (%)† | 173/194 (89) | 109/124 (88) |
| Occurrences, n/N (%) | ||
| 1 | 47/194 (24) | 33/124 (27) |
| 2 | 29/194 (15) | 27/124 (22) |
| ≥3 | 118/194 (61) | 64/124 (52) |
| Grade 2 or higher OEFs . | DREAMM-7 BVd (N = 242) . | DREAMM-8 BPd (N = 150) . |
|---|---|---|
| Overall grade ≥2 OEFs, n (%) | 209 (86) | 131 (87) |
| Time to onset of first grade ≥2 event, median (range), d | 43 (15-611) | 32 (18-533) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 86 (5-774) | 109 (15-746) |
| First event resolved, n/N (%)† | 169/209 (81) | 113/131 (86) |
| Occurrences, n/N (%) | ||
| 1 | 63/209 (30) | 36/131 (27) |
| 2 | 35/209 (17) | 20/131 (15) |
| ≥3 | 111/209 (53) | 75/131 (57) |
| Grade ≥2 corneal examination finding, n (%) | 198 (82) | 120 (80) |
| Time to onset of first grade ≥2 event, median (range), d | 44 (15-967) | 47 (18-672) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 96 (8-802) | 92 (15-746) |
| First event resolved, n/N (%)† | 172/198 (87) | 108/120 (90) |
| Occurrences, n/N (%) | ||
| 1 | 63/198 (32) | 37/120 (31) |
| 2 | 33/198 (17) | 18/120 (15) |
| ≥3 | 102/198 (52) | 65/120 (54) |
| Grade ≥2 BCVA change, n (%) | 194 (80) | 124 (83) |
| Time to onset of first grade ≥2 event, median (range), d | 53 (16-627) | 59 (21-704) |
| Time to resolution of first grade ≥2 event, median (range), d∗ | 51 (4-481) | 57 (8-548) |
| First event resolved, n/N (%)† | 173/194 (89) | 109/124 (88) |
| Occurrences, n/N (%) | ||
| 1 | 47/194 (24) | 33/124 (27) |
| 2 | 29/194 (15) | 27/124 (22) |
| ≥3 | 118/194 (61) | 64/124 (52) |
Duration is the time from onset of any KVA scale event (grade ≥2) until the event is resolved (grade ≤1). Duration is missing for events that have not resolved at the data cutoff.
Resolved was defined as achieving grade ≤1.
Overall grade 2 or higher OEFs across DREAMM-7 and DREAMM-8 (pooled)
| Overall grade 2 or higher OEFs∗ . | DREAMM-7 BVd and DREAMM-8 BPd (N = 392) . |
|---|---|
| Time to resolution of any grade ≥2 event, median (range), d† | 85 (2-813) |
| Total grade ≥2 occurrences, N | 1192 |
| Occurrences resolved, n/N (%)‡ | 995/1192 (83) |
| Occurrences ongoing as of last follow-up, n/N (%) | 197/1192 (17) |
| On treatment and follow-up ongoing | 94/1192 (8) |
| Discontinued treatment and follow-up ongoing | 43/1192 (4) |
| Discontinued treatment and follow-up ended | 60/1192 (5) |
| Overall grade 2 or higher OEFs∗ . | DREAMM-7 BVd and DREAMM-8 BPd (N = 392) . |
|---|---|
| Time to resolution of any grade ≥2 event, median (range), d† | 85 (2-813) |
| Total grade ≥2 occurrences, N | 1192 |
| Occurrences resolved, n/N (%)‡ | 995/1192 (83) |
| Occurrences ongoing as of last follow-up, n/N (%) | 197/1192 (17) |
| On treatment and follow-up ongoing | 94/1192 (8) |
| Discontinued treatment and follow-up ongoing | 43/1192 (4) |
| Discontinued treatment and follow-up ended | 60/1192 (5) |
Post hoc pooled analysis.
Duration is the time from onset of any KVA scale event (grade ≥2) until the event is resolved (grade ≤1). Duration is missing for events that have not resolved at the data cutoff.
Resolved was defined as achieving grade ≤1.
With protocol-driven dose modifications, 34% of patients with normal baseline had bilateral reduction in visual acuity to 20/50 or worse and 1% to 2% to 20/200 or worse at any point during treatment (supplemental Table 4).1,2 The median time to onset of the first bilateral reduction in visual acuity to 20/50 or worse was ∼11 weeks (range, 2-108) with BVd and ∼16 weeks (range, 4-109) with BPd. The first visual acuity reduction to 20/50 or worse resolved to normal baseline in 94% and 84% of patients in the BVd and BPd arms, respectively, and the median time to resolution was ∼9 weeks (range, 1-130) and ∼8 weeks (range, 2-64), respectively. The median time to improvement was ∼3 weeks in both studies (supplemental Table 4). In these patients, the average proportion of time spent on treatment with a visual acuity of 20/50 or worse was low (BVd, 11%; BPd, 14%; supplemental Table 5). On average, resolution of visual acuity occurred faster than resolution of grade ≥2 corneal examination findings (Table 1; supplemental Table 4). Few patients had multiple occurrences of visual acuity reduction to 20/50 or worse; 25 of 242 in the BVd arm and 11 of 150 in the BPd arm had ≥3 occurrences (supplemental Table 4).
Ocular events were managed with dose modifications and generally reversible with adequate follow-up, enabling patients to continue treatment. In a post hoc pooled analysis of belamaf-treated patients in DREAMM-7 and DREAMM-8, 995 of 1192 grade ≥2 OEF occurrences (83%) had resolved (Table 2). Ocular occurrences that had not resolved at the data cutoff were mainly in patients who were still on treatment or in follow-up or those who died or withdrew consent before resolution could be documented.
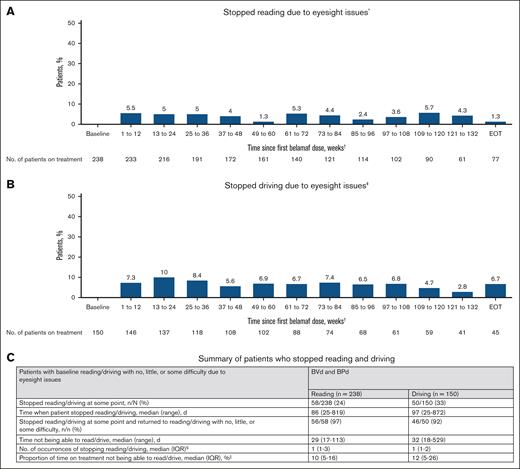
In a pooled analysis of DREAMM-7 and DREAMM-8, 348 of 392 patients (89%) had normal baseline corrected visual acuity and were routinely evaluated for reading and driving abilities at the time of their ocular examination. Most patients did not report having to stop these activities at any point during the study (Figure 2A-B); 180 of 238 (76%) did not stop reading, and 100 of 150 (67%) did not stop driving (Figure 2C). Patients who stopped reading stopped a median of 1 time (interquartile range, 1-3), which accounted for a median of 10% of their time on treatment. Patients who stopped driving stopped a median of 1 time (interquartile range, 1-2), which accounted for a median of 12% of their time on treatment. Of those who stopped reading or driving at some point, nearly all (97% for reading and 92% for driving) returned to normal baseline (Figure 2C).
Reading and driving in patients with normal baseline BCVA (20/25 or better in ≥1 eye) who received BVd in DREAMM-7 and BPd in DREAMM-8. Post hoc pooled analysis (BVd and BPd) of reading (A) and driving (B) in patients who had BCVA of 20/25 or better in ≥1 eye at baseline by time intervals since the first belamaf dose. (C) Summary of patients who stopped reading and driving. ∗Patients who could read with little or no difficulty or read with some difficulty at baseline, mainly due to eyesight issues, were considered, whereas those who stopped reading due to other reasons during follow-up were excluded. †The first 2.5 years of treatment are shown due to the limited number of patients at risk beyond this time point. ‡Patients who could drive with little or no difficulty or drive with some difficulty at baseline, mainly due to eyesight issues, were considered, whereas those who stopped driving due to other reasons during follow-up were excluded. §Occurrences before the treatment end date are considered. ||Time not being able to read/drive is the sum of days from when the patient stopped reading/driving to reading/driving with no, little, or some difficulty or the end of belamaf treatment, whichever is earlier, divided by the duration of belamaf treatment. EOT, end of treatment; IQR, interquartile range.
Reading and driving in patients with normal baseline BCVA (20/25 or better in ≥1 eye) who received BVd in DREAMM-7 and BPd in DREAMM-8. Post hoc pooled analysis (BVd and BPd) of reading (A) and driving (B) in patients who had BCVA of 20/25 or better in ≥1 eye at baseline by time intervals since the first belamaf dose. (C) Summary of patients who stopped reading and driving. ∗Patients who could read with little or no difficulty or read with some difficulty at baseline, mainly due to eyesight issues, were considered, whereas those who stopped reading due to other reasons during follow-up were excluded. †The first 2.5 years of treatment are shown due to the limited number of patients at risk beyond this time point. ‡Patients who could drive with little or no difficulty or drive with some difficulty at baseline, mainly due to eyesight issues, were considered, whereas those who stopped driving due to other reasons during follow-up were excluded. §Occurrences before the treatment end date are considered. ||Time not being able to read/drive is the sum of days from when the patient stopped reading/driving to reading/driving with no, little, or some difficulty or the end of belamaf treatment, whichever is earlier, divided by the duration of belamaf treatment. EOT, end of treatment; IQR, interquartile range.
Overview of dose modifications
Overall, 88% of patients in the BVd arm had a belamaf dose delay, and 72% in the DVd arm had a daratumumab dose delay; 93% of patients in the BPd arm had a belamaf dose delay, and 83% in the PVd arm had a bortezomib dose delay (supplemental Table 6). In the BVd arm, 69% of patients had belamaf dose reductions. Dose reductions were not permitted for daratumumab. In the BPd arm, 58% of patients had belamaf dose reductions, and in the PVd arm, 40% had bortezomib dose reductions.2 An analysis of investigator-reported dose modifications of any component of study treatment due to ocular events in the BVd and BPd arms showed that ∼80% of patients had interruptions/dose delays, 40% to 60% had dose reductions, and 9% had discontinuations (supplemental Table 7).1,2
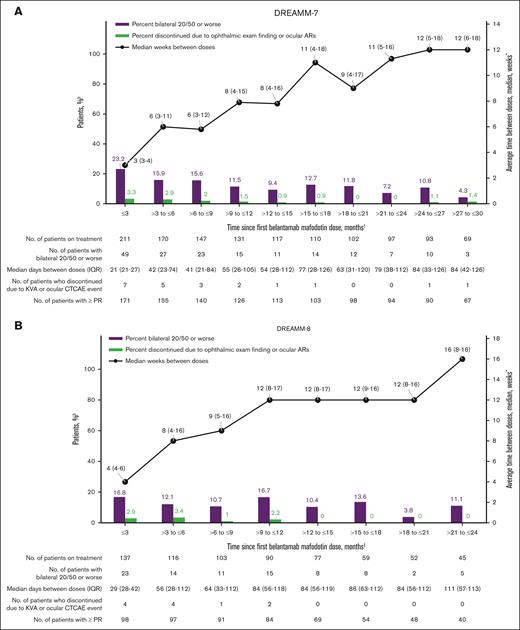
The median time between belamaf doses in DREAMM-7 and DREAMM-8 increased from the initial protocol-defined 3 and 4 weeks, respectively, to a median of 8 to 12 weeks in both studies by 9 months; however, interpatient variability was high (Figure 3). In DREAMM-8, protocol-recommended reduction in dosing frequency to every 8 weeks resulted in a proactive extension of dosing intervals, leading to a longer median dosing interval than that in DREAMM-7. The relative dose intensity of belamaf, which is the ratio of the amount of drug administered to the amount planned for a fixed time period, was 51% and 52% in DREAMM-7 and DREAMM-8, respectively, mostly due to per-protocol belamaf dose modifications.1,2 The median relative dose intensity of belamaf decreased at later time points in both studies (supplemental Table 8) because median dosing intervals increased with time on treatment. Patients in the BVd and BPd arms received a median of 9.0 and 6.0 infusions of belamaf per patient, respectively (supplemental Table 9); the median total exposure to any study treatment was 15.9 months in the BVd arm and 16.5 months in the BPd arm.1,2
Median dosing interval and ocular events. (A-B) Post hoc analyses that included only treated patients who had BCVA of 20/25 or better in ≥1 eye at baseline in DREAMM-7 (n = 211; A) and DREAMM-8 (n = 137; B). The graph only shows the occurrence of bilateral 20/50 or worse BCVA changes, overall median of the average time between belamaf doses, and discontinuation of belamaf due to ocular events in each time interval while patients remained on belamaf treatment. The incidence rate at each time point is based on the number of patients who remained on treatment at that time point and does not reflect the overall risk of developing bilateral worsening of 20/50 or greater or discontinuing due to an ocular event at any time during belamaf treatment. The increased time between doses is due to AEs, including ocular events, and other non–AE-related reasons. This graph does not detail any efficacy parameter over time. ∗Median of average days between doses for each patient per interval was used and converted to weeks. †For DREAMM-7, events during the initial 2.5 years of treatment are shown due to limited number of patients at risk beyond this time point. ‡For DREAMM-8, the first 2 years of treatment are shown due to limited number of patients at risk beyond this time point. CTCAE, Common Terminology Criteria for Adverse Events; IQR, interquartile range.
Median dosing interval and ocular events. (A-B) Post hoc analyses that included only treated patients who had BCVA of 20/25 or better in ≥1 eye at baseline in DREAMM-7 (n = 211; A) and DREAMM-8 (n = 137; B). The graph only shows the occurrence of bilateral 20/50 or worse BCVA changes, overall median of the average time between belamaf doses, and discontinuation of belamaf due to ocular events in each time interval while patients remained on belamaf treatment. The incidence rate at each time point is based on the number of patients who remained on treatment at that time point and does not reflect the overall risk of developing bilateral worsening of 20/50 or greater or discontinuing due to an ocular event at any time during belamaf treatment. The increased time between doses is due to AEs, including ocular events, and other non–AE-related reasons. This graph does not detail any efficacy parameter over time. ∗Median of average days between doses for each patient per interval was used and converted to weeks. †For DREAMM-7, events during the initial 2.5 years of treatment are shown due to limited number of patients at risk beyond this time point. ‡For DREAMM-8, the first 2 years of treatment are shown due to limited number of patients at risk beyond this time point. CTCAE, Common Terminology Criteria for Adverse Events; IQR, interquartile range.
Impact of dose modification on safety
In patients with normal baseline visual acuity, the prevalence of a reduction to bilateral 20/50 or worse (Figure 3) and ocular ARs (supplemental Figure 2) were generally low, with the highest prevalence in the first 3 months.
In patients with normal baseline visual acuity, discontinuation rates due to ocular events remained low at all time points; discontinuations occurred even more infrequently after the first few months in both trials (Figure 3). Overall, 22 patients (9%) discontinued BVd due to ocular events after a median of 5 doses (range, 2-37) of belamaf; ocular events leading to discontinuation resolved in 15 and were ongoing in 7 at the data cutoff, with follow-up ending at the time the patient died or at study withdrawal (supplemental Table 10). The 14 patients (9%) who discontinued BPd due to ocular events did so after a median of 3 doses (range, 1-7) of belamaf; ocular events leading to discontinuation resolved in all 14 patients at the data cutoff.
Impact of dose modifications on efficacy
As described above, the median time to onset of the first grade ≥2 OEF was ∼5 to 6 weeks. The median time to response (partial response [PR] or better) was ∼6 (range, 3-36) and ∼5 weeks (range, 4-40) with BVd and BPd, respectively, and the median time to best response was ∼19 (range, 3-140) and ∼24 weeks (range, 4-112), respectively. Most patients who had a response (PR or better) on BVd or BPd required dose modifications. Overall, 97% and 99% of responders in DREAMM-7 and DREAMM-8, respectively, had a dose delay, with most (74%) having ≥3 delays and a median duration of delay of ∼8 weeks (BVd [range, 0.14-105]; BPd [range, 0.14-140]; Table 3). In the BVd and BPd arms, 91% and 92% of patients with grade ≥2 OEFs, respectively, continued belamaf, with a median of 8 and 5 additional doses after the onset of the first event; 93% and 88% who continued belamaf had a PR or better (supplemental Table 11).
Characteristics of belamaf dose modifications in responders
| Belantamab mafodotin dose modifications . | DREAMM-7 Responders (n = 201) . | DREAMM-8 Responders (n = 120) . | ||
|---|---|---|---|---|
| Dose interval >3 weeks (+3-day window) . | Dose reduction . | Dose interval >4 weeks (+3-day window) . | Dose reduction . | |
| Any dose modification, n (%) | 194 (97) | 150 (75) | 119 (>99) | 83 (69) |
| Dose modifications per patient, n (%) | ||||
| 0 | 7 (3) | 47 (23) | 1 (<1) | 35 (29) |
| 1 | 25 (12) | 132 (66) | 15 (13) | 80 (67) |
| 2 | 21 (10) | 15 (7) | 16 (13) | 3 (3) |
| ≥3 | 148 (76) | 3 (1) | 88 (74) | 0 |
| Not evaluable∗ | — | 4 (2) | — | 2 (2) |
| Dose modifications, n | 1093 | 172 | 595 | 86 |
| Duration of dose delay, median (range), d | 57 (1-732) | Not applicable | 53 (1-980) | Not applicable |
| Belantamab mafodotin dose modifications . | DREAMM-7 Responders (n = 201) . | DREAMM-8 Responders (n = 120) . | ||
|---|---|---|---|---|
| Dose interval >3 weeks (+3-day window) . | Dose reduction . | Dose interval >4 weeks (+3-day window) . | Dose reduction . | |
| Any dose modification, n (%) | 194 (97) | 150 (75) | 119 (>99) | 83 (69) |
| Dose modifications per patient, n (%) | ||||
| 0 | 7 (3) | 47 (23) | 1 (<1) | 35 (29) |
| 1 | 25 (12) | 132 (66) | 15 (13) | 80 (67) |
| 2 | 21 (10) | 15 (7) | 16 (13) | 3 (3) |
| ≥3 | 148 (76) | 3 (1) | 88 (74) | 0 |
| Not evaluable∗ | — | 4 (2) | — | 2 (2) |
| Dose modifications, n | 1093 | 172 | 595 | 86 |
| Duration of dose delay, median (range), d | 57 (1-732) | Not applicable | 53 (1-980) | Not applicable |
Post hoc analysis.
Not evaluable means the patient did not receive any drug in any succeeding time period after the first dose.
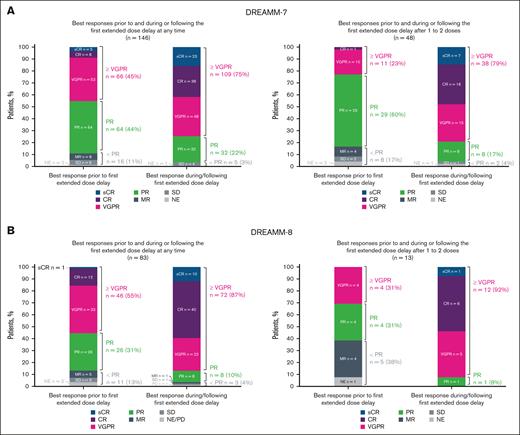
In patients requiring extended dose delays (>2 cycles), responses were achieved, maintained, or deepened during or after the first dose delay, including in those who had a dose delay after 1 to 2 doses of belamaf (Figure 4). Most patients with an extended dose delay had a PR or better before the first extended dose delay: 89% in the BVd arm and 87% in the BPd arm. The percentage of patients with a very good PR or better increased from 45% to 75% and from 55% to 87% after the first extended dose delay in DREAMM-7 and DREAMM-8, respectively. In patients who had an extended dose delay after 1 to 2 doses of belamaf, few had a response worse than PR or a nonevaluable response before the dose delay: 8 in the BVd arm and 5 in the BPd arm. Most of these patients subsequently had a PR or better: 6 in the BVd arm and 5 in the BPd arm.
Summary of best response after the first extended dose delay of belamaf. (A-B) Post hoc analysis of DREAMM-7 (A) and DREAMM-8 (B) evaluating best response in patients on treatment before the first extended dose delay and after the first extended dose delay at any time (left graph) or after 1 to 2 doses (right graph). The first extended dose delay was defined as lasting >2 cycles, which in DREAMM-7 was >42 days and in DREAMM-8 was >56 days. CR, complete response; MR, minimal response; NE, not evaluable; PD, progressive disease; sCR, stringent complete response; SD, stable disease; VGPR, very good PR.
Summary of best response after the first extended dose delay of belamaf. (A-B) Post hoc analysis of DREAMM-7 (A) and DREAMM-8 (B) evaluating best response in patients on treatment before the first extended dose delay and after the first extended dose delay at any time (left graph) or after 1 to 2 doses (right graph). The first extended dose delay was defined as lasting >2 cycles, which in DREAMM-7 was >42 days and in DREAMM-8 was >56 days. CR, complete response; MR, minimal response; NE, not evaluable; PD, progressive disease; sCR, stringent complete response; SD, stable disease; VGPR, very good PR.
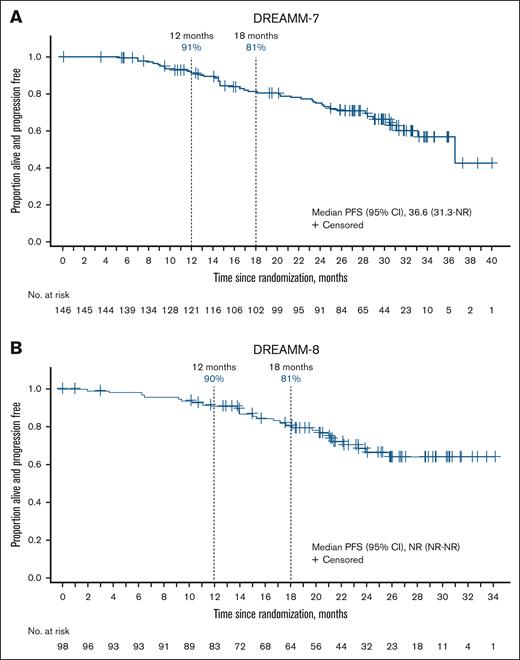
In both studies, patients who experienced ≥1 extended belamaf dose delay (≥12 weeks) had robust PFS (Figure 5). Patients treated with BVd with dose delays of ≥12 weeks (n = 146) had a median PFS of 36.6 months (95% CI, 31.3 to not reached) and estimated 12- and 18-month PFS rates of 91% and 81%, respectively. Median PFS in patients treated with BPd with ≥1 dose delay of ≥12 weeks (n = 98) was not reached, and the estimated 12- and 18-month PFS rates were 90% and 81%, respectively.
PFS in patients with ≥1 extended belamaf dose delay of ≥12 weeks. (A-B) Post hoc analysis evaluating patients who had ≥1 belamaf dose delay of ≥12 weeks in (A) DREAMM-7 (BVd, n = 146) and (B) DREAMM-8 (BPd, n = 98). NR, not reached.
PFS in patients with ≥1 extended belamaf dose delay of ≥12 weeks. (A-B) Post hoc analysis evaluating patients who had ≥1 belamaf dose delay of ≥12 weeks in (A) DREAMM-7 (BVd, n = 146) and (B) DREAMM-8 (BPd, n = 98). NR, not reached.
In both trials, an overlay of results from patients with normal baseline visual acuity showed that median intervals between dosing increased at later time points; prevalence of visual acuity worsening and discontinuation rates due to ocular events remained low (Figure 3).
Discussion
Most patients treated with belamaf triplets in the DREAMM-7 and DREAMM-8 trials experienced grade 2 or 3 ocular events that were managed with protocol-recommended dose modifications; therefore, patients could remain on belamaf treatment and derive robust efficacy benefit. Dose modifications contributed to an increase in the median dosing intervals of belamaf in patients with normal baseline visual acuity the longer the patients were on therapy. In these patients, prevalences of a clinically meaningful visual acuity reduction to 20/50 or worse and ocular ARs were generally low, with the highest prevalence in the first 3 months of belamaf treatment. Notably, visual impairments were generally reversible with dose modifications in patients with adequate follow-up data. Although multiple grade ≥2 OEFs were reported, they were not always associated with visual impairment, and the median duration of occurrences was consistent and predictable.
Importantly, efficacy was maintained despite dose modifications. Almost all patients who responded to treatment experienced ≥1 dose delay. Responses were rapid, and most patients experienced a PR or better before an extended dose delay; most who had not had a response subsequently achieved a response during or after the dose delay. Responses were maintained or deepened after the first extended dose delay, including in those with early dose delays after 1 to 2 doses of belamaf. Dose delays were common and could begin as early as cycle 2, with the median time between doses extending to every 8 and 12 weeks in DREAMM-7 and DREAMM-8, respectively. Extended dosing schedules after cycle 1 will likely be common in future treatment regimens with belamaf. After a grade ≥2 OEF, most patients in the BVd and BPd arms continued treatment and had high best overall response rates. Robust PFS data are reported for patients who required ≥1 extended dose delay of ≥12 weeks in both studies. These findings are consistent with those of the DREAMM-2 and DREAMM-3 studies of belamaf monotherapy in patients in later lines, in which responses were maintained during belamaf dose delays.13,14 A potential reason for the robust efficacy of belamaf despite extended dosing intervals is its multimodal mechanism of action that not only includes direct cytotoxic activity through the MMAF payload but also antibody-dependent cell-mediated cytotoxicity/phagocytosis and immunogenic cell death, which are associated with sustained antimyeloma effects.15
Additionally, most patients evaluated were able to continue reading and driving throughout treatment; of the patients who stopped at some point during treatment, 97% and 92% returned to normal baseline reading and driving, respectively. Previous results for patient-reported outcomes showed no differences in global quality of life, role functioning, physical functioning, or fatigue between the belamaf combination arms (BVd and BPd) and their respective controls (DVd and PVd) over time.1,2,16,17
Belamaf combinations are a convenient treatment option that can be given in an outpatient setting. Unlike other B-cell maturation antigen–targeted therapies, including chimeric antigen receptor T cells and bispecific monoclonal antibodies, belamaf triplets do not require administration and monitoring in specialized medical centers, because rates of opportunistic infections were infrequent, and serious immune conditions such as fatal cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome were not reported.1,2
Use of belamaf in a real-world setting does not require complex AE management (ie, hospitalization and prophylactic IV immunoglobulin use). Ocular events are the predominant AEs; these can be managed with belamaf dose modifications, based on the OEFs determined by an eye care professional before each of the first 4 doses of belamaf and as clinically indicated thereafter. Treating physicians should establish a partnership with an eye care professional and anticipate ocular events early in treatment. Our data support that belamaf dose modifications can help manage ocular events and preserve clinical benefit. The median time to resolution of grade ≥2 OEFs pooled in both trials was ∼12 weeks (range, 0.3-116); therefore, health care professionals may use this approximate time to resolution to help guide the scheduling of the next ocular examination and the restart of belamaf dosing. Dose modifications may lead to extended dosing schedules, resulting in less frequent therapy and ocular examinations. It is recommended to adjust the dosing interval based on individual patient tolerability.
A limitation of this study is the open-label trial design due to differences in dosing administration and schedule and ocular assessments in the belamaf combination arms compared with the control arms. Additionally, the data presented are from exploratory post hoc analyses. However, prospective trials that include an extended dosing interval and other strategies to mitigate ocular events are ongoing or planned.18,19 The phase 3 DREAMM-10 study will evaluate belamaf (combined with lenalidomide and dexamethasone) administration at 1.9 mg/kg every 8 weeks for 24 weeks, then 1.9 mg/kg every 12 weeks thereafter, in transplant-ineligible patients with newly diagnosed multiple myeloma.20 In addition, because the cytotoxic payload (eg, mafodotin) may be responsible for ocular events, the DREAMM-20 study will evaluate the safety, tolerability, and clinical activity of escalating doses of belantamab, the unconjugated B-cell maturation antigen monoclonal antibody, as a single agent and at different dose ratios with belamaf (delivered as a separate drug) in patients with relapsed/refractory multiple myeloma.19
Conclusion
Belamaf, at the doses used in DREAMM-7 and DREAMM-8, has a favorable benefit-risk profile in patients with multiple myeloma. In practice, most patients are anticipated to have grade ≥2 OEFs after 2 to 3 doses that will require an extended dosing interval. Many patients received an extended dosing schedule (8- to 12-week interval) that may reflect the time for OEF resolution. Although ocular events were common, they were effectively managed with dose modifications and were generally reversible with adequate follow-up, allowing for patients to remain on treatment and derive robust efficacy benefit. Starting with a higher dose and subsequently titrating the dose intensity (primarily by extending dosing intervals), per individual patient tolerability, results in a favorable benefit-risk ratio. Furthermore, insights into the onset and duration of grade ≥2 OEFs can help guide frequency of ocular monitoring.
Acknowledgments
The authors thank the patients who volunteered to participate in the trial, their families, their caregivers, and the physicians and nurses who cared for the patients and supported this clinical trial. The authors acknowledge the contributions of the DREAMM-7 and DREAMM-8 study teams.
Drug linker technology was licensed from Seagen Inc; monoclonal antibody was produced using POTELLIGENT Technology, which was licensed from BioWa. Medical writing and editorial assistance were provided by Jarin Chu (Nucleus Global, an Inizio company), in accordance with the International Committee of Medical Journal Editors and Good Publication Practice guidelines, and funded by GSK.
The DREAMM-7 and DREAMM-8 studies were funded by GSK.
Authorship
Contribution: M.V.M., K.K., V.H., and M.A.D. contributed to study concept or design and data acquisition; S.T., H.Q., P.R., M.B., L.P., M.H., V.Z., S.D., T.J., C.W., P.J.H., V.V., M.P.d.L., G.A.M., I.S., J.R., M.C., C.C., C.F., and K.S. contributed to data acquisition; R.R., A.P.-J., and Z.W. contributed to study concept or design, data analysis, and data interpretation; H.B. contributed to data acquisition, data analysis, and data interpretation; J.W., X.L.Z., E.L., L.E., N.S., and P.P. contributed to data analysis and data interpretation; J.B.O. and P.M. contributed to data interpretation; and all authors were not precluded from accessing data in the study and accept responsibility to submit for publication.
Conflict-of-interest disclosure: M.V.M. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Janssen, Bristol Myers Squibb (BMS), GSK, Sanofi, AbbVie, Kite, Stemline, and Pfizer; and participation on a data safety monitoring or advisory board for Janssen, BMS, Amgen, Sanofi, GSK, Roche, Pfizer, AbbVie, Kite, and Stemline. S.T. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, GSK, Janssen, Pfizer, and Sanofi; consulting or advisory role at BMS, GSK, and Roche; and research funding from Amgen, BMS, Genentech, GSK, Janssen, K36 Therapeutics, Pfizer, and Roche. M.B. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from BMS and Janssen; and consulting or advisory role at GSK, Takeda, Amgen, Janssen, and Menarini. S.D. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Amgen, GSK, Janssen, and Takeda. T.J. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events and/or research grants from Sanofi, Janssen, BMS, Pfizer, and GSK. C.W. reports payment or honoraria for lectures, presentations, and speakers’ bureaus from AstraZeneca, CSL, GSK, Alexion, Bayer, and Drivetime Radio; and was the education and planning committee chair for the International Society on Thrombosis and Haemostasis 2024 Congress. P.J.H. reports participation on a data safety monitoring or advisory board for the Australasian Leukaemia & Lymphoma Group trials; unpaid leadership or fiduciary roles in advisory boards for Antengene, Gilead, iTeos Therapeutics, Janssen, and Pfizer; and research and medical writing support from Novartis. V.V. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AbbVie, Amgen, AstraZeneca, BeiGene, Gilead Sciences, Janssen, Novartis, Roche, Sanofi, and Takeda; consulting or advisory role for AbbVie, AstraZeneca, BeiGene, Gilead Sciences, Janssen Oncology, Novartis, Sanofi, and Takeda; payment or honoraria for speakers bureaus from AbbVie, Amgen, AstraZeneca, BeiGene, Biocad, BMS, Janssen, Merck & Co, Inc, Novartis, Roche, Sanofi, and Takeda; and travel, accommodations, and expenses from AstraZeneca. I.S. reports consulting fees from BMS, Amgen, Janssen-Cilag, Takeda, Sanofi, and GSK; honoraria from Amgen, Janssen-Cilag, Takeda, BMS, and Sanofi; support for attending meetings and/or travel from Janssen-Cilag, BMS, and Sanofi; and participation on a data safety monitoring board or advisory board for Janssen-Cilag, Takeda, Sanofi, and GSK. J.R. reports consulting fees from GSK, Johnson & Johnson, and Sanofi; honoraria from Johnson & Johnson, Pfizer, BMS, Sanofi, and GSK; support for attending meetings and/or travel from Johnson & Johnson and Sanofi; and participation on a data safety monitoring board or advisory board for GSK, Johnson & Johnson, Sanofi, BMS, and Pfizer. C.C. reports stock or stock options in GSK; and advisory board speaker fees from AbbVie, Amgen, Astellas, BeiGene, BMS, Glycomimetics, GSK, Immunogen, Janssen, Jazz, Karyopharm, Menarini-Stemline, Oncopeptides, Pfizer, Sanofi, Servier, Stemline, and Takeda. K.S. reports honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Janssen Pharmaceutical K.K., Sanofi, and BMS. R.R., Z.W., H.B., J.W., X.L.Z., E.L., L.E., P.P., and P.M. report employment and/or stock or stock options at GSK. A.P.-J. and N.S. report employment and/or stock or stock options at GSK; and travel support from GSK. J.B.O. reports issued or pending patents and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, educational events, or travel support from GSK; and employment and/or stock or stock options at GSK. V.H. reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AbbVie, Amgen, BMS, GSK, Janssen, Sanofi, Pfizer, and Takeda; and participation on a data safety monitoring or advisory board for BMS, GSK, Janssen, Kite, Regeneron, and Sanofi. M.A.D. reports honoraria from Amgen, Sanofi, Regeneron, Menarini, Takeda, GSK, BMS, Janssen, BeiGene, Swixx, and AstraZeneca. The remaining authors declare no competing financial interests.
The current affiliation for X.L.Z. is ONO PHARMA USA, Waltham, MA.
Correspondence: Meletios Athanasios Dimopoulos, Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Alexandra Hospital, 80 Vasilisis Sofias, 11528, Athens, Greece; email: mdimop@med.uoa.gr.
References
Author notes
M.V.M. and S.T. contributed equally to this work.
This study has been presented, in part, at the International Myeloma Society (IMS) 2024 (P-396 and P-413), 26 September 2024.
Complete deidentified patient data sets generated and analyzed for this study along with supporting study documents are available to others for research purposes on approval of request. Data will become available on study completion and can be requested at https://www.gsk-studyregister.com/en/.
The full-text version of this article contains a data supplement.