Key Points
The association of race and ethnicity with worse postrelapse B-ALL survival is largely accounted for by disease and socioeconomic factors.
Hispanic patients had higher mortality following B-ALL relapse even after accounting for known prognostic factors such as early relapse.
Visual Abstract
Pediatric Hispanic and Black patients with newly diagnosed B-cell acute lymphoblastic leukemia (B-ALL) experience worse overall survival (OS). We hypothesized that differential outcomes by race and ethnicity following relapse may contribute to disparities. We examined 2053 patients with ALL enrolled in frontline Children’s Oncology Group trials from 1996 to 2014 who relapsed. We assessed the association of race and ethnicity, disease characteristics, and socioeconomic status with relapse survival predictors and postrelapse OS. For noninfant B-ALL, postrelapse OS (P = .002) and disease-related prognosticators such as time to relapse (P = .0002) differed by race and ethnicity. After adjusting for disease and patient characteristics, the OS association with overall race and ethnicity was attenuated, and lost statistical significance; Hispanic ethnicity specifically remained associated with worse OS (hazard ratio [HR], 1.19; 95% confidence interval [CI], 1.01-1.41). Patients from highest annual median household income ZIP codes (>$85 000, approximately the highest quartile of patients) had better 5-year OS than those from the lowest (<$50 000; HR, 0.79; 95% CI, 0.63-0.99). Non-Hispanic Black and Hispanic patients more commonly lived in lower-income ZIP codes. For T-cell ALL, race, ethnicity, and socioeconomic status were not associated with OS. Worse postrelapse outcomes among racial and ethnic minority patients are largely driven by the prevalence of adverse disease-related factors at time of relapse, with a persistent disparity observed in Hispanic patients. The greatest impact in decreasing racial and ethnic B-ALL outcome disparities may be achieved by targeting frontline treatment interventions to address increased relapse among Black and Hispanic patients, and by developing and enabling equitable access to effective relapse treatments such as novel immunotherapies.
Introduction
Pediatric Hispanic and Black patients with acute lymphoblastic leukemia (ALL) have historically experienced worse outcomes compared with non-Hispanic (NH) White patients.1 Although modern risk-adapted therapy has improved 5-year event-free survival (EFS) for children with ALL to 85% to 90%,2 we recently showed that Black and Hispanic patients with B-cell ALL (B-ALL) continue to experience worse outcomes compared with NH White patients.3 Overall survival (OS) disparities were more pronounced compared with disparities in EFS, potentially indicating a reduced ability to salvage minority patients following relapse. In Hodgkin lymphoma, racial and ethnic disparities in OS are driven by postrelapse mortality.4 In contrast, the reasons for disparate outcomes in B-ALL are only partially understood. Identifying underlying contributors will help inform interventions aimed at mitigating survival disparities.
Outcomes for children and young adults with ALL following relapse have historically been poor.5 We hypothesized that disparities in ALL outcomes observed in the frontline setting would be further exacerbated at relapse, thus contributing to the worse OS for Black and Hispanic children and young adults with ALL.3 We recently assembled a large cohort of children and young adults with B- and T-lineage ALL enrolled in frontline Children’s Oncology Group (COG) trials who experienced relapse. During this time frame, before the advent of highly effective immunotherapy for relapsed/refractory B-ALL, most patients were treated with chemotherapy-based regimens with or without transplantation. Using this data set, we identified clinical and biological prognostic factors predicting survival for patients in first relapse.6 Several factors present at original diagnosis (including age, cytogenetics, T-cell ALL [T-ALL] immunophenotype, and end-induction minimal residual disease [MRD], first incorporated in risk stratification in AALL03B1,7 which began enrollment in January 2004) and at relapse (time to relapse and site of relapse) were associated with OS after relapse.6 Herein we specifically examine the impact that race and ethnicity have on survival following relapse, in the context of other disease and socioeconomic factors.
Methods
Patients and clinical trials
Patients with newly diagnosed B-ALL or T-ALL enrolled in 12 frontline COG clinical trials (Children’s Cancer Group [CCG] 1991,8 Pediatric Oncology Group [POG] 9404,9 POG 9407,10 POG 9904,11 POG 9905,11 POG 9906,12 COG AALL0232,13 COG AALL0331,14 COG AALL0434,15 COG AALL0631,16 COG AALL07P4,17 COG AALL08P118) from June 1996 to July 2014 who subsequently experienced relapse as a first event were included in this study. Details of these clinical trials are summarized in supplemental Table 1.6 The inclusion criteria used for trial eligibility are described in each trial publication.8-18 Relapse was defined morphologically: bone marrow relapse was defined by ≥25% morphologic blasts or ≥5% blasts with concomitant extramedullary relapse; central nervous system (CNS) relapse was defined by CNS 3 status (≥5 white blood cells [WBC] per microliter cerebrospinal fluid, with blasts on cytospin) or clinical signs of CNS leukemia. Patients or guardians provided informed consent for trial participation. Data collected at first relapse included time from initial diagnosis to relapse (time to relapse), relapse site, and enrollment in a COG relapse ALL trial (including AALL01P2,19 AALL02P2,20 AALL0433,21 AALL07P1,22 AALL1331,23 and AALL1821). Date of death was also captured. Patients were treated in Australia, Canada, New Zealand, Switzerland, and the United States (US). Patients with B-ALL diagnosed from birth to 1 year of age constituted the “infant ALL” cohort, whereas patients aged >1 year comprised the “B-ALL cohort.” Non-B-ALL infants aged <1 year were excluded from our analysis.
The trial protocols were approved by the National Cancer Institute, the Pediatric Central Institutional Review Board, and each trial center’s institutional review board. All patients or a parent/guardian provided written informed consent for clinical trial enrollment. The data and safety monitoring committees of the independent COG and the legacy CCG and POG met regularly to review trial safety and efficacy data according to their charters and standard operating procedures.
Race and ethnicity
Race and ethnicity were collected by institutional submission and are reported as a composite measure in keeping with prior COG studies. Although meant to reflect self-reporting, sites were not required to confirm this assumption. Categories included Hispanic (of all races, subdivided in supplemental Table 2), NH White, NH Black, NH Asian, NH Other, and Other/Unknown, with NH White patients serving as the reference group.
Potential predictors
COG risk stratification algorithms are described in each trial publication.8-18 Relapses within 18 months of initial diagnosis are defined as “early,” whereas those occurring ≥36 months from diagnosis are defined as “late.” Relapses between 18 and 36 months from diagnosis are defined as “intermediate.” Cytogenetic groups were defined as follows: favorable (double trisomy of chromosomes 4 and 10, ETV6::RUNX1, and trisomy of chromosome 17 for POG 9904, POG 9905, and POG 9906) vs unfavorable (KMT2A-rearranged, hypodiploid, intrachromosomal amplification of chromosome 21, and BCR::ABL1; notably, these protocols predated routine screening for BCR::ABL1-like ALL) vs neutral (all others). We performed a sensitivity analysis to evaluate the impact of year of relapse on our results.
For US patients, data on insurance status (private, Medicaid only, or Other/Unknown) were collected per site report. Median household income based on ZIP code at initial diagnosis from the 2020 US census was used as a measure of area-based socioeconomic status for US patients; it was subdivided into categories approximating quartiles: ≤$50 000, $50 001 to $65 000, $65 001 to $85 000, >$85 000, “US Unknown,” and non-US. Of note, US $50 000 is roughly twice the federal poverty line for a family of 4. Non-US patients were excluded from analyses of insurance status and income. The size of the COG institution at original diagnosis was broken down by total enrollments across the 12 frontline COG trials analyzed (≤50, 51-100, 101-200, >200). We also separately examined patients according to whether treatment occurred at a US or non-US institution.
Statistical analyses
Postrelapse OS was defined as the time between the date of the first relapse and the date of death; patients alive at last follow-up were censored. The cutoff for survival data was 30 June 2021. OS probabilities were calculated using the Kaplan-Meier method with Greenwood standard errors. Analyses of OS after relapse in relation to patient and disease characteristics were based on log-rank tests and univariate and multivariable Cox regression models.24 For the construction of reduced multivariable models, a stepwise backward model selection procedure was used to eliminate any adjusted variable that was not significant at P ≤ .20 while keeping the major variable of interest, race and ethnicity, in the model.25 Analyses were performed separately for B-ALL, T-ALL, and infant ALL given the previously described differences in disparities and as per prior studies.3,6 All P values reported are 2-sided, and a P ≤ .05 was considered statistically significant. Statistical analyses were performed using Stata software (StataCorp).26
Results
During the study period, 16 115 patients enrolled in frontline COG clinical trials, with 12.7% (2053) experiencing relapse during or after frontline therapy.6 Five-year EFS ranged from 79.9% for Hispanic patients to 85.3% for NH White patients, and median time to relapse ranged from 20.5 months for NH Other patients to 33.4 months for NH White patients (Table 1). The median follow-up from relapse was 70.5 months across all groups (Table 1).
Survival and follow-up by race and ethnicity of 16 115 patients included in this report
| Race and ethnicity . | No. eligible . | 5-year EFS ± SE . | No. of relapses∗ (% per protocol) . | Time to relapse† in months, median (range) . | Median follow-up in months from diagnosis‡ . | Median follow-up in months from relapse§ . |
|---|---|---|---|---|---|---|
| Hispanic of all races | 3190 | 79.9% ± 0.7% | 492 (15.4%) | 29.8 (0.9-176.0) | 122.2 | 72.7 |
| NH White | 9584 | 85.3% ± 0.4% | 1147 (12.0%) | 33.4 (0.3-186.0) | 122.2 | 69.8 |
| NH Black | 1014 | 80.3% ± 1.3% | 145 (14.3%) | 25.3 (0.7-108.1) | 116.4 | 70.1 |
| NH Asian | 639 | 83.7% ± 1.5% | 65 (10.2%) | 30.4 (1.2-111.5) | 117.2 | 63.5 |
| NH Other | 176 | 81.4% ± 3.0% | 20 (11.4%) | 20.5 (3.4-84.0) | 121.1 | 59.0 |
| Other/Unknown | 1512 | 82.6% ± 1.0% | 184 (12.2%) | 26.9 (0.3-139.5) | 122.2 | 74.0 |
| Total | 16 115 | 83.5% ± 0.3% | 2053 (12.7%) | 31.0 (0.3-186.0) | 121.7 | 70.5 |
| Race and ethnicity . | No. eligible . | 5-year EFS ± SE . | No. of relapses∗ (% per protocol) . | Time to relapse† in months, median (range) . | Median follow-up in months from diagnosis‡ . | Median follow-up in months from relapse§ . |
|---|---|---|---|---|---|---|
| Hispanic of all races | 3190 | 79.9% ± 0.7% | 492 (15.4%) | 29.8 (0.9-176.0) | 122.2 | 72.7 |
| NH White | 9584 | 85.3% ± 0.4% | 1147 (12.0%) | 33.4 (0.3-186.0) | 122.2 | 69.8 |
| NH Black | 1014 | 80.3% ± 1.3% | 145 (14.3%) | 25.3 (0.7-108.1) | 116.4 | 70.1 |
| NH Asian | 639 | 83.7% ± 1.5% | 65 (10.2%) | 30.4 (1.2-111.5) | 117.2 | 63.5 |
| NH Other | 176 | 81.4% ± 3.0% | 20 (11.4%) | 20.5 (3.4-84.0) | 121.1 | 59.0 |
| Other/Unknown | 1512 | 82.6% ± 1.0% | 184 (12.2%) | 26.9 (0.3-139.5) | 122.2 | 74.0 |
| Total | 16 115 | 83.5% ± 0.3% | 2053 (12.7%) | 31.0 (0.3-186.0) | 121.7 | 70.5 |
EFS, event-free survival (from time of original diagnosis); SE, standard error.
Percentage of total eligible patients (patients who enrolled in any included protocol who experienced relapse as first event) in this row provided in the second column of the table.
Time in months from initial diagnosis to relapse among relapsed patients.
Median follow-up time in months from initial diagnosis among relapsed patients who were alive as of the last follow-up date.
Median follow-up time in months from relapse diagnosis among relapsed patients who were alive as of the last follow-up date.
B-ALL
Several factors known to be associated with worse postrelapse OS differed by race and ethnicity (Table 2) in this cohort of patients with relapsed ALL, including time to relapse (P = .0002), WBC count at initial diagnosis (P = .034), and CNS status at initial diagnosis (P = .027). In general, NH White patients were more likely to have favorable disease prognosticators compared with Hispanic, NH Black, or NH Asian patients. For example, a lower proportion of NH White patients (16.9%) had early relapse at <18 months compared with Hispanic (24.3%, P < .0001) and NH Black (29.6%, P = .002) patients, but not NH Asian (23.1%, P = .47) patients. WBC count at initial diagnosis was more frequently <50 000 cells per μL among NH White patients (79.8%) compared with Hispanic (73.0%, P = .005) patients, but not NH Black (73.0%, P = .13) or NH Asian (69.2%, P = .17) patients. The main exception to the above pattern of worse disease prognosticators among Hispanic patients was in end-induction MRD during frontline treatment (overall P = .04) (Table 2); a lower proportion of NH White (38.5%) patients had end-induction MRD <0.01% at day 29 following frontline induction compared with Hispanic (43.5%, P < .0001) or NH Black (51.9%, P = .002) patients, but not NH Asian (26.8%, P = .47) patients.
Demographics and disease characteristics of patients with noninfant B-ALL experiencing relapse after frontline COG clinical trials by race and ethnicity
| Characteristics . | Race/ethnicity grouping . | P value . | ||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 452) . | NH White (N = 957) . | NH Black (N = 115) . | NH Asian (N = 52) . | NH Other (N = 16) . | Other/Unknown (N = 123) . | Total (N = 1715) . | ||
| Age at diagnosis, n (%) | .58∗ | |||||||
| 1-9 years | 306 (67.7) | 686 (71.7) | 77 (67.0) | 39 (75.0) | 10 (62.5) | 84 (68.3) | 1202 (70.1) | |
| 10-15 years | 93 (20.6) | 181 (18.9) | 29 (25.2) | 10 (19.2) | 3 (18.8) | 28 (22.8) | 344 (20.1) | |
| 16+ years | 53 (11.7) | 90 (9.4) | 9 (7.8) | 3 (5.8) | 3 (18.8) | 11 (8.9) | 169 (9.9) | |
| Age at relapse, n (%) | .64∗ | |||||||
| 1-9 years | 231 (51.1) | 478 (49.9) | 67 (58.3) | 30 (57.7) | 8 (50.0) | 61 (49.6) | 875 (51.0) | |
| 10-15 years | 120 (26.5) | 286 (29.9) | 23 (20.0) | 14 (26.9) | 5 (31.3) | 34 (27.6) | 482 (28.1) | |
| 16+ years | 101 (22.3) | 193 (20.2) | 25 (21.7) | 8 (15.4) | 3 (18.8) | 28 (22.8) | 358 (20.9) | |
| Cytogenetic group†, n (%) | .13∗ | |||||||
| Favorable | 95 (26.4) | 223 (31.4) | 32 (34.8) | 12 (30.0) | 7 (53.8) | 22 (23.9) | 391 (29.9) | |
| Unfavorable | 45 (12.5) | 114 (16.1) | 12 (13.0) | 7 (17.5) | 1 (7.7) | 11 (12.0) | 190 (14.5) | |
| Neutral | 220 (61.1) | 373 (52.5) | 48 (52.2) | 21 (52.5) | 5 (38.5) | 59 (64.1) | 726 (55.5) | |
| Unknown | 92 | 247 | 23 | 12 | 3 | 31 | 408 | |
| Duration in frontline trial (months) | .11‡ | |||||||
| N | 449 | 953 | 114 | 52 | 16 | 121 | 1705 | |
| Mean | 20.6 | 21.4 | 20 | 18.8 | 13.0 | 21.3 | 20.9 | |
| Median | 24.1 | 26.2 | 24.5 | 20.5 | 11.6 | 26.2 | 25.9 | |
| Range | 0.1-45.5 | 0.0-42.2 | 0.2-40.5 | 0.4-40.8 | 0.2-29.3 | 0.0-40.7 | 0.0-45.5 | |
| Enrolled in selected ALL studies§at relapse, n (%) | .67∗ | |||||||
| No | 372 (82.3) | 772 (80.7) | 94 (81.7) | 46 (88.5) | 14 (87.5) | 97 (78.9) | 1395 (81.3) | |
| Yes | 80 (17.7) | 185 (19.3) | 21 (18.3) | 6 (11.5) | 2 (12.5) | 26 (21.1) | 320 (18.7) | |
| Sex, n (%) | .50∗ | |||||||
| Male | 278 (61.5) | 558 (58.3) | 76 (66.1) | 29 (55.8) | 9 (56.3) | 69 (56.1) | 1019 (59.4) | |
| Female | 174 (38.5) | 399 (41.7) | 39 (33.9) | 23 (44.2) | 7 (43.8) | 54 (43.9) | 696 (40.6) | |
| Time to relapse, n (%) | .0002∗ | |||||||
| <18 months | 110 (24.3) | 162 (16.9) | 34 (29.6) | 12 (23.1) | 5 (31.3) | 26 (21.1) | 349 (20.3) | |
| 18-35 months | 165 (36.5) | 290 (30.3) | 35 (30.4) | 16 (30.8) | 5 (31.3) | 35 (28.5) | 546 (31.8) | |
| ≥36 months | 177 (39.2) | 505 (52.8) | 46 (40.0) | 24 (46.2) | 6 (37.5) | 62 (50.4) | 820 (47.8) | |
| Sites of relapse, n (%) | .60∗ | |||||||
| Isolated BM | 267 (59.1) | 552 (57.7) | 66 (57.4) | 29 (55.8) | 10 (62.5) | 79 (64.2) | 1003 (58.5) | |
| Combined BM (±CNS) | 59 (13.1) | 139 (14.5) | 13 (11.3) | 7 (13.5) | 4 (25.0) | 14 (11.4) | 236 (13.8) | |
| Isolated CNS | 104 (23.0) | 202 (21.1) | 30 (26.1) | 9 (17.3) | 1 (6.3) | 25 (20.3) | 371 (21.6) | |
| Unknown | 2 (0.4) | 4 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 7 (0.4) | |
| Other | 20 (4.4) | 60 (6.3) | 6 (5.2) | 7 (13.5) | 1 (6.3) | 4 (3.3) | 98 (5.7) | |
| WBC count at initial diagnosis (x 103cells per μL), n (%) | .034∗ | |||||||
| <50 | 330 (73.0) | 764 (79.8) | 84 (73.0) | 36 (69.2) | 13 (81.3) | 99 (80.5) | 1326 (77.3) | |
| 50-100 | 45 (10.0) | 87 (9.1) | 17 (14.8) | 8 (15.4) | 0 (0.0) | 12 (9.8) | 169 (9.9) | |
| >100 | 77 (17.0) | 106 (11.1) | 14 (12.2) | 8 (15.4) | 3 (18.8) | 12 (9.8) | 220 (12.8) | |
| CNS status at initial diagnosis, n (%) | .027∗ | |||||||
| CNS 1 | 367 (81.6) | 816 (85.9) | 94 (81.7) | 42 (80.8) | 15 (93.8) | 115 (93.5) | 1449 (84.9) | |
| CNS 2 | 72 (16.0) | 115 (12.1) | 17 (14.8) | 10 (19.2) | 0 (0.0) | 8 (6.5) | 222 (13.0) | |
| CNS 3 | 11 (2.4) | 19 (2.0) | 4 (3.5) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 35 (2.1) | |
| Unknown/missing | 2 | 7 | 0 | 0 | 0 | 0 | 9 | |
| NCI risk group, n (%) | .48∗ | |||||||
| Standard risk | 243 (53.8%) | 555 (58.0%) | 57 (49.6%) | 28 (53.8%) | 9 (56.3%) | 69 (56.1%) | 961 (56.0%) | |
| High risk | 209 (46.2%) | 402 (42.0%) | 58 (50.4%) | 24 (46.2%) | 7 (43.8%) | 54 (43.9%) | 754 (44.0%) | |
| Frontline induction day 29 MRD category, n (%) | .040∗ | |||||||
| <0.01%|| | 167 (43.5) | 300 (38.5) | 54 (51.9) | 11 (26.8) | 5 (35.7) | 50 (46.7) | 587 (41.1) | |
| 0.01%-0.099% | 121 (31.5) | 270 (34.7) | 33 (31.7) | 16 (39.0) | 4 (28.6) | 26 (24.3) | 470 (32.9) | |
| 0.1%-0.99% | 69 (18.0) | 135 (17.3) | 14 (13.5) | 8 (19.5) | 5 (35.7) | 18 (16.8) | 249 (17.4) | |
| ≥1% | 27 (7.0) | 74 (9.5) | 3 (2.9) | 6 (14.6) | 0 (0.0) | 13 (12.1) | 123 (8.6) | |
| Unknown | 68 | 178 | 11 | 11 | 2 | 16 | 286 | |
| Characteristics . | Race/ethnicity grouping . | P value . | ||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 452) . | NH White (N = 957) . | NH Black (N = 115) . | NH Asian (N = 52) . | NH Other (N = 16) . | Other/Unknown (N = 123) . | Total (N = 1715) . | ||
| Age at diagnosis, n (%) | .58∗ | |||||||
| 1-9 years | 306 (67.7) | 686 (71.7) | 77 (67.0) | 39 (75.0) | 10 (62.5) | 84 (68.3) | 1202 (70.1) | |
| 10-15 years | 93 (20.6) | 181 (18.9) | 29 (25.2) | 10 (19.2) | 3 (18.8) | 28 (22.8) | 344 (20.1) | |
| 16+ years | 53 (11.7) | 90 (9.4) | 9 (7.8) | 3 (5.8) | 3 (18.8) | 11 (8.9) | 169 (9.9) | |
| Age at relapse, n (%) | .64∗ | |||||||
| 1-9 years | 231 (51.1) | 478 (49.9) | 67 (58.3) | 30 (57.7) | 8 (50.0) | 61 (49.6) | 875 (51.0) | |
| 10-15 years | 120 (26.5) | 286 (29.9) | 23 (20.0) | 14 (26.9) | 5 (31.3) | 34 (27.6) | 482 (28.1) | |
| 16+ years | 101 (22.3) | 193 (20.2) | 25 (21.7) | 8 (15.4) | 3 (18.8) | 28 (22.8) | 358 (20.9) | |
| Cytogenetic group†, n (%) | .13∗ | |||||||
| Favorable | 95 (26.4) | 223 (31.4) | 32 (34.8) | 12 (30.0) | 7 (53.8) | 22 (23.9) | 391 (29.9) | |
| Unfavorable | 45 (12.5) | 114 (16.1) | 12 (13.0) | 7 (17.5) | 1 (7.7) | 11 (12.0) | 190 (14.5) | |
| Neutral | 220 (61.1) | 373 (52.5) | 48 (52.2) | 21 (52.5) | 5 (38.5) | 59 (64.1) | 726 (55.5) | |
| Unknown | 92 | 247 | 23 | 12 | 3 | 31 | 408 | |
| Duration in frontline trial (months) | .11‡ | |||||||
| N | 449 | 953 | 114 | 52 | 16 | 121 | 1705 | |
| Mean | 20.6 | 21.4 | 20 | 18.8 | 13.0 | 21.3 | 20.9 | |
| Median | 24.1 | 26.2 | 24.5 | 20.5 | 11.6 | 26.2 | 25.9 | |
| Range | 0.1-45.5 | 0.0-42.2 | 0.2-40.5 | 0.4-40.8 | 0.2-29.3 | 0.0-40.7 | 0.0-45.5 | |
| Enrolled in selected ALL studies§at relapse, n (%) | .67∗ | |||||||
| No | 372 (82.3) | 772 (80.7) | 94 (81.7) | 46 (88.5) | 14 (87.5) | 97 (78.9) | 1395 (81.3) | |
| Yes | 80 (17.7) | 185 (19.3) | 21 (18.3) | 6 (11.5) | 2 (12.5) | 26 (21.1) | 320 (18.7) | |
| Sex, n (%) | .50∗ | |||||||
| Male | 278 (61.5) | 558 (58.3) | 76 (66.1) | 29 (55.8) | 9 (56.3) | 69 (56.1) | 1019 (59.4) | |
| Female | 174 (38.5) | 399 (41.7) | 39 (33.9) | 23 (44.2) | 7 (43.8) | 54 (43.9) | 696 (40.6) | |
| Time to relapse, n (%) | .0002∗ | |||||||
| <18 months | 110 (24.3) | 162 (16.9) | 34 (29.6) | 12 (23.1) | 5 (31.3) | 26 (21.1) | 349 (20.3) | |
| 18-35 months | 165 (36.5) | 290 (30.3) | 35 (30.4) | 16 (30.8) | 5 (31.3) | 35 (28.5) | 546 (31.8) | |
| ≥36 months | 177 (39.2) | 505 (52.8) | 46 (40.0) | 24 (46.2) | 6 (37.5) | 62 (50.4) | 820 (47.8) | |
| Sites of relapse, n (%) | .60∗ | |||||||
| Isolated BM | 267 (59.1) | 552 (57.7) | 66 (57.4) | 29 (55.8) | 10 (62.5) | 79 (64.2) | 1003 (58.5) | |
| Combined BM (±CNS) | 59 (13.1) | 139 (14.5) | 13 (11.3) | 7 (13.5) | 4 (25.0) | 14 (11.4) | 236 (13.8) | |
| Isolated CNS | 104 (23.0) | 202 (21.1) | 30 (26.1) | 9 (17.3) | 1 (6.3) | 25 (20.3) | 371 (21.6) | |
| Unknown | 2 (0.4) | 4 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 7 (0.4) | |
| Other | 20 (4.4) | 60 (6.3) | 6 (5.2) | 7 (13.5) | 1 (6.3) | 4 (3.3) | 98 (5.7) | |
| WBC count at initial diagnosis (x 103cells per μL), n (%) | .034∗ | |||||||
| <50 | 330 (73.0) | 764 (79.8) | 84 (73.0) | 36 (69.2) | 13 (81.3) | 99 (80.5) | 1326 (77.3) | |
| 50-100 | 45 (10.0) | 87 (9.1) | 17 (14.8) | 8 (15.4) | 0 (0.0) | 12 (9.8) | 169 (9.9) | |
| >100 | 77 (17.0) | 106 (11.1) | 14 (12.2) | 8 (15.4) | 3 (18.8) | 12 (9.8) | 220 (12.8) | |
| CNS status at initial diagnosis, n (%) | .027∗ | |||||||
| CNS 1 | 367 (81.6) | 816 (85.9) | 94 (81.7) | 42 (80.8) | 15 (93.8) | 115 (93.5) | 1449 (84.9) | |
| CNS 2 | 72 (16.0) | 115 (12.1) | 17 (14.8) | 10 (19.2) | 0 (0.0) | 8 (6.5) | 222 (13.0) | |
| CNS 3 | 11 (2.4) | 19 (2.0) | 4 (3.5) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 35 (2.1) | |
| Unknown/missing | 2 | 7 | 0 | 0 | 0 | 0 | 9 | |
| NCI risk group, n (%) | .48∗ | |||||||
| Standard risk | 243 (53.8%) | 555 (58.0%) | 57 (49.6%) | 28 (53.8%) | 9 (56.3%) | 69 (56.1%) | 961 (56.0%) | |
| High risk | 209 (46.2%) | 402 (42.0%) | 58 (50.4%) | 24 (46.2%) | 7 (43.8%) | 54 (43.9%) | 754 (44.0%) | |
| Frontline induction day 29 MRD category, n (%) | .040∗ | |||||||
| <0.01%|| | 167 (43.5) | 300 (38.5) | 54 (51.9) | 11 (26.8) | 5 (35.7) | 50 (46.7) | 587 (41.1) | |
| 0.01%-0.099% | 121 (31.5) | 270 (34.7) | 33 (31.7) | 16 (39.0) | 4 (28.6) | 26 (24.3) | 470 (32.9) | |
| 0.1%-0.99% | 69 (18.0) | 135 (17.3) | 14 (13.5) | 8 (19.5) | 5 (35.7) | 18 (16.8) | 249 (17.4) | |
| ≥1% | 27 (7.0) | 74 (9.5) | 3 (2.9) | 6 (14.6) | 0 (0.0) | 13 (12.1) | 123 (8.6) | |
| Unknown | 68 | 178 | 11 | 11 | 2 | 16 | 286 | |
Bold values indicate P < .05.
BM, bone marrow; NCI, National Cancer Institute.
P values calculated using the chi-square test.
Cytogenetic group was defined as the following: favorable (double trisomy 4/10, ETV6::RUNX1) vs unfavorable (KMT2A-rearranged, hypodiploid <44, intrachromosomal amplification of chromosome 21, BCR::ABL1) vs neutral (all others).
For duration of frontline trial, P value is calculated using the Kruskal-Wallis test.
The COG relapse ALL studies included: AALL01P2, AALL02P2, AALL0433, AALL07P1, AALL1331, and AALL1821.
This category included patients with MRD <0.01% and a small number of patients whose MRD was reported as negative using an assay with a sensitivity of 1 in 1000.
Several socioeconomic factors and other characteristics differed by race and ethnicity as well, including treating institution size (P = .001), being treated outside the US (P < .0001), US insurance status (P < .0001), and US ZIP code–based median household income (P < .0001) (Table 3). Compared with NH White and NH Asian patients, Hispanic and NH Black patients were more commonly insured by Medicaid only (NH White 17.7%, compared with: Hispanic 41.2%; NH Black 42.5%; NH Asian 18.2%) and lived in an area with median household income ≤$50 000 (NH White 19.5%, compared with: Hispanic 29%; NH Black 48.7%; NH Asian 9.1%) (Table 3).
Additional socioeconomic factors and other characteristics of patients with noninfant B-ALL experiencing relapse after frontline COG clinical trials by race and ethnicity
| Characteristics . | Race/ethnicity grouping . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 452) . | NH White (N = 957) . | NH Black (N = 115) . | NH Asian (N = 52) . | NH Other (N = 16) . | Other/Unknown (N = 123) . | Total (N = 1715) . | P value . | |
| Institution size (based on frontline trial enrollment)∗, n (%) | .001 | |||||||
| Institution size: ≤50 | 55 (12.2) | 183 (19.2) | 27 (23.5) | 9 (17.3) | 2 (12.5) | 20 (16.3) | 296 (17.3) | |
| Institution size: 51-100 | 138 (30.5) | 295 (30.9) | 32 (27.8) | 12 (23.1) | 9 (56.3) | 23 (18.7) | 509 (29.7) | |
| Institution size: 101-200 | 161 (35.6) | 329 (34.5) | 42 (36.5) | 19 (36.5) | 3 (18.8) | 57 (46.3) | 611 (35.7) | |
| Institution size: >200 | 98 (21.7) | 148 (15.5) | 14 (12.2) | 12 (23.1) | 2 (12.5) | 23 (18.7) | 297 (17.3) | |
| Missing | 0 | 2 | 0 | 0 | 0 | 0 | 2 | |
| Treatment site location†, n (%) | <.0001 | |||||||
| US | 452 (100.0) | 836 (87.4) | 113 (98.3) | 44 (84.6) | 15 (93.8) | 100 (81.3) | 1560 (91.0) | |
| Outside US | 0 (0.0) | 121 (12.6) | 2 (1.7) | 8 (15.4) | 1 (6.3) | 23 (18.7) | 155 (9.0) | |
| Insurance status, n (%) | <.0001 | |||||||
| US Private | 167 (36.9) | 586 (70.1) | 36 (31.9) | 30 (68.2) | 10 (66.7) | 41 (41.0) | 870 (55.8) | |
| US Medicaid | 186 (41.2) | 148 (17.7) | 48 (42.5) | 8 (18.2) | 2 (13.3) | 28 (28.0) | 420 (26.9) | |
| US Other | 60 (13.3) | 52 (6.2) | 21 (18.6) | 4 (9.1) | 2 (13.3) | 10 (10.0) | 149 (9.6) | |
| US, Unknown | 39 (8.6) | 50 (6.0) | 8 (7.1) | 2 (4.5) | 1 (6.7) | 21 (21.0) | 121 (7.8) | |
| Non-US | 0 | 121 | 2 | 8 | 1 | 23 | 155 | |
| Area-based median household income category based on quartiles‡, n (%) | <.0001 | |||||||
| US, ≤$50 000 | 131 (29.0) | 163 (19.5) | 55 (48.7) | 4 (9.1) | 3 (20.0) | 18 (18.0) | 374 (24.0) | |
| US, $50 001-$65 000 | 137 (30.3) | 250 (29.9) | 25 (22.1) | 8 (18.2) | 4 (26.7) | 28 (28.0) | 452 (29.0) | |
| US, $65 001-$85 000 | 107 (23.7) | 214 (25.6) | 13 (11.5) | 14 (31.8) | 5 (33.3) | 26 (26.0) | 379 (24.3) | |
| US, ≥$85 000 | 71 (15.7) | 203 (24.3) | 16 (14.2) | 18 (40.9) | 2 (13.3) | 25 (25.0) | 335 (21.5) | |
| US, Unknown | 6 (1.3) | 6 (0.7) | 4 (3.5) | 0 (0.0) | 1 (6.7) | 3 (3.0) | 20 (1.3) | |
| Non-US | 0 | 121 | 2 | 8 | 1 | 23 | 155 | |
| Characteristics . | Race/ethnicity grouping . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 452) . | NH White (N = 957) . | NH Black (N = 115) . | NH Asian (N = 52) . | NH Other (N = 16) . | Other/Unknown (N = 123) . | Total (N = 1715) . | P value . | |
| Institution size (based on frontline trial enrollment)∗, n (%) | .001 | |||||||
| Institution size: ≤50 | 55 (12.2) | 183 (19.2) | 27 (23.5) | 9 (17.3) | 2 (12.5) | 20 (16.3) | 296 (17.3) | |
| Institution size: 51-100 | 138 (30.5) | 295 (30.9) | 32 (27.8) | 12 (23.1) | 9 (56.3) | 23 (18.7) | 509 (29.7) | |
| Institution size: 101-200 | 161 (35.6) | 329 (34.5) | 42 (36.5) | 19 (36.5) | 3 (18.8) | 57 (46.3) | 611 (35.7) | |
| Institution size: >200 | 98 (21.7) | 148 (15.5) | 14 (12.2) | 12 (23.1) | 2 (12.5) | 23 (18.7) | 297 (17.3) | |
| Missing | 0 | 2 | 0 | 0 | 0 | 0 | 2 | |
| Treatment site location†, n (%) | <.0001 | |||||||
| US | 452 (100.0) | 836 (87.4) | 113 (98.3) | 44 (84.6) | 15 (93.8) | 100 (81.3) | 1560 (91.0) | |
| Outside US | 0 (0.0) | 121 (12.6) | 2 (1.7) | 8 (15.4) | 1 (6.3) | 23 (18.7) | 155 (9.0) | |
| Insurance status, n (%) | <.0001 | |||||||
| US Private | 167 (36.9) | 586 (70.1) | 36 (31.9) | 30 (68.2) | 10 (66.7) | 41 (41.0) | 870 (55.8) | |
| US Medicaid | 186 (41.2) | 148 (17.7) | 48 (42.5) | 8 (18.2) | 2 (13.3) | 28 (28.0) | 420 (26.9) | |
| US Other | 60 (13.3) | 52 (6.2) | 21 (18.6) | 4 (9.1) | 2 (13.3) | 10 (10.0) | 149 (9.6) | |
| US, Unknown | 39 (8.6) | 50 (6.0) | 8 (7.1) | 2 (4.5) | 1 (6.7) | 21 (21.0) | 121 (7.8) | |
| Non-US | 0 | 121 | 2 | 8 | 1 | 23 | 155 | |
| Area-based median household income category based on quartiles‡, n (%) | <.0001 | |||||||
| US, ≤$50 000 | 131 (29.0) | 163 (19.5) | 55 (48.7) | 4 (9.1) | 3 (20.0) | 18 (18.0) | 374 (24.0) | |
| US, $50 001-$65 000 | 137 (30.3) | 250 (29.9) | 25 (22.1) | 8 (18.2) | 4 (26.7) | 28 (28.0) | 452 (29.0) | |
| US, $65 001-$85 000 | 107 (23.7) | 214 (25.6) | 13 (11.5) | 14 (31.8) | 5 (33.3) | 26 (26.0) | 379 (24.3) | |
| US, ≥$85 000 | 71 (15.7) | 203 (24.3) | 16 (14.2) | 18 (40.9) | 2 (13.3) | 25 (25.0) | 335 (21.5) | |
| US, Unknown | 6 (1.3) | 6 (0.7) | 4 (3.5) | 0 (0.0) | 1 (6.7) | 3 (3.0) | 20 (1.3) | |
| Non-US | 0 | 121 | 2 | 8 | 1 | 23 | 155 | |
Bold values indicate P < .05.
Institution size based on number of enrollments from each institution (out of the 16 115 total enrollments) in the 12 frontline COG trials.
Non-US countries included Australia, Canada, New Zealand, and Switzerland.
Area-based median household income based on ZIP code for US patients only.
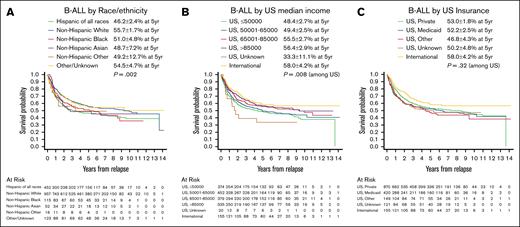
For children with B-ALL, postrelapse OS differed by race and ethnicity (5-year OS ranging from 46.2% ± 2.4% for Hispanic to 55.7% ± 1.7% for NH White; P = .002) and by area-based median household income (5-year OS ranging from 48.4% ± 2.7% for US median income ≤$50 000 to 56.4% ± 2.9% for US median income >$85 000; P = .008) (Figure 1A-B; Table 4). However, after adjusting for disease-related and patient characteristics (WBC count at initial diagnosis, frontline end-induction MRD, cytogenetic group, treatment at a non-US center, age at relapse, sites of relapse, time to relapse) in multivariable analysis, the magnitudes of association between both race and area-based income and OS were attenuated and were no longer statistically significant. An association between Hispanic ethnicity and lower postrelapse OS (compared with the reference group, NH White patients) was attenuated on multivariable analysis but maintained statistical significance (univariate hazard ratio [HR], 1.39; 95% confidence interval [CI], 1.19-1.63; P < .0001; multivariable HR, 1.19; 95% CI, 1.01-1.41; P = .04). NH Black patients had lower OS; however, the difference in OS was not statistically significant (univariate HR, 1.28; 95% CI, 0.98-1.67; P = .08; multivariable HR, 1.16; 95% CI, 0.88-1.53; P = .29) (Table 4). Additionally, although overall US median household income was not associated with OS, we observed an effect at the extremes of socioeconomic status categories. There was a protective association between the highest category of US median household income (>$85 000) vs the lowest income category (<$50 000) and OS, which persisted after adjustment (univariate HR, 0.73; 95% CI, 0.58-0.9; P = .004; multivariable HR, 0.79; 95% CI, 0.63-0.99; P = .04). Perhaps unsurprisingly, patients who relapsed most recently (from 2013-2021) had better OS after relapse (HR, 0.65; 95% CI, 0.44-0.96; P = .031). However, addition of year of relapse did not influence any of the other variables in our model (supplemental Table 3), especially the primary exposures of interest (ie, race, ethnicity, socioeconomic factors), and was thus not included in the main multivariable analysis (Table 4).
OS after relapse for patients with B-ALL, based on race and ethnicity, median household income, and insurance status. OS displayed by Kaplan-Meier curves for B-ALL stratified by (A) race and ethnicity, (B) area-based median household income based on ZIP code, and (C) insurance status. Legends display 5-year OS probability calculated by Kaplan-Meier method with Greenwood standard errors to determine 95% CI. The displayed P values are based on univariate Cox regression models.
OS after relapse for patients with B-ALL, based on race and ethnicity, median household income, and insurance status. OS displayed by Kaplan-Meier curves for B-ALL stratified by (A) race and ethnicity, (B) area-based median household income based on ZIP code, and (C) insurance status. Legends display 5-year OS probability calculated by Kaplan-Meier method with Greenwood standard errors to determine 95% CI. The displayed P values are based on univariate Cox regression models.
B-ALL Cox regression reduced model for postrelapse OS
| Variables . | No. of patients (percentage of total B-ALL cohort) . | No. of events (percentage of N, number of patients) . | Univariate analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P value∗ . | P value† . | HR (95% CI) . | P value∗ . | P value† . | |||
| Age at relapse | ||||||||
| 1-9 years | 875 (51.0%) | 381 (43.5%) | 1 | <.0001 | 1 | <.0001 | ||
| 10-15 years | 482 (28.1%) | 229 (47.5%) | 1.23 (1.04-1.45) | .015 | 1.36 (1.15-1.62) | .0004 | ||
| 16+ years | 358 (20.9%) | 199 (55.6%) | 1.61 (1.36-1.91) | <.0001 | 1.67 (1.38-2.01) | <.0001 | ||
| Cytogenetic group‡ | ||||||||
| Favorable | 391 (22.8%) | 112 (28.6%) | 0.45 (0.37-0.56) | <.0001 | <.0001 | 0.65 (0.52-0.81) | .0002 | .0001 |
| Neutral | 726 (42.3%) | 377 (51.9%) | 1 | 1 | ||||
| Unfavorable | 190 (11.1%) | 115 (60.5%) | 1.47 (1.19-1.81) | .0003 | 1.11 (0.89-1.39) | .37 | ||
| Unknown | 408 (23.8%) | 205 (50.2%) | 0.92 (0.78-1.09) | .36 | 1.08 (0.88-1.33) | .448 | ||
| Non-US | ||||||||
| No | 1560 (91.0%) | 749 (48.0%) | 1 | .032 | 1 | .057 | ||
| Yes | 155 (9.0%) | 60 (38.7%) | 0.75 (0.58-0.98) | — | 0.75 (0.55-1.01) | — | ||
| US median household income | ||||||||
| US ≤$50 000 | 374 (24.0%) | 196 (52.4%) | 1 | .008 (.010 trend test) | 1 | .30 (.19 trend test) | ||
| US $50 001-$65 000 | 452 (29.0%) | 228 (50.4%) | 0.91 (0.75-1.1) | .32 | 0.93 (0.77-1.13) | .47 | ||
| US $65 001-$85 000 | 379 (24.3%) | 170 (44.9%) | 0.77 (0.63-0.95) | .013 | 0.87 (0.7-1.07) | .19 | ||
| US >$85 000 | 335 (21.5%) | 143 (42.7%) | 0.73 (0.58-0.9) | .004 | 0.79 (0.63-0.99) | .038 | ||
| US Unknown | 20 (1.3%) | 12 (60.0%) | 1.36 (0.76-2.43) | .31 | 0.84 (0.46-1.51) | .56 | ||
| Race/ethnicity | ||||||||
| Hispanic all race | 452 (26.4%) | 242 (53.5%) | 1.39 (1.19-1.63) | <.0001 | .002 | 1.19 (1.01-1.41) | .043 | .45 |
| NH White | 957 (55.8%) | 418 (43.7%) | 1 | 1 | ||||
| NH Black | 115 (6.7%) | 61 (53.0%) | 1.28 (0.98-1.67) | .075 | 1.16 (0.88-1.53) | .29 | ||
| NH Asian | 52 (3.0%) | 26 (50.0%) | 1.25 (0.84-1.85) | .274 | 1.16 (0.78-1.73) | .47 | ||
| NH Other | 16 (0.9%) | 8 (50.0%) | 1.34 (0.66-2.69) | .42 | 1.01 (0.49-2.05) | .99 | ||
| Other/Unknown | 123 (7.2%) | 54 (43.9%) | 1.03 (0.77-1.36) | .86 | 1.07 (0.80-1.43) | .64 | ||
| Sites of relapse | ||||||||
| Isolated BM | 1003 (58.5%) | 537 (53.5%) | 1 | <.0001 | 1 | <.0001 | ||
| Combined BM (±CNS) | 236 (13.8%) | 96 (40.7%) | 0.65 (0.52-0.81) | .0001 | 0.73 (0.58-0.91) | .005 | ||
| Isolated CNS | 371 (21.6%) | 140 (37.7%) | 0.53 (0.44-0.64) | <.0001 | 0.32 (0.26-0.39) | <.0001 | ||
| Unknown | 7 (0.4%) | 2 (28.6%) | 0.73 (0.18-2.94) | .66 | 0.47 (0.11-1.94) | .30 | ||
| Other | 98 (5.7%) | 34 (34.7%) | 0.5 (0.35-0.71) | <.0001 | 0.54 (0.38-0.77) | .0006 | ||
| Time to relapse | ||||||||
| <18 months | 349 (20.3%) | 256 (73.4%) | 1 | <.0001 | 1 | <.0001 | ||
| 18-35 months | 546 (31.8%) | 285 (52.2%) | 0.46 (0.39-0.55) | <.0001 | 0.44 (0.37-0.53) | <.0001 | ||
| ≥36 months | 820 (47.8%) | 268 (32.7%) | 0.26 (0.22-0.31) | <.0001 | 0.18 (0.15-0.22) | <.0001 | ||
| WBC count at initial diagnosis (cells per μL) | ||||||||
| <50 000 | 1326 (77.3%) | 605 (45.6%) | 1 | <.0001 | 1 | .060 | ||
| 50 000-100 000 | 169 (9.9%) | 69 (40.8%) | 0.89 (0.69-1.14) | .35 | 0.87 (0.68-1.13) | .30 | ||
| >100 000 | 220 (12.8%) | 135 (61.4%) | 1.6 (1.33-1.93) | <.0001 | 1.23 (1.00-1.50) | .051 | ||
| Frontline induction day 29 MRD category | ||||||||
| <0.01%§ | 587 (41.1%) | 246 (41.9%) | 1 | <.0001 | 1 | .012 | ||
| 0.01% - 0.099% | 470 (32.9%) | 197 (41.9%) | 1.02 (0.85-1.23) | .83 | 0.97 (0.80-1.18) | .79 | ||
| 0.1%-0.99% | 249 (17.4%) | 141 (56.6%) | 1.54 (1.26-1.90) | <.0001 | 1.27 (1.03-1.57) | .027 | ||
| ≥1% | 123 (8.6%) | 87 (70.7%) | 2.22 (1.74-2.84) | <.0001 | 1.45 (1.12-1.88) | .005 | ||
| Unknown | 286 | 138 (48.3%) | 1.22 (0.99-1.50) | .060 | 1.15 (0.91-1.46) | .25 | ||
| Variables . | No. of patients (percentage of total B-ALL cohort) . | No. of events (percentage of N, number of patients) . | Univariate analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P value∗ . | P value† . | HR (95% CI) . | P value∗ . | P value† . | |||
| Age at relapse | ||||||||
| 1-9 years | 875 (51.0%) | 381 (43.5%) | 1 | <.0001 | 1 | <.0001 | ||
| 10-15 years | 482 (28.1%) | 229 (47.5%) | 1.23 (1.04-1.45) | .015 | 1.36 (1.15-1.62) | .0004 | ||
| 16+ years | 358 (20.9%) | 199 (55.6%) | 1.61 (1.36-1.91) | <.0001 | 1.67 (1.38-2.01) | <.0001 | ||
| Cytogenetic group‡ | ||||||||
| Favorable | 391 (22.8%) | 112 (28.6%) | 0.45 (0.37-0.56) | <.0001 | <.0001 | 0.65 (0.52-0.81) | .0002 | .0001 |
| Neutral | 726 (42.3%) | 377 (51.9%) | 1 | 1 | ||||
| Unfavorable | 190 (11.1%) | 115 (60.5%) | 1.47 (1.19-1.81) | .0003 | 1.11 (0.89-1.39) | .37 | ||
| Unknown | 408 (23.8%) | 205 (50.2%) | 0.92 (0.78-1.09) | .36 | 1.08 (0.88-1.33) | .448 | ||
| Non-US | ||||||||
| No | 1560 (91.0%) | 749 (48.0%) | 1 | .032 | 1 | .057 | ||
| Yes | 155 (9.0%) | 60 (38.7%) | 0.75 (0.58-0.98) | — | 0.75 (0.55-1.01) | — | ||
| US median household income | ||||||||
| US ≤$50 000 | 374 (24.0%) | 196 (52.4%) | 1 | .008 (.010 trend test) | 1 | .30 (.19 trend test) | ||
| US $50 001-$65 000 | 452 (29.0%) | 228 (50.4%) | 0.91 (0.75-1.1) | .32 | 0.93 (0.77-1.13) | .47 | ||
| US $65 001-$85 000 | 379 (24.3%) | 170 (44.9%) | 0.77 (0.63-0.95) | .013 | 0.87 (0.7-1.07) | .19 | ||
| US >$85 000 | 335 (21.5%) | 143 (42.7%) | 0.73 (0.58-0.9) | .004 | 0.79 (0.63-0.99) | .038 | ||
| US Unknown | 20 (1.3%) | 12 (60.0%) | 1.36 (0.76-2.43) | .31 | 0.84 (0.46-1.51) | .56 | ||
| Race/ethnicity | ||||||||
| Hispanic all race | 452 (26.4%) | 242 (53.5%) | 1.39 (1.19-1.63) | <.0001 | .002 | 1.19 (1.01-1.41) | .043 | .45 |
| NH White | 957 (55.8%) | 418 (43.7%) | 1 | 1 | ||||
| NH Black | 115 (6.7%) | 61 (53.0%) | 1.28 (0.98-1.67) | .075 | 1.16 (0.88-1.53) | .29 | ||
| NH Asian | 52 (3.0%) | 26 (50.0%) | 1.25 (0.84-1.85) | .274 | 1.16 (0.78-1.73) | .47 | ||
| NH Other | 16 (0.9%) | 8 (50.0%) | 1.34 (0.66-2.69) | .42 | 1.01 (0.49-2.05) | .99 | ||
| Other/Unknown | 123 (7.2%) | 54 (43.9%) | 1.03 (0.77-1.36) | .86 | 1.07 (0.80-1.43) | .64 | ||
| Sites of relapse | ||||||||
| Isolated BM | 1003 (58.5%) | 537 (53.5%) | 1 | <.0001 | 1 | <.0001 | ||
| Combined BM (±CNS) | 236 (13.8%) | 96 (40.7%) | 0.65 (0.52-0.81) | .0001 | 0.73 (0.58-0.91) | .005 | ||
| Isolated CNS | 371 (21.6%) | 140 (37.7%) | 0.53 (0.44-0.64) | <.0001 | 0.32 (0.26-0.39) | <.0001 | ||
| Unknown | 7 (0.4%) | 2 (28.6%) | 0.73 (0.18-2.94) | .66 | 0.47 (0.11-1.94) | .30 | ||
| Other | 98 (5.7%) | 34 (34.7%) | 0.5 (0.35-0.71) | <.0001 | 0.54 (0.38-0.77) | .0006 | ||
| Time to relapse | ||||||||
| <18 months | 349 (20.3%) | 256 (73.4%) | 1 | <.0001 | 1 | <.0001 | ||
| 18-35 months | 546 (31.8%) | 285 (52.2%) | 0.46 (0.39-0.55) | <.0001 | 0.44 (0.37-0.53) | <.0001 | ||
| ≥36 months | 820 (47.8%) | 268 (32.7%) | 0.26 (0.22-0.31) | <.0001 | 0.18 (0.15-0.22) | <.0001 | ||
| WBC count at initial diagnosis (cells per μL) | ||||||||
| <50 000 | 1326 (77.3%) | 605 (45.6%) | 1 | <.0001 | 1 | .060 | ||
| 50 000-100 000 | 169 (9.9%) | 69 (40.8%) | 0.89 (0.69-1.14) | .35 | 0.87 (0.68-1.13) | .30 | ||
| >100 000 | 220 (12.8%) | 135 (61.4%) | 1.6 (1.33-1.93) | <.0001 | 1.23 (1.00-1.50) | .051 | ||
| Frontline induction day 29 MRD category | ||||||||
| <0.01%§ | 587 (41.1%) | 246 (41.9%) | 1 | <.0001 | 1 | .012 | ||
| 0.01% - 0.099% | 470 (32.9%) | 197 (41.9%) | 1.02 (0.85-1.23) | .83 | 0.97 (0.80-1.18) | .79 | ||
| 0.1%-0.99% | 249 (17.4%) | 141 (56.6%) | 1.54 (1.26-1.90) | <.0001 | 1.27 (1.03-1.57) | .027 | ||
| ≥1% | 123 (8.6%) | 87 (70.7%) | 2.22 (1.74-2.84) | <.0001 | 1.45 (1.12-1.88) | .005 | ||
| Unknown | 286 | 138 (48.3%) | 1.22 (0.99-1.50) | .060 | 1.15 (0.91-1.46) | .25 | ||
Bold values indicate P < .05.
BM, bone marrow.
P value for each subcategory of a variable in comparison with the reference group.
P value for the overall test for a variable.
Cytogenetic group was defined as the following: favorable (double trisomy 4/10, ETV6::RUNX1) vs unfavorable (KMT2A-rearranged, hypodiploid <44, intrachromosomal amplification of chromosome 21, BCR::ABL1) vs neutral (all others).
This category included patients with MRD <0.01% and a small number of patients whose MRD was reported as negative using an assay with a sensitivity of 1 in 1000.
As reported in our prior publication,6 clinical and disease characteristics were found to drive OS in the multivariable analysis, including age at relapse (P < .0001), cytogenetic group (P < .0001), sites of relapse (P < .0001), time to relapse (P < .0001), and frontline end-induction MRD (P = .01) (Table 4). A better OS was observed in patients treated at non-US sites by univariate analysis (P = .03), although this was not statistically significant on multivariable analysis (P = .06) (Table 4). US insurance status was not associated with OS in univariate analysis (P = .32) (Figure 1C).
T-ALL
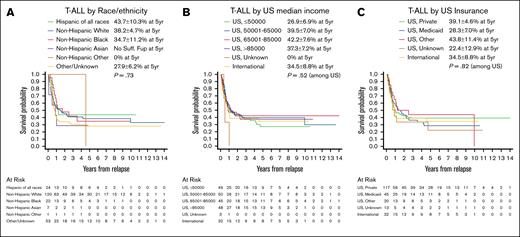
In contrast, within the cohort of patients with T-ALL who relapsed, no clinical or disease characteristics differed by race or ethnicity (Table 5). In terms of socioeconomic factors, race and ethnicity were associated with US insurance status (P = .04) and being treated at a non-US institution (P < .0001), with Hispanic and NH Black patients more commonly being insured by Medicaid only (NH White 17.3%, compared with: Hispanic 39.1%, NH Black 36.4%, NH Asian 0%) (Table 5). Race and ethnicity (P = .73) and area-based median household income (P = .52) were not associated with OS in relapsed T-ALL (Figure 2A-B; supplemental Table 4), nor was US insurance category (P = .82) (Figure 2C). Clinical and disease characteristics again largely drove postrelapse OS in the multivariable analysis, as demonstrated in our prior publication (supplemental Table 4).6
Demographics, disease characteristics, socioeconomic factors, and other characteristics of patients with T-ALL experiencing relapse after frontline COG clinical trials by race and ethnicity
| Characteristics . | New race/ethnicity grouping . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 24) . | NH White (N = 120) . | NH Black (N = 22) . | NH Asian (N = 7) . | NH Other (N = 1) . | Other/Unknown (N = 53) . | Total (N = 227) . | P value . | |
| Age at diagnosis, n (%) | .058∗ | |||||||
| 1-9 years | 17 (70.8) | 76 (63.3) | 17 (77.3) | 5 (71.4) | 0 (0.0) | 29 (54.7) | 144 (63.4) | |
| 10-15 years | 6 (25.0) | 32 (26.7) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 17 (32.1) | 57 (25.1) | |
| 16+ years | 1 (4.2) | 12 (10.0) | 3 (13.6) | 2 (28.6) | 1 (100.0) | 7 (13.2) | 26 (11.5) | |
| Age at relapse, n (%) | .061∗ | |||||||
| 1-9 years | 10 (41.7) | 60 (50.0) | 15 (68.2) | 5 (71.4) | 0 (0.0) | 21 (39.6) | 111 (48.9) | |
| 10-15 years | 12 (50.0) | 39 (32.5) | 3 (13.6) | 0 (0.0) | 0 (0.0) | 20 (37.7) | 74 (32.6) | |
| 16+ years | 2 (8.3) | 21 (17.5) | 4 (18.2) | 2 (28.6) | 1 (100.0) | 12 (22.6) | 42 (18.5) | |
| Cytogenetic group, n (%) | .15∗ | |||||||
| Favorable | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (2.2) | |
| Unfavorable | 0 (0.0) | 4 (12.1) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 8 (17.8) | |
| Neutral | 1 (100.0) | 29 (87.9) | 2 (50.0) | 1 (100.0) | 0 (0.0) | 3 (50.0) | 36 (80.0) | |
| Unknown | 23 | 87 | 18 | 6 | 1 | 47 | 182 | |
| Duration in frontline trial (months) | .28† | |||||||
| N | 21 | 110 | 20 | 7 | 1 | 12 | 171 | |
| Mean | 19 | 12.3 | 10.5 | 13.3 | 1 | 12.3 | 12.9 | |
| Median | 19.2 | 9.5 | 5.2 | 0.9 | 1 | 7.1 | 9.4 | |
| Range | 0.9-40.1 | 0.2-40.3 | 0.4-39.5 | 0.8-40.1 | 1.0-1.0 | 0.1-40.7 | 0.1-40.7 | |
| Enrolled in selected ALL studies at relapse, n (%) | .48∗ | |||||||
| No | 24 (100.0) | 111 (92.5) | 21 (95.5) | 7 (100.0) | 1 (100.0) | 52 (98.1) | 216 (95.2) | |
| Yes | 0 (0.0) | 9 (7.5) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 11 (4.8) | |
| Sex, n (%) | .36∗ | |||||||
| Male | 17 (70.8) | 91 (75.8) | 16 (72.7) | 6 (85.7) | 0 (0.0) | 44 (83.0) | 174 (76.7) | |
| Female | 7 (29.2) | 29 (24.2) | 6 (27.3) | 1 (14.3) | 1 (100.0) | 9 (17.0) | 53 (23.3) | |
| Institution size (based on frontline trial enrollment)‡, n (%) | .93∗ | |||||||
| Institution size: ≤50 | 4 (16.7) | 16 (13.4) | 3 (13.6) | 0 (0.0) | 0 (0.0) | 10 (18.9) | 33 (14.6) | |
| Institution size: 51-100 | 7 (29.2) | 38 (31.9) | 10 (45.5) | 2 (28.6) | 0 (0.0) | 18 (34.0) | 75 (33.2) | |
| Institution size: 101-200 | 8 (33.3) | 43 (36.1) | 6 (27.3) | 4 (57.1) | 1 (100.0) | 19 (35.8) | 81 (35.8) | |
| Institution size: >200 | 5 (20.8) | 22 (18.5) | 3 (13.6) | 1 (14.3) | 0 (0.0) | 6 (11.3) | 37 (16.4) | |
| Missing | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Non-US, n (%) | <.0001∗ | |||||||
| No | 23 (95.8) | 104 (86.7) | 22 (100.0) | 1 (14.3) | 0 (0.0) | 45 (84.9) | 195 (85.9) | |
| Yes | 1 (4.2) | 16 (13.3) | 0 (0.0) | 6 (85.7) | 1 (100.0) | 8 (15.1) | 32 (14.1) | |
| Insurance status, n (%) | .041∗ | |||||||
| US Private | 9 (39.1) | 72 (69.2) | 13 (59.1) | 1 (100.0) | 0 (0.0) | 22 (48.9) | 117 (60.0) | |
| US Medicaid | 9 (39.1) | 18 (17.3) | 8 (36.4) | 0 (0.0) | 0 (0.0) | 10 (22.2) | 45 (23.1) | |
| US Other | 2 (8.7) | 7 (6.7) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 10 (22.2) | 20 (10.3) | |
| US, Unknown | 3 (13.0) | 7 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (6.7) | 13 (6.7) | |
| Non-US | 1 | 16 | 0 | 6 | 1 | 8 | 32 | |
| Area-based median household income category based on quartiles, n (%) | .19∗ | |||||||
| US, ≤$50 000 | 7 (30.4) | 21 (20.2) | 10 (45.5) | 0 (0.0) | 0 (0.0) | 11 (24.4) | 49 (25.1) | |
| US, $50 001-$65 000 | 8 (34.8) | 24 (23.1) | 1 (4.5) | 1 (100.0) | 0 (0.0) | 16 (35.6) | 50 (25.6) | |
| US, $65 001-$85 000 | 6 (26.1) | 26 (25.0) | 4 (18.2) | 0 (0.0) | 0 (0.0) | 9 (20.0) | 45 (23.1) | |
| US, $85 000 | 2 (8.7) | 31 (29.8) | 7 (31.8) | 0 (0.0) | 0 (0.0) | 8 (17.8) | 48 (24.6) | |
| US, Unknown | 0 (0.0) | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 3 (1.5) | |
| Non-US | 1 | 16 | 0 | 6 | 1 | 8 | 32 | |
| Time to relapse, n (%) | .20∗ | |||||||
| <18 months | 11 (45.8) | 78 (65.0) | 18 (81.8) | 5 (71.4) | 0 (0.0) | 35 (66.0) | 147 (64.8) | |
| 18-35 months | 7 (29.2) | 20 (16.7) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 10 (18.9) | 39 (17.2) | |
| ≥36 months | 6 (25.0) | 22 (18.3) | 2 (9.1) | 2 (28.6) | 1 (100.0) | 8 (15.1) | 41 (18.1) | |
| Sites of relapse, n (%) | .37∗ | |||||||
| Isolated BM | 9 (37.5) | 49 (40.8) | 9 (40.9) | 5 (71.4) | 0 (0.0) | 19 (35.8) | 91 (40.1) | |
| Combined BM (±CNS) | 6 (25.0) | 18 (15.0) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 9 (17.0) | 35 (15.4) | |
| Isolated CNS | 5 (20.8) | 40 (33.3) | 8 (36.4) | 1 (14.3) | 0 (0.0) | 16 (30.2) | 70 (30.8) | |
| Unknown | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.7) | 4 (1.8) | |
| Other | 4 (16.7) | 12 (10.0) | 3 (13.6) | 1 (14.3) | 1 (100.0) | 6 (11.3) | 27 (11.9) | |
| WBC count at initial diagnosis (cells per μL), n (%) | .29∗ | |||||||
| <50 000 | 9 (37.5) | 39 (32.5) | 10 (45.5) | 4 (57.1) | 1 (100.0) | 12 (22.6) | 75 (33.0) | |
| 50 000-100 000 | 3 (12.5) | 19 (15.8) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 5 (9.4) | 28 (12.3) | |
| >100 000 | 12 (50.0) | 62 (51.7) | 11 (50.0) | 3 (42.9) | 0 (0.0) | 36 (67.9) | 124 (54.6) | |
| CNS status at initial diagnosis, n (%) | .68∗ | |||||||
| CNS 1 | 15 (62.5) | 75 (63.0) | 17 (81.0) | 6 (85.7) | 1 (100.0) | 38 (73.1) | 152 (67.9) | |
| CNS 2 | 6 (25.0) | 24 (20.2) | 3 (14.3) | 1 (14.3) | 0 (0.0) | 10 (19.2) | 44 (19.6) | |
| CNS 3 | 3 (12.5) | 20 (16.8) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 4 (7.7) | 28 (12.5) | |
| Unknown/missing | 0 | 1 | 1 | 0 | 0 | 1 | 3 | |
| Characteristics . | New race/ethnicity grouping . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hispanic of all races (N = 24) . | NH White (N = 120) . | NH Black (N = 22) . | NH Asian (N = 7) . | NH Other (N = 1) . | Other/Unknown (N = 53) . | Total (N = 227) . | P value . | |
| Age at diagnosis, n (%) | .058∗ | |||||||
| 1-9 years | 17 (70.8) | 76 (63.3) | 17 (77.3) | 5 (71.4) | 0 (0.0) | 29 (54.7) | 144 (63.4) | |
| 10-15 years | 6 (25.0) | 32 (26.7) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 17 (32.1) | 57 (25.1) | |
| 16+ years | 1 (4.2) | 12 (10.0) | 3 (13.6) | 2 (28.6) | 1 (100.0) | 7 (13.2) | 26 (11.5) | |
| Age at relapse, n (%) | .061∗ | |||||||
| 1-9 years | 10 (41.7) | 60 (50.0) | 15 (68.2) | 5 (71.4) | 0 (0.0) | 21 (39.6) | 111 (48.9) | |
| 10-15 years | 12 (50.0) | 39 (32.5) | 3 (13.6) | 0 (0.0) | 0 (0.0) | 20 (37.7) | 74 (32.6) | |
| 16+ years | 2 (8.3) | 21 (17.5) | 4 (18.2) | 2 (28.6) | 1 (100.0) | 12 (22.6) | 42 (18.5) | |
| Cytogenetic group, n (%) | .15∗ | |||||||
| Favorable | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (2.2) | |
| Unfavorable | 0 (0.0) | 4 (12.1) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 8 (17.8) | |
| Neutral | 1 (100.0) | 29 (87.9) | 2 (50.0) | 1 (100.0) | 0 (0.0) | 3 (50.0) | 36 (80.0) | |
| Unknown | 23 | 87 | 18 | 6 | 1 | 47 | 182 | |
| Duration in frontline trial (months) | .28† | |||||||
| N | 21 | 110 | 20 | 7 | 1 | 12 | 171 | |
| Mean | 19 | 12.3 | 10.5 | 13.3 | 1 | 12.3 | 12.9 | |
| Median | 19.2 | 9.5 | 5.2 | 0.9 | 1 | 7.1 | 9.4 | |
| Range | 0.9-40.1 | 0.2-40.3 | 0.4-39.5 | 0.8-40.1 | 1.0-1.0 | 0.1-40.7 | 0.1-40.7 | |
| Enrolled in selected ALL studies at relapse, n (%) | .48∗ | |||||||
| No | 24 (100.0) | 111 (92.5) | 21 (95.5) | 7 (100.0) | 1 (100.0) | 52 (98.1) | 216 (95.2) | |
| Yes | 0 (0.0) | 9 (7.5) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 1 (1.9) | 11 (4.8) | |
| Sex, n (%) | .36∗ | |||||||
| Male | 17 (70.8) | 91 (75.8) | 16 (72.7) | 6 (85.7) | 0 (0.0) | 44 (83.0) | 174 (76.7) | |
| Female | 7 (29.2) | 29 (24.2) | 6 (27.3) | 1 (14.3) | 1 (100.0) | 9 (17.0) | 53 (23.3) | |
| Institution size (based on frontline trial enrollment)‡, n (%) | .93∗ | |||||||
| Institution size: ≤50 | 4 (16.7) | 16 (13.4) | 3 (13.6) | 0 (0.0) | 0 (0.0) | 10 (18.9) | 33 (14.6) | |
| Institution size: 51-100 | 7 (29.2) | 38 (31.9) | 10 (45.5) | 2 (28.6) | 0 (0.0) | 18 (34.0) | 75 (33.2) | |
| Institution size: 101-200 | 8 (33.3) | 43 (36.1) | 6 (27.3) | 4 (57.1) | 1 (100.0) | 19 (35.8) | 81 (35.8) | |
| Institution size: >200 | 5 (20.8) | 22 (18.5) | 3 (13.6) | 1 (14.3) | 0 (0.0) | 6 (11.3) | 37 (16.4) | |
| Missing | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Non-US, n (%) | <.0001∗ | |||||||
| No | 23 (95.8) | 104 (86.7) | 22 (100.0) | 1 (14.3) | 0 (0.0) | 45 (84.9) | 195 (85.9) | |
| Yes | 1 (4.2) | 16 (13.3) | 0 (0.0) | 6 (85.7) | 1 (100.0) | 8 (15.1) | 32 (14.1) | |
| Insurance status, n (%) | .041∗ | |||||||
| US Private | 9 (39.1) | 72 (69.2) | 13 (59.1) | 1 (100.0) | 0 (0.0) | 22 (48.9) | 117 (60.0) | |
| US Medicaid | 9 (39.1) | 18 (17.3) | 8 (36.4) | 0 (0.0) | 0 (0.0) | 10 (22.2) | 45 (23.1) | |
| US Other | 2 (8.7) | 7 (6.7) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 10 (22.2) | 20 (10.3) | |
| US, Unknown | 3 (13.0) | 7 (6.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (6.7) | 13 (6.7) | |
| Non-US | 1 | 16 | 0 | 6 | 1 | 8 | 32 | |
| Area-based median household income category based on quartiles, n (%) | .19∗ | |||||||
| US, ≤$50 000 | 7 (30.4) | 21 (20.2) | 10 (45.5) | 0 (0.0) | 0 (0.0) | 11 (24.4) | 49 (25.1) | |
| US, $50 001-$65 000 | 8 (34.8) | 24 (23.1) | 1 (4.5) | 1 (100.0) | 0 (0.0) | 16 (35.6) | 50 (25.6) | |
| US, $65 001-$85 000 | 6 (26.1) | 26 (25.0) | 4 (18.2) | 0 (0.0) | 0 (0.0) | 9 (20.0) | 45 (23.1) | |
| US, $85 000 | 2 (8.7) | 31 (29.8) | 7 (31.8) | 0 (0.0) | 0 (0.0) | 8 (17.8) | 48 (24.6) | |
| US, Unknown | 0 (0.0) | 2 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 3 (1.5) | |
| Non-US | 1 | 16 | 0 | 6 | 1 | 8 | 32 | |
| Time to relapse, n (%) | .20∗ | |||||||
| <18 months | 11 (45.8) | 78 (65.0) | 18 (81.8) | 5 (71.4) | 0 (0.0) | 35 (66.0) | 147 (64.8) | |
| 18-35 months | 7 (29.2) | 20 (16.7) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 10 (18.9) | 39 (17.2) | |
| ≥36 months | 6 (25.0) | 22 (18.3) | 2 (9.1) | 2 (28.6) | 1 (100.0) | 8 (15.1) | 41 (18.1) | |
| Sites of relapse, n (%) | .37∗ | |||||||
| Isolated BM | 9 (37.5) | 49 (40.8) | 9 (40.9) | 5 (71.4) | 0 (0.0) | 19 (35.8) | 91 (40.1) | |
| Combined BM (±CNS) | 6 (25.0) | 18 (15.0) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 9 (17.0) | 35 (15.4) | |
| Isolated CNS | 5 (20.8) | 40 (33.3) | 8 (36.4) | 1 (14.3) | 0 (0.0) | 16 (30.2) | 70 (30.8) | |
| Unknown | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (5.7) | 4 (1.8) | |
| Other | 4 (16.7) | 12 (10.0) | 3 (13.6) | 1 (14.3) | 1 (100.0) | 6 (11.3) | 27 (11.9) | |
| WBC count at initial diagnosis (cells per μL), n (%) | .29∗ | |||||||
| <50 000 | 9 (37.5) | 39 (32.5) | 10 (45.5) | 4 (57.1) | 1 (100.0) | 12 (22.6) | 75 (33.0) | |
| 50 000-100 000 | 3 (12.5) | 19 (15.8) | 1 (4.5) | 0 (0.0) | 0 (0.0) | 5 (9.4) | 28 (12.3) | |
| >100 000 | 12 (50.0) | 62 (51.7) | 11 (50.0) | 3 (42.9) | 0 (0.0) | 36 (67.9) | 124 (54.6) | |
| CNS status at initial diagnosis, n (%) | .68∗ | |||||||
| CNS 1 | 15 (62.5) | 75 (63.0) | 17 (81.0) | 6 (85.7) | 1 (100.0) | 38 (73.1) | 152 (67.9) | |
| CNS 2 | 6 (25.0) | 24 (20.2) | 3 (14.3) | 1 (14.3) | 0 (0.0) | 10 (19.2) | 44 (19.6) | |
| CNS 3 | 3 (12.5) | 20 (16.8) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 4 (7.7) | 28 (12.5) | |
| Unknown/missing | 0 | 1 | 1 | 0 | 0 | 1 | 3 | |
Bold values indicate P < .05.
Area-based median household income was based on ZIP code for US patients only.
BM, bone marrow.
P values calculated using the chi-square test.
For duration of frontline trial, P value is calculated using the Kruskal-Wallis test.
Institution size based on number of enrollments from each institution (out of the 16 115 total enrollments) in the 12 frontline COG trials.
OS after relapse for patients with T-ALL, based on race and ethnicity, median household income, and insurance status. OS displayed by Kaplan-Meier curves for T-ALL stratified by (A) race and ethnicity, (B) area-based median household income based on ZIP code, and (C) insurance status. Legends display 5-year OS probability calculated by Kaplan-Meier method with Greenwood standard errors to determine 95% CI. The displayed P values are based on univariate Cox regression models.
OS after relapse for patients with T-ALL, based on race and ethnicity, median household income, and insurance status. OS displayed by Kaplan-Meier curves for T-ALL stratified by (A) race and ethnicity, (B) area-based median household income based on ZIP code, and (C) insurance status. Legends display 5-year OS probability calculated by Kaplan-Meier method with Greenwood standard errors to determine 95% CI. The displayed P values are based on univariate Cox regression models.
Infant ALL
Within the comparatively small cohort of patients with infant ALL who relapsed, there was an association between race, ethnicity, and cytogenetics (P = .0003), although all but 2 patients with known cytogenetics had “unfavorable” cytogenetics (supplemental Table 5); no other factors were associated with race or ethnicity. Race and ethnicity were not associated with OS in relapsed infant ALL by univariate analysis (P = .72) (supplemental Figure 1); however, this association was observed after adjustment in multivariable analysis (P = .027) (supplemental Table 6). This seems to be driven by the small group of NH Black patients (n = 8) with prolonged OS compared with NH White patients (HR, 0.25; 95% CI, 0.10-0.67; P = .006) (supplemental Table 6). As reported in our prior publication,6 sex, sites of relapse, and time to relapse were associated with OS in multivariable analysis (supplemental Table 6). Neither area-based median income (P = .42) nor US insurance status (P = .51) were associated with OS in univariate analysis (supplemental Figure 1B-C).
Discussion
Using the largest available cohort of pediatric and young adult patients with relapsed ALL, we found an association between race and ethnicity and OS among patients with B-ALL that was no longer significant for NH Black patients when controlling for disease factors known to portend poor OS after first relapse, including early relapse, higher WBC count at initial diagnosis, and CNS involvement at initial diagnosis. Our multivariable analysis suggests that these disease-related and socioeconomic factors likely drive the association between race and ethnicity and postrelapse OS. However, Hispanic patients had worse postrelapse OS compared with NH White patients, and this disparity in outcomes, although attenuated, persisted even after accounting for relevant measured confounders on multivariable analysis. Additionally, it should be highlighted that although not statistically significant, disparities for NH Black patients in multivariable analyses are similar in magnitude to those for Hispanic patients. Thus, a lack of statistical significance relative to NH White patients could be related to limited statistical power due to smaller sample size.
There are several potential reasons why race is not an independent risk factor for worse postrelapse OS after accounting for confounding factors. Compared with our previous study in the frontline setting,3 we may have better accounted for socioeconomic factors by considering median household income by ZIP code. Patients with income in the highest category (>$85 000 per year) had better OS. This suggests that individual and/or community-level resources may enable a better chance of surviving relapse and is in line with previous literature showing that living in a low-income area is correlated with higher risk of early relapse.27 Interestingly, US insurance status was not associated with postrelapse OS, in contrast to previous studies of those treated in the frontline setting, where it has been shown to be an important predictor of survival.3,28 Because insurance status was determined at diagnosis (and not reassessed at relapse), it is possible that it is not an accurate reflection of access to relapse care (or an accurate proxy for low household income), particularly given that data suggest that insurance status changes over the course of cancer treatment.29 Alternatively, perhaps insurance status is less relevant in the relapse setting given that patients typically have already established relationships with oncology care. Relatively few patients were enrolled in COG trials for relapse, and it is thus unclear whether referral to tertiary care centers for enrollment in clinical trials for relapse benefited patients with relapsed B-ALL.
The association between Hispanic ethnicity and worse postrelapse survival was not fully attenuated in multivariable analysis. This suggests that the Hispanic population may have additional unmeasured drivers of worse outcomes compared with other groups. This survival difference and the higher presenting initial WBC count may be partially accounted for by the increased frequency of Philadelphia chromosome–like ALL in Hispanic patients30 given that this genomic subset profile was not consistently captured across these studies. Recent studies demonstrate a clear link of genetic ancestry with molecular subtypes and prognosis.31 There may be additional disease-related or socioeconomic factors that are specific to or more prevalent in the Hispanic population, such as preferred language and obesity, that are not adequately measured using the data available in our current analysis. Additionally, host factors that affect aspects of treatment such as chemotherapy tolerance or toxicity may play an important role in outcomes. Future prospective clinical trials must collect comprehensive social and demographic information to allow us to better understand how social determinants of health impact patient outcomes.
Hispanic and NH Black patients had several worse disease-related predictors of postrelapse survival, such as shorter time to relapse. One notable exception in our relapsed cohort is that Hispanic and NH Black patients more frequently achieved an MRD-negative remission following frontline induction. Although end-induction MRD status is highly prognostic for patients with B-ALL as a measure of responsiveness to frontline chemotherapy, we found that a higher proportion of Hispanic and NH Black patients experienced relapse despite initially achieving an MRD-negative remission at the end of induction. Again, this may be partially explained by these studies not capturing the Philadelphia chromosome–like ALL phenotype, particularly in Hispanic patients, which has been shown to be an independent negative prognostic factor in prior studies that have included MRD.32 Relapse following MRD-negative remission could be related to increased toxicity (leading to decreased intensity of therapy). Relapse could also be linked to disproportionate financial toxicity among Hispanic and NH Black patients, who tend to have lower income, which could potentially affect their ability to adhere to treatment recommendations.33-35 MRD at the end of induction may not be prognostic after isolated CNS relapse, which accounts for almost 25% of the relapses among NH White, NH Black, and Hispanic patients. In addition, it is possible that Hispanic and NH Black patients experience increased nonrelapse mortality during postrelapse treatment, such as stem cell transplantation.36 Additional investigations into the relevance of end-induction MRD in predicting postrelapse OS in minority populations should be incorporated into future clinical trials and retrospective studies.
Given that many of the factors that impact postrelapse OS were determined at the time of diagnosis, our data suggest that to improve outcomes at relapse, we must incorporate strategies during frontline treatment that minimize risk of early relapse or prevent relapse altogether. Potential interventions could include enhanced risk classification algorithms, further intensification of therapy, and better supportive care. Additionally, the incorporation of novel treatment modalities such as immunotherapy in the frontline setting may close the gap in outcomes between racial and ethnic groups. Indeed, in the recently completed frontline ALL clinical trial AALL1731, Hispanic patients who received blinatumomab experienced a substantial improvement in disease-free survival time and in time to relapse compared with those who received conventional chemotherapy.37 In the new era of frontline blinatumomab, it is crucial that future clinical trials collect comprehensive data on social determinants of health38,39 so that factors that may continue to impact outcomes can be identified and potentially modified or mitigated.
Patients who relapsed in this analysis likely largely received chemotherapy-based salvage regimens. In addition to the incorporation of blinatumomab into the frontline setting,37 immunotherapy as a broad treatment modality has proven highly effective for relapsed B-ALL in the past 10 years. These immunotherapeutic approaches include bispecific T-cell engagers (ie, blinatumomab),23 antibody-drug conjugates (ie, inotuzumab),40 and chimeric antigen receptor T cells (ie, tisagenlecleucel).41 Our present study sets a recent benchmark for outcomes in relapsed ALL by race and ethnicity to which future analyses of current and future novel immunotherapies can be compared. It will be important to determine if similar disparities are observed for patients who experienced relapse during the immunotherapy era, if these disparities are attenuated, or if perhaps new disparities emerge. Immunotherapy as a treatment modality is likely to be effective across leukemias with variable risk features in the relapse setting, including race and ethnicity, as has been suggested by some small single-institution studies.42 However, it is also conceivable that as certain subsets of patients increasingly gain access to these novel therapies and increasingly survive following relapse, greater disparities between races and ethnicities may become evident as patients encounter disparate barriers to accessing novel therapies.43,44
There are several limitations of our analysis. Only patients who enrolled in COG treatment trials at initial diagnosis were included, leading to possible selection bias. It is possible that racial and ethnic disparities are more prominent among patients who were not initially treated in clinical trials. It is possible that other changes in frontline therapy, such as a shift to more sensitive measures of MRD using next-generation sequencing, may also impact the magnitude of disparities, particularly if access to these new technologies is uneven. Additionally, the variables of race and ethnicity in our analysis rely on institutional reporting, which may or may not reflect self-reported race and ethnicity. The categories are also based on standard reporting in the US rather than racial, ethnic, or cultural groups that may be more relevant in other countries such as Canada, Australia, or New Zealand. The impact of sites using their own perception of race and ethnicity vs self-report on our results is unknown. Furthermore, our analysis of socioeconomic status is based on US census data from 2020, which may not accurately reflect the demographics of the location at the time the clinical trial was conducted; we did not have individual self-reported details of income or other social determinants of health for individual patients enrolled in the clinical trial. Information on other potentially important factors, such as access to a transplant center or supportive care practices, was not available and could not be included in our analyses. Finally, although this represents, to our knowledge, the largest cohort of pediatric patients with relapsed ALL available, the number of patients who experienced relapse is much smaller than the overall number of patients treated in the frontline setting. Accordingly, there may be lower power to detect modest differences in outcomes, especially once the cohorts are parsed into multiple subsets. In particular, the numbers of patients in the T-ALL and infant ALL cohorts are very small, and thus care should be taken in interpreting the results for these subgroups.
In conclusion, in B-ALL, race and ethnicity are known to be associated with greater risk of relapse. Among pediatric and young adult patients who were enrolled in COG frontline clinical trials, a greater proportion of patients who were Hispanic and NH Black had host and disease factors that portend poorer survival after relapse. A notable exception was end-induction MRD status, where it appears a higher proportion of Hispanic and NH Black patients experience worse survival despite initially achieving an MRD-negative remission after induction. It will be important to observe how outcome disparities evolve as immunotherapy is incorporated into frontline treatment for most children with B-ALL.
Acknowledgments
The authors acknowledge all the patients who participated in COG and acute lymphoblastic leukemia therapeutic trials for teaching them how to treat the next generation of children better. The authors thank additional study chairs for the frontline clinical trials, including Yousif Matloub, Barbara Asselin, ZoAnn Dreyer, Paul Martin, W. Paul Bowman, Eric Larsen, Kelly Maloney, Stuart Winter, Joanne Hilden, Anne Angiolillo, Leonard Mattano, and Kimberly Dunsmore. The authors also thank all the COG centers and their research coordinators, nurses, and physicians for supplying data for analysis.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10 CA13539, U10 CA29139, U10 CA98543, and U10 CA180886 (COG, Children’s Cancer Group, and POG Chair’s grants); U10 CA98413 and U10 CA180899 (COG Statistics and Data Center grants); and U24 CA114766 and U24-CA196173 (COG Specimen Banking) to the COG. Research was also supported by St. Baldrick's Foundation funding, a Minority Young Investigator Award from the COG Foundation/Children’s Cancer Research Fund, and Sandoz Inc. E.A.R. is a KiDS of New York University (NYU) Foundation Professor at NYU Langone Health. M.L.L. is the Aldarra Foundation Endowed Chair in Pediatric Cancer Research, established by Bill and June Boeing. S.G. is supported by the Garron Family Chair in Childhood Cancer Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.A.L., S.R.R., D.B., S.G., and L.E.W. conceived the study concept and design, and performed data analyses and data interpretation of the results; L.J., A.D., X.X., and M.D. conducted the statistical analyses; J.A.K., M.S., N.A.H., and A.J.C. conducted the cytogenetic analyses; M.B. and B.L.W. conducted the minimal residual disease analyses; J.A.L., S.G., and L.E.W. wrote the manuscript, which was edited and approved by all authors; no nonauthor wrote the first draft or any part of the article; and all authors contributed critically to the manuscript and reviewed the final manuscript, and have agreed to be coauthors.
Conflict-of-interest disclosure: J.A.L. has received honoraria for career advice provided through the Health Professional Student Association. L.J. reports consulting for Pfizer (data safety monitoring committee [DSMC]). S.R.R. reports consulting for Pfizer (DSMC) and AbbVie (steering committee). S.P.H. reports consulting for Novartis; honoraria from Amgen, Jazz Pharmaceuticals, and Servier; and common stock ownership in Amgen. E.A.R. reports consulting for Bristol Myers Squibb (data safety monitoring board). M.L.L. reports consulting for Jazz Pharmaceuticals (advisory board). D.B. receives research funding to her institution from Wugen Inc. The remaining authors declare no competing financial interests.
Correspondence: John A. Ligon, Division of Hematology and Oncology, Department of Pediatrics, University of Florida Health Cancer Center, University of Florida, PO Box 100298, Gainesville, FL 32610-0298; email: john.ligon@ufl.edu.
References
Author notes
S.G. and L.E.W. contributed equally to this study and are joint senior authors.
Deidentified data from each of the included COG trials are available on request to the relevant trial committees. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org.
The full-text version of this article contains a data supplement.