Key Points
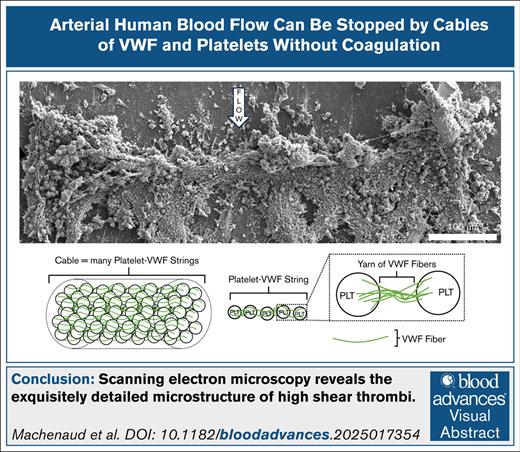
Shear-induced platelet aggregation thrombi are built from platelet-VWF strings forming a strong transverse cable.
VWF-VWF bonds are the weakest link in thrombus strength, making them key targets for clot lysis.
Visual Abstract
Arterial thrombi formed under high shear rates (∼15 000/s) must withstand substantial forces to occlude arteries, as occurs in myocardial infarction or ischemic stroke, driving the need to understand the structural features responsible for their strength. We used scanning electron microscopy and immunofluorescence microscopy to characterize the structure of in vitro high-shear thrombi. We show that the occlusive portion of the thrombus consists of a thick, transverse cable spanning the channel width. The cable is made of hundreds of parallel strings of platelets joined by yarns of von Willebrand factor (VWF) fibers identified by immunofluorescence microscopy. The elements composing the occlusive structure—cable, strings, yarns, and fibers, listed in hierarchical order—are oriented in the direction of tension to confer high breakage strength and stability to resist the upstream pressure. This structural hierarchy supports VWF-VWF bonding as a potential new target for the prevention and lysis of occlusive arterial thrombi.
Introduction
Arterial thrombosis is the occlusion of arterial blood flow by a clot leading to fatal pathologies such as myocardial infarction and ischemic stroke.1 Blood flow in a stenosed artery induces high wall shear stress that could cause atherosclerotic plaque rupture.2 The ruptured plaque cap exposes collagen fibers, offering a thrombogenic surface for von Willebrand factor (VWF) and platelets.3 High wall shear rates (from 3000/s up to 400 000/s)2 unfold VWF fibers to expose their A1 domains that bind to the GP1b platelet surface receptor.4 VWF can also self-associate in high shear rate flow.5 To reach occlusion, adherent platelets activate and release ultra long VWF (ulVWF) fibers that are 50× more concentrated locally than in circulating blood.6 After platelet activation, the platelet αIIbβ3 receptor undergoes conformational change to bind the VWF C1 domain with high affinity,7 enabling thrombus stabilization.8 This process leading to arterial occlusion is called shear-induced platelet aggregation (SIPA) and differs from the Virchow’s coagulation triad requiring blood stagnation to form RBC-rich, fibrin-rich thrombi.9 The terms “high-shear thrombi” and “SIPA thrombi” are used interchangeably in this study.
SIPA thrombi are VWF-rich, platelet-rich, white in appearance,10 and strong enough to resist arterial pressure (up to 180 mm Hg) while occluding arteries.11 In comparison, coagulation thrombi are too weak to withstand arterial pressure forces.11 Prior histological images of SIPA thrombi revealed a microscopic structure composed of long thin “fingers” of platelets and VWF, extending from the wall toward the lumen.10 SIPA thrombi are similar to arterial thrombi in that they both have a high content of activated platelets.12-14
In this study, we explore the microscopic structure contributing to the high strength of SIPA thrombi. We hypothesize that their structure is organized to withstand the high arterial hemodynamic forces.
We use a combination of high-resolution scanning electron microscopy (SEM) and fluorescence light microscopy, including immunohistochemistry, to characterize the detailed structure of SIPA thrombi. The new SEM images of SIPA thrombi reveal an exquisite hierarchy of structural elements formed from human whole blood over collagen, observed at the time of occlusion.
Methods
Experimental design
SIPA thrombi were formed from heparinized human whole blood in a microfluidic system. After occlusion, thrombi were fixed and imaged with SEM. Thrombi were also imaged by immunofluorescence to identify VWF and fibrin(ogen). Comparison images of thrombi formed with low-dose or high-dose heparin elucidate the role of heparin-sensitive blood components.
Whole human blood
Blood was obtained from consenting healthy volunteers after approval by the Georgia Tech Institutional Review Board (approval number 17315). All participants were older than 18 years and had not taken antiplatelet medications for at least 10 days before the blood collection. Blood was drawn by a professional phlebotomist into a 60 mL syringe containing low dose heparin (3.5 IU/mL) to prevent blood clotting during transport. High-dose heparin (15 IU/mL) was used to assure exclusion of fibrin formation, or DNA filaments released by white blood cells (WBCs).15
High-shear-rate microfluidic thrombosis-on-a-chip system
Thrombi were formed by perfusing whole human blood through the stenotic chamber of a microfluidic PDMS chip bonded to a glass slide via vacuum forces16 (supplemental Figure 1). The chamber was coated overnight with 100 μg/mL equine collagen fibrils type I (Chrono-Log Corp) to render the glass slide surface thrombogenic. The flow was driven by a constant pressure head (∼20 mm Hg), inducing high initial wall shear rate (∼15 000/s) in the stenosis. The dimensions of the stenotic chamber were 48 × 640 × 750 μm (height × width × length; supplemental Figure 1). The channel occluded in ∼2 minutes when the blood flow rate fell to <5% of the initial flow rate. Five minutes after occlusion, the glass slide was retrieved from the PDMS chip making the SIPA thrombus ready for imaging.
SEM imaging
After PDMS chip unbinding, the glass slide with the attached thrombus was rinsed in cacodylate buffer (50 mM sodium cacodylate, 150 mM NaCl, pH 7.4) 2 times for 10 minutes, in excess volume. Then the sample was fixed overnight in a tube containing 2% glutaraldehyde in the same cacodylate buffer. The sample was dehydrated using a series of ethanol solutions, beginning at 30% and progressing to absolute ethanol. Following dehydration, the sample was immersed into hexamethyldisilane, air-dried, and sputter-coated with gold-palladium using a Polaron E5100 sputter coater. High-resolution images were taken using a Quanta FEG 250 scanning electron microscope (ThermoFisher Scientific, Hillsboro, OR).
Low-magnification (100× to 200×) images were taken to observe thrombi in the stenotic portion. Intermediate-magnification (500× to 1000×) images showed details of the occlusive part of the thrombi and the islets downstream. High magnification (2000×) revealed how platelets were joined together on the occlusive part and the islets.
Results
SIPA thrombus structure consisted of a thick transverse cable made of platelet-VWF strings
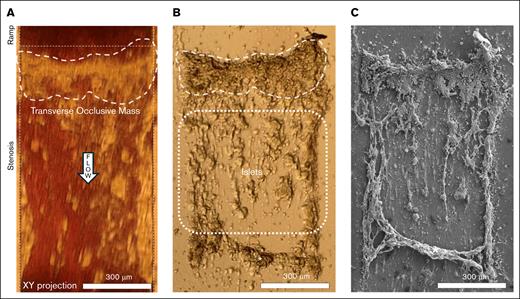
At occlusion, light microscopy showed that a 200-μm thick transverse mass formed at the upstream end of the stenosis, spanning the 640-μm wide stenotic test section (Figure 1A). After sample fixation, islets previously hidden by red blood cells became visible (Figure 1B). The contours of the thick occluding mass and islets downstream were visualized. A crosscurrent mass downstream of the islets appeared on light microscopy (marked with “∗” in Figure 1B).
Blood flow was impeded by a mass adopting the structure of a thick transverse cable. (A) Light microscopy showed the formation of a large transverse occlusive mass that spanned the entire cross-section (640 μm). Blood flow direction is indicated by the “flow” arrow. This mass was the most flow-resisting part of the thrombus. (B) Light microscopy of air-exposed thrombus showed islets attached to the glass slide downstream to the cable, previously obscured by blood. ∗A crosscurrent mass downstream to the islets, likely obscured by blood, could only be observed after glass slide removal. (C) Low-magnification SEM of thrombi revealed that the occlusive mass adopted the structure of a thick transverse cable unseen with light microscopy.
Blood flow was impeded by a mass adopting the structure of a thick transverse cable. (A) Light microscopy showed the formation of a large transverse occlusive mass that spanned the entire cross-section (640 μm). Blood flow direction is indicated by the “flow” arrow. This mass was the most flow-resisting part of the thrombus. (B) Light microscopy of air-exposed thrombus showed islets attached to the glass slide downstream to the cable, previously obscured by blood. ∗A crosscurrent mass downstream to the islets, likely obscured by blood, could only be observed after glass slide removal. (C) Low-magnification SEM of thrombi revealed that the occlusive mass adopted the structure of a thick transverse cable unseen with light microscopy.
SEM at low magnification revealed that the occluding mass was a transverse cable made of a fibrous structure not visualized well at the light microscopy resolution (Figure 1C). This 200-μm thick cable spanned the width of the stenotic chamber. Intermediate magnification showed that the transverse cable was concave in shape on the upstream side, likely from flow forces (Figure 2A). The cable thickness varied across its length (40-200 μm).
The structural hierarchy of the occlusive mass consisted of a 200-μm-thick transverse cable made of parallel strings of PLTs linked by 1 to 0.05-μm-thin yarns made up of VWF. (A) Intermediate-magnification SEM showed that the transverse cable spanning the width of the stenosis was concave in shape. The thickness of the cable varied across the channel (40-200 μm). (B) High-magnification SEM (dashed square in panel A) revealed beads linked with one another like strings on beads (dashed line). These beads were identified as PLTs based on their diameter (1-2 μm) and appearance. The strings were parallel to each other and oriented perpendicular to the flow, similar to the cable. (C) A PLT linked by fibers. The thinnest fiber detected by SEM had a diameter of 0.05 μm. (D) A PLT linked by thicker yarns (0.5-1 μm). This PLT appeared to be degranulated, indicating that the yarns of VWF may have come from PLT α-granules. (E) Confocal microscopy 40-μm-high z-slice of a separate and representative (n = 3) high-shear thrombus labeled for VWF (green), fibrin (red), and DNA (blue). The transverse cable was rich in VWF and lacked fibrin, illustrating that the strings of PLTs were linked by yarns of VWF. (F) Directionality analysis of the transverse cable confirmed the transverse orientation of the PLT-VWF strings. (G) Schematic of the structural hierarchy of the occlusive transverse cable as being composed of strings of PLTs joined by yarns of VWF fibers. PLT, platelet.
The structural hierarchy of the occlusive mass consisted of a 200-μm-thick transverse cable made of parallel strings of PLTs linked by 1 to 0.05-μm-thin yarns made up of VWF. (A) Intermediate-magnification SEM showed that the transverse cable spanning the width of the stenosis was concave in shape. The thickness of the cable varied across the channel (40-200 μm). (B) High-magnification SEM (dashed square in panel A) revealed beads linked with one another like strings on beads (dashed line). These beads were identified as PLTs based on their diameter (1-2 μm) and appearance. The strings were parallel to each other and oriented perpendicular to the flow, similar to the cable. (C) A PLT linked by fibers. The thinnest fiber detected by SEM had a diameter of 0.05 μm. (D) A PLT linked by thicker yarns (0.5-1 μm). This PLT appeared to be degranulated, indicating that the yarns of VWF may have come from PLT α-granules. (E) Confocal microscopy 40-μm-high z-slice of a separate and representative (n = 3) high-shear thrombus labeled for VWF (green), fibrin (red), and DNA (blue). The transverse cable was rich in VWF and lacked fibrin, illustrating that the strings of PLTs were linked by yarns of VWF. (F) Directionality analysis of the transverse cable confirmed the transverse orientation of the PLT-VWF strings. (G) Schematic of the structural hierarchy of the occlusive transverse cable as being composed of strings of PLTs joined by yarns of VWF fibers. PLT, platelet.
High-magnification SEM (Figure 2B) showed the transverse cable to be made of strings of beads (Figure 2B, white dashed line). The beads were identified as platelets based on their diameter (∼2 μm) and appearance.17 Although resting platelets have a discoid shape,18 here the platelets appeared spherical, spread and even fused (Figures 2B-D and 3A), suggesting an advanced state of activation.18 The strings were parallel to each other and oriented perpendicular to the flow, as shown by quantitative analysis of the directionality of the transverse cable (Figure 2F). WBCs were also identified in the cable based on their spherical shape and their diameter (8-12 μm).19
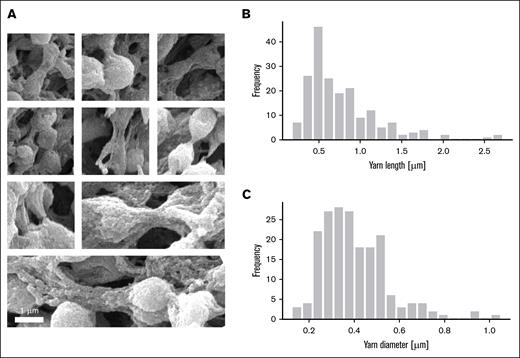
High-resolution SEM images enable measurements of the dimensions of yarns. (A) Mosaic of high-magnification SEM images showing a variety of yarns bridging adjacent platelets. (B) Distribution of measured yarn length. Average yarn length, 0.77 ± 0.44 μm (N = 191). (C) Distribution of measured yarn diameter. Average yarn diameter, 0.40 ± 0.15 μm (N = 191). Length and diameter were measured using ImageJ on SEM images.
High-resolution SEM images enable measurements of the dimensions of yarns. (A) Mosaic of high-magnification SEM images showing a variety of yarns bridging adjacent platelets. (B) Distribution of measured yarn length. Average yarn length, 0.77 ± 0.44 μm (N = 191). (C) Distribution of measured yarn diameter. Average yarn diameter, 0.40 ± 0.15 μm (N = 191). Length and diameter were measured using ImageJ on SEM images.
Even higher magnification showed that yarns linked platelets (Figure 2C-D). The smallest visible thickness of the yarns (0.05 μm) observed with SEM (Figure 2C) were ∼4000× thinner than the thickest portion of the transverse cable (200 μm). In summary, the transverse cable was made of long strings of platelets serially linked by yarns, and yarns were made of entangled fibers. A schematic of the transverse cable structural hierarchy spanning 3 orders of magnitude in size is shown in Figure 2G. A diagram of the structural hierarchy of the cable is provided in supplemental Figure 7.
The composition of yarns was determined by fluorescence microscopy. Thrombi were stained for VWF (green), fibrin(ogen) (red), and DNA (blue; Figure 2E). Immunofluorescence images revealed the high-shear thrombi to be rich in VWF while lacking fibrin. DNA staining only identified nuclei of WBCs without extracellular DNA. We concluded the strings to be platelet-VWF complexes. Thus, SEM and immunofluorescence showed that the thick (40-200 μm) occlusive transverse cable spanning the width of the channel (640 μm) was composed of parallel oriented platelet-VWF strings (1-2 μm), in which platelets were linked by yarns of VWF fibers (Figure 2G).
Mechanical analysis showed VWF-VWF bonding as the weakest link
The transverse cable must be of sufficient strength to withstand arterial pressures and occlude blood flow. The cable was made of VWF yarns linking platelets. The rupture strength of the cable thus of VWF yarns was measured to be 4.4 kPa,11 given as force per unit area. Using SEM images of yarns (Figure 3A), we found an average yarn diameter of 0.40 ± 0.15 μm (Figure 3C), therefore a cross-sectional area of 0.126 μm2. Thus, the rupture force of the average yarn is (4.4 kPa) × (0.126 μm2) = 550 pN. This macro rupture force was then compared to the following estimates derived from microscopic analysis also using SEM.
Single VWF fiber diameter is 4.4 nm.20 The average yarn diameter seen in SEM is 0.40 μm, so each yarn likely contains at least 90 VWF fibers (400/4.4; supplemental Figure 3A). This estimate is based on surface fibers visible in the SEM image. If each VWF fiber forms one or more evenly distributed bonds (supplemental Figure 4), then 90 fibers provide at least 90 bonds. Platelets form long-term bonds to VWF via αIIbβ3-C1 (∼50 pN per bond,21 supplemental Figure 2A) giving a rupture force of 4500 pN (90 × 50) which is greater than the yarn rupture force of 550 pN. In comparison, a VWF fiber can connect to another VWF fiber through hydrogen bonding estimated at ∼4.6 pN per bond22 (supplemental Figure 2B). VWF-VWF bonding would provide a rupture force of 414 pN (90 × 4.6). Thus, the VWF-VWF bonding is the weakest link in the chain. Note that the estimated 414 pN is less than the measured rupture force of 550 pN. To make up for this macro rupture force, VWF-VWF yarns would likely recruit more VWF-VWF bonds. A more complete analysis is proposed in the supplemental Methods and supplemental Figure 3.
The geometrical analysis (supplemental Figure 3A) suggested that yarn should be composed of on average at least 90 VWF fibers. Using that number of VWF fibers per yarn (90) as input, we were able to output the minimum volume fraction (1.1%) and the minimum mass concentration of VWF fibers (107 μg/mL) in the clot (supplemental Material). As the average mass concentration of VWF in blood is 10 μg/mL, the SIPA thrombus has at least 10 times more VWF than the concentration of soluble VWF in normal blood.
To estimate platelet density, we used the SEM-measured average yarn length using high-magnification images (Figure 3A) along geometrical considerations (supplemental Figure 4). The average yarn length (0.77 ± 0.44 μm; Figure 3B) was used to define a volume of reference. We then estimate the platelet density as 0.09/μm3.
WBCs were filtered by the transverse cable
We observed that WBCs were more prevalent on the upstream side of the thrombus than on the downstream side. We quantified this difference by counting the large cells (8-12 μm) on either side of the transverse cable (Figure 4A, red circles) with the dividing line positioned at the midpoint along the ridge of the cable. The transverse cable was divided into 10 slices, each 10 μm wide. The average number of WBCs per slice upstream was significantly higher than downstream (23.4 ± 8.4 vs 15.4 ± 6.6; P = .037; Figure 4B).23 Over the entire stenosis, WBCs were most dense on the transverse cable than elsewhere (19.4 ± 8.5 vs 2.3 ± 2.2; P = 1 × 10–11; Figure 4C). These results indicated that WBCs were caught by the transverse cable, predominantly on its upstream side, leading to a higher concentration there. We interpreted the upstream concentration to reflect that the transverse cable acted as a physical filter to capture large WBCs on the upstream side of the occluding thrombus. High-magnification images of WBCs were provided for illustrative purposes (Figure 4D-F).
The transverse cable was found to be dense with WBCs. (A) SEM of the transverse cable densely decorated with WBCs (red circles). (B) Density of WBCs on the thrombus. The upstream of the transverse cable was denser in WBCs (mean ± standard deviation [SD], n = 10 for both upstream and downstream; t(18) = 2.25; P = .037, 2-tailed t test). (C) Density of WBCs on the transverse cable per 10μm slice. The transverse cable was rich in WBCs, whereas the rest of the thrombus area was sparce of WBCs (mean ± SD, n = 20 for transverse cable and n = 76 for rest; P = 1 × 10–11, Mann-Whitney U test), indicating that the cable filtered WBCs like a net. (D) High-magnification images showing upstream WBCs on the cable; (E) downstream on the cable; and (F) in the rest region, where WBC is attached to collagen and an islet.
The transverse cable was found to be dense with WBCs. (A) SEM of the transverse cable densely decorated with WBCs (red circles). (B) Density of WBCs on the thrombus. The upstream of the transverse cable was denser in WBCs (mean ± standard deviation [SD], n = 10 for both upstream and downstream; t(18) = 2.25; P = .037, 2-tailed t test). (C) Density of WBCs on the transverse cable per 10μm slice. The transverse cable was rich in WBCs, whereas the rest of the thrombus area was sparce of WBCs (mean ± SD, n = 20 for transverse cable and n = 76 for rest; P = 1 × 10–11, Mann-Whitney U test), indicating that the cable filtered WBCs like a net. (D) High-magnification images showing upstream WBCs on the cable; (E) downstream on the cable; and (F) in the rest region, where WBC is attached to collagen and an islet.
Islets merged transversally via platelet-VWF strings
Light microscopy images showed the presence of islets downstream to the transverse cable (Figure 1B). Platelets were connected by long yarns of VWF immobilized on the collagen surface (Figure 5A). The platelet marked with asterisk is on top of an agglomerate (∼5 platelets) and has finger-like projections, likely filopodia, resembling spread and fused platelets in a highly activated state.18 Larger agglomerates with dozens of platelets were observed (Figure 5B). Platelets’ membrane looked perforated, characteristic of activation and degranulation.24 Larger islets (>15 μm) with hundreds to thousands of platelets were round and could reach 50 μm in diameter (Figure 5C), providing a greater thrombogenic surface for capturing blood cells.
Islets downstream of the occluding cable could merge transversally. SEM images show various morphologies of aggregates spread on the collagen layer. (A) Platelet (marked with “∗”) on top of yarn oriented in the flow direction, from flow-elongated VWF immobilized on the collagen layer. (B) Aggregates of platelets forming a short, rounded islet. Most platelets appeared in a degranulated state, similar to the one shown in the dashed square, indicative of platelet α-granule release. (C) Larger rounded islet showing uniform growth of an islet. (D) Islet linked to a cable made of platelet-VWF strings (straight dashed lines), likely the result of streamwise growth induced by flow forces. (E) Two islets that merged in the streamwise direction. (F) Two islets that merged in the transverse direction, possibly representing mechanism for the transverse cable growth. Islets came in various size (longest dimension: 20-100 μm). In panels A-F, a uniform looking material (black arrows in images) was spread on the glass slide and attached to the foundation of agglomerates or islets, likely spread platelets.
Islets downstream of the occluding cable could merge transversally. SEM images show various morphologies of aggregates spread on the collagen layer. (A) Platelet (marked with “∗”) on top of yarn oriented in the flow direction, from flow-elongated VWF immobilized on the collagen layer. (B) Aggregates of platelets forming a short, rounded islet. Most platelets appeared in a degranulated state, similar to the one shown in the dashed square, indicative of platelet α-granule release. (C) Larger rounded islet showing uniform growth of an islet. (D) Islet linked to a cable made of platelet-VWF strings (straight dashed lines), likely the result of streamwise growth induced by flow forces. (E) Two islets that merged in the streamwise direction. (F) Two islets that merged in the transverse direction, possibly representing mechanism for the transverse cable growth. Islets came in various size (longest dimension: 20-100 μm). In panels A-F, a uniform looking material (black arrows in images) was spread on the glass slide and attached to the foundation of agglomerates or islets, likely spread platelets.
Islets were seen either isolated (Figure 5C), grown (Figure 5D), or merged (Figure 5E-F). Some islets were trailed by thin streamwise cables (Figure 5D) that we interpreted to stem from VWF released by platelet α-granules.25 These islets likely served as posts for released VWF to tether and extend with flow, capturing platelets to form platelet-VWF strings. We named this mechanism “streamwise growth” (Figure 5D). Islets were observed merged streamwise (Figure 5E) or transversely (Figure 5F), linked by platelet-VWF cables aligned with the merging direction. We named these mechanisms “streamwise merging” and “transverse merging.”
We interpret the images of platelets, agglomerates, and then islets to be snapshots of the transverse cable growth, as emphasized by black arrows between images (Figure 5). Specifically, the transverse merging of islets (Figure 5F) appears to be the mechanism for growth of the transverse cable.
A second macroscopic structure resembled a rope with few platelets made of woven strands of thinner yarns of VWF fibers. (A) Low-magnification SEM revealed that the crosscurrent mass adopted the structure of a rope made of strands of yarn (black arrows; diameter 10-15 μm) suspended above the glass slide. We surmise this rope formed in the streamwise direction at the edge of the chamber and was dislodged transversally after one side broke. (B) Higher magnification revealed a structure abundant in yarns of VWF, with few platelets compared to the occlusive transverse cable seen in Figure 4A. (C) SEM of representative example of the densest region of sheared (15 000/s; 10 minutes) collagen fibers in separate flow experiments without whole blood (n = 2). (D) Schematic of the structural hierarchy of the rope structure.
A second macroscopic structure resembled a rope with few platelets made of woven strands of thinner yarns of VWF fibers. (A) Low-magnification SEM revealed that the crosscurrent mass adopted the structure of a rope made of strands of yarn (black arrows; diameter 10-15 μm) suspended above the glass slide. We surmise this rope formed in the streamwise direction at the edge of the chamber and was dislodged transversally after one side broke. (B) Higher magnification revealed a structure abundant in yarns of VWF, with few platelets compared to the occlusive transverse cable seen in Figure 4A. (C) SEM of representative example of the densest region of sheared (15 000/s; 10 minutes) collagen fibers in separate flow experiments without whole blood (n = 2). (D) Schematic of the structural hierarchy of the rope structure.
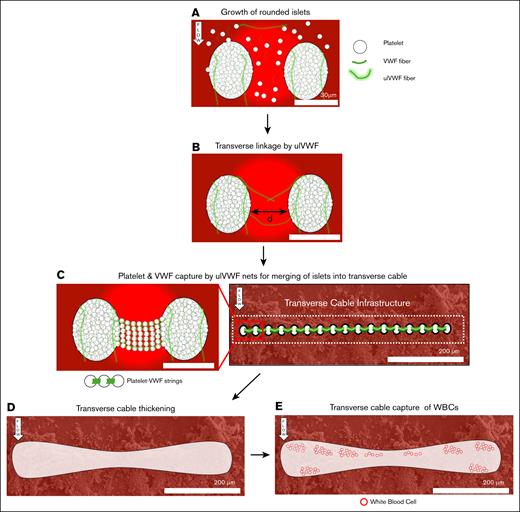
Proposed mechanism for the growth of the strong occlusive transverse cable. (A) Flowing aggregates of platelets and soluble VWF are captured on the collagen-coated glass slide forming rounded islets. ulVWF released by shear-activated platelets enhance growth of islets by catching more platelets. (B) Close islets are then linked by multiple, joined VWF fibers or ulVWF. We expect the maximum distance between 2 islets to be less than twice the length of ulVWFs (distance < 30 μm). (C) The transverse linkage between 2 islets forms a net that traps incoming platelets and VWF, aligning them with the linkage to form platelet-VWF strings. Islets located at the entrance of the stenosis merge transversally with one another and form the transverse cable infrastructure. (D) The transverse cable thickens likely by streamwise growth and streamwise merging. (E) The thicker transverse cable then physically arrests incoming WBCs.
Proposed mechanism for the growth of the strong occlusive transverse cable. (A) Flowing aggregates of platelets and soluble VWF are captured on the collagen-coated glass slide forming rounded islets. ulVWF released by shear-activated platelets enhance growth of islets by catching more platelets. (B) Close islets are then linked by multiple, joined VWF fibers or ulVWF. We expect the maximum distance between 2 islets to be less than twice the length of ulVWFs (distance < 30 μm). (C) The transverse linkage between 2 islets forms a net that traps incoming platelets and VWF, aligning them with the linkage to form platelet-VWF strings. Islets located at the entrance of the stenosis merge transversally with one another and form the transverse cable infrastructure. (D) The transverse cable thickens likely by streamwise growth and streamwise merging. (E) The thicker transverse cable then physically arrests incoming WBCs.
High-magnification SEM allowed us to look more closely at islet. Islets merged with each other (Figure 5E-F) via short cables (supplemental Figure 5A) of platelets (supplemental Figure 5B) joined by yarns (supplemental Figure 5C). Those linking islets were oriented streamwise (supplemental Figure 5A, top) or transversely (supplemental Figure 5A, bottom). Fluorescence microscopy demonstrated the yarns to be made of VWF and not fibrin or DNA (supplemental Figure 5G). A platelet-VWF string was identified (supplemental Figure 5E) as 2 platelets (white arrows) connected by yarn of VWF fibers (black arrows). Analysis of the fluorescence showed that VWF was more concentrated at the platelets (white arrows) than in the middle of the yarns (black arrows). Platelets were not permeabilized, thus VWF staining likely marked mostly surface fibers, indicating VWF wrapped around each platelet.
A second large structure resembling a rope formed in high-shear flow
A crosscurrent mass downstream was observed (marked with “∗”, Figure 1B). Although not seen in other SIPA thrombi, we acknowledge this alternative woven structure with few platelets (Figure 6). This crosscurrent mass resembled a loose rope made of 2 woven thick strands (black arrows, 10-20 μm diameter) of thinner yarn (0.5-1 μm; Figure 6A). This rope appeared suspended, highlighted by bright contrast above and below the rope. The rope likely formed in the streamwise direction at the edge of the chamber and reoriented transversally after the upstream head detached. Only a few platelets were visible in the central part of the rope (Figure 6B). The woven thick strands were composed of entangled yarns of VWF fibers that likely self-associated to form this 600-μm-long structure. Note that the collagen fibers on the glass surface had a different appearance (Figure 6C). Fibrillar collagen could not match the yarn density. The structural hierarchy of the rope is shown in Figure 6D and compared to the cable in supplemental Figure 7.
SIPA thrombi formed with high heparin
The occluding thrombus formed with high heparin concentration exhibited the same appearance as the low heparin thrombus presented so far in this paper (supplemental Figure 6). The stenosis was occluded by a transverse cable made of platelets joined by VWF yarns (supplemental Figure 6A-B). Thinner cables joining islets were observed. The platelet-VWF strings composing the transverse cable were oriented perpendicular to the flow (supplemental Figure 6C). WBCs were denser at the upstream side of the transverse cable (supplemental Figure 6D), and denser at the transverse cable vs the rest (supplemental Figure 6E). However, no rope was seen in this specimen. In summary, a high concentration of heparin did not alter the structural hierarchy of SIPA thrombi, and the fluorescence study confirmed sparse fibrin and DNA as the structural components in SIPA thrombi. These results indicate that SIPA occlusions can form independently of blood coagulation and NETosis.
Discussion
This study investigated the microscopic structure of arterial-like thrombi formed in high-shear flow in microfluidics from heparinized whole human blood. This is the first study to expose the structural hierarchy of in vitro SIPA thrombi at the submicron scale, spanning 3 orders of magnitude. SEM revealed that the occlusive mass of thrombi was organized into a transverse cable (∼200 μm) made of hundreds of parallel strings of beads (∼2 μm) joined by yarns (∼0.5 μm). Beads were identified as platelets due to their size and appearance. The ultra-high density of platelets found in this study (0.09/μm3) was similar to that found in a life-sized artery in vitro high-shear thrombus SEM10 (0.05/μm3). Yarns were identified as entangled VWF fibers using immunofluorescence labeling. The lack of fibrin or DNA on immunofluorescence images and no change in appearance with high-dose heparin further verify the yarns as VWF.
The analysis of the transverse cable structural hierarchy (cable, string, yarn, or fiber) (Figure 2) and the morphologies of islet aggregates (Figure 5) hint at the mechanism of formation and growth (Figure 7). First, agglomerates of platelets and ultra large VWF (ulVWF) are captured on the collagen layer. Platelet accumulation can occur within milliseconds in stenotic arteries.26 Second, closely spaced islets may be spanned by ulVWF which can reach 15μm in length.27 Transverse linkage is further helped by islets growing laterally via platelet capture. Third, ulVWF bridging islets at the entrance of the stenosis form a net capturing more platelets and VWF. Islets coalesce this way to initiate transverse cable formation. Fourth, thickening of the transverse cable occurs by merging or by capturing more platelets and VWF. Fifth, the cable can grow to span the whole cross-section, enough to capture WBCs. This proposed mechanism suggests that “Transverse Merging” arises from the bridging of closely spaced islets by ulVWF. Platelets alpha granules are necessary for SIPA occlusion in a mouse carotid model.25 Thus, occlusion is not reached if platelets are depleted of their ulVWF. Future work should combine SEM, immunofluorescence, and computational fluid dynamics to test the “Transverse Merging” hypothesis. This growth mechanism may be extended to the general occlusion of other adhesive colloids and beads under high shear forming a robust structure.
A similar cable structure was seen in mouse hemostatic plugs in Tomaiuolo et al,28 but most closely resembles the structure in SEM pictures from Wu et al.3 They showed platelets aggregates formed under intermediate shear rate (2600/s) from blood of patients with VWD with recombinant or mutant VWF. The links connecting their aggregates resemble our cables. They showed that with mutant VWF lacking the A1 domain, platelet aggregates did not form, emphasizing the platelet-VWF GP1b-A1 axis for high-shear thrombus formation. In Wu et al,3 high-dose heparin (20 IU/mL) ruled out fibrin therefore supporting that the thick cables observed on our SEM images are platelet-VWF strings. The platelet-VWF string structure had been described by others29-31 and have been reported as longer than 500 μm.
In addition to the GP1b-A1 axis, the platelet-VWF αIIbβ3-C1 axis is crucial for occlusion, as αIIbβ3 inhibition with eptifibatide32 or abciximab33 prevented occlusive thrombi formation for over 25 minutes under high shear (>6000/s) with heparinized blood. N-acetyl-cysteine targeting VWF disulfide-rich structures similarly prevented occlusion of a parallel-plate microfluidic chamber by high-shear thrombus.34
The cable structure described here also resembles the VWF fibers captured on microposts reported by Herbig et al.35 Our woven VWF strands and their VWF fibers formed under high shear rates have a similar appearance. Their micro-post structure was 10 to 20 μm wide, the width of the VWF strands seen in our SEM images. Although their VWF structure formed within 5 minutes, our occlusive cable formed under 2 minutes likely accelerated by platelet volume of platelets and the platelet internal store of VWF. Nonetheless, both their VWF fibers and our cable rely on VWF self-association, reinforcing the idea that VWF-VWF bonding can create thick and long structures within seconds. A key difference between our SIPA thrombi and other previously reported VWF complexes elongated in the flow direction4,30,31,35 is that their structures remain nonocclusive; whereas, in our case, platelet-released ulVWF enables channel occlusion within 2 minutes.
WBCs were dense in SIPA thrombi and concentrated on the upstream side of the transverse cable, suggesting it filtered and captured WBCs. Platelet α-granules express P-selectin36 which binds WBC P-selectin glycoprotein ligand-1.37 Platelets appeared highly activated and degranulated in our SEM images, suggesting a concentration burst of released α-granules in the vicinity of the thrombus that could lead to WBC arrest. Neutrophil extracellular traps (NETs) were not visualized by SEM nor detected on fluorescence images. Possibly, NETs were lysed by heparin.15 Without heparin, NETs could promote faster platelet-VWF–rich occlusive cable formation by capturing platelets15 and binding to VWF A1 domain.38 In addition, neither fibrin nor NETs were seen here. Both the appearance of NETs and formation of fibrin are beyond the time scale of this study. Fibrin forming over a timescale >2 minutes may interact with NETs in aging thrombi (>30 minutes),39 as modulation of NETosis by fibrin has been described previously.40 Still, WBCs presence upstream of the transverse cable was consistent ex vivo observations: WBCs accumulate in platelet-rich areas or at the border between RBC-rich and platelet-rich areas.41 This differential concentration of WBC could be used to orient harvested thrombi as to upstream and downstream orientation.
High-resolution SEM images revealed that VWF yarns in SIPA thrombi align in the direction of principal stress, which in solid mechanics is widely accepted to optimize a material’s resistance to breakage.42 SIPA thrombi formed in large stenotic glass capillaries also adopted a transverse structure to resist shear forces and pressure.10 Using a prior result on SIPA thrombi breakage strength11 and yarn dimensions measured in this study, we analyzed the thrombus rupture limit. We investigated 2 modes of rupture: rupture of platelet-VWF αIIbβ3-C1 bonds on the platelet surface, or rupture of VWF-VWF bonds within the yarns. The analysis showed that the VWF-VWF bond is likely to be the weakest link in SIPA thrombi. Although stability was thought to be rely on platelet-VWF bonds as with αIIbβ3,8 our findings suggest VWF-VWF bonds and yarns of VWF to be as crucial for thrombus strength. VWF-VWF bonding and VWF yarns represent a potential target for strategies aimed at breaking down arterial thrombi. N,N’-diacetyl-L-cystine can disrupt VWF disulfide bonds and has been shown to induce lysis and in vitro recanalization of SIPA thrombi.43 Also, an anti-VWF aptamer inhibiting VWF-mediated platelet adhesion is being developed for treatment of arterial thrombosis.44
A limitation of the current study was the absence of a comparison of SIPA thrombi structure made without heparin. Using selective inhibitors like DNase-1 or PPACK would help reveal the role of NETs, respectively, on SIPA thrombi structure. We expect slight changes in structural hierarchy or in occlusion time, since VWF fibers were sufficient to quickly form a robust occlusive structure. A second limitation was the rectangular geometry of the in vitro model and its 50-μm chamber height. Kim et al used a life-size artery model with whole blood flowing at high shear rate to form a 2.5-mm-thick macroscopic clot.10 SEM images of this large clot lacked the resolution and details seen here. Our 50-μm-high parallel-plate microfluidic channel yielded exquisitely high-resolution images of the cable structure. Our single-surface collagen model mimics the first 50 μm of growth of the 2.5-mm-thick macroscopic clot, corresponding to a chord of the circular section at the wall of a large cylindrical artery. Our microfluidic thrombus shares the platelet density and the structure of cables seen in the large artery model. A last limitation is in our mechanical analysis and is the assumption that every fiber in yarns contributes to their strength. Some VWF fibers may merely decorate platelets leading to fewer VWF-platelet bonds. In this case, platelet-VWF bonding may be limiting. We think that the possibility of having large amounts of noncontributing fibers to be possible but unlikely.
In conclusion, we describe the structural hierarchy of platelet-VWF thrombi formed under pathologically high shear rates in a microfluidic model. Our results show that the SIPA thrombus has a structure of surface aggregates of platelets connected by many VWF fibers. The platelet-VWF strings can span adjacent islands into a large cable structure that occludes flow. The macroscopic cable structure is highly organized along lines of tension that would provide linear strength over large distances. It is amazing that this occluding structure self-organizes in minutes to provide sufficient strength to occlude arteries under high systolic pressures. Our results show that SIPA thrombus strength depends heavily on the VWF-VWF bonds, suggesting a novel target for thrombolysis in the treatment of arterial thrombosis.
Authorship
Contribution: E.M., D.K., J.W.W., R.I.L., and C.N. conceptualized the study; E.M., D.K., J.W.W., R.I.L., and C.N. developed the methodology; E.M. and C.N. performed the investigation; E.M., D.K., J.W.W., R.I.L., and C.N. visualized the data; D.K., J.W.W., and R.I.L. supervised the study; E.M. and D.K. wrote the original draft; and E.M., D.K., J.W.W., R.I.L., and C.N. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Ku, George W. Woodruff School of Mechanical Engineering, Georgia Institute of Technology, 315 Ferst Dr NW, Atlanta, GA 30332; email: david.ku@me.gatech.edu.
References
Author notes
Original data are available upon reasonable request from the corresponding author, David Ku (david.ku@me.gatech.edu).
The full-text version of this article contains a data supplement.


![The transverse cable was found to be dense with WBCs. (A) SEM of the transverse cable densely decorated with WBCs (red circles). (B) Density of WBCs on the thrombus. The upstream of the transverse cable was denser in WBCs (mean ± standard deviation [SD], n = 10 for both upstream and downstream; t(18) = 2.25; P = .037, 2-tailed t test). (C) Density of WBCs on the transverse cable per 10μm slice. The transverse cable was rich in WBCs, whereas the rest of the thrombus area was sparce of WBCs (mean ± SD, n = 20 for transverse cable and n = 76 for rest; P = 1 × 10–11, Mann-Whitney U test), indicating that the cable filtered WBCs like a net. (D) High-magnification images showing upstream WBCs on the cable; (E) downstream on the cable; and (F) in the rest region, where WBC is attached to collagen and an islet.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/23/10.1182_bloodadvances.2025017354/1/m_blooda_adv-2025-017354-gr4.jpeg?Expires=1767203285&Signature=y8D7TsawrRiLa3g~FdGMk7bXI1NxTvPSKNMJve2cZ7epBsiOUDtzbd8CAZLnD8jypKNViYg29tTJGUg~eBVYp7TENs~vpbutVVV9cThvHdYwI81vaF472~32dILa3QAz02LRyQ3gBfGotnsCUAhx9MnLru1aM-tlBIWwGIlsuqrxjb60tVp~Dr9HbX4cORGR8pSY1LhJBfHJlGBycYdRXwsxus2hfUCAHQyGhv6fuCX2Rnv5IP~T0sgxRDc6FYG8sVWATnnDP1AS6DTgb~2qQ0YYHGKHPRuInPqtPSnj8SEGVi~1mxij9AD3BXw2MGHnH5Tu~aOdiKo-twxS4fc7tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)