In this issue of Blood Advances, Machenaud et al1 uncover in their recent work that the occlusive portion of shear-induced thrombi formed under flow consists of a thick transverse platelet–von Willebrand factor (VWF) cable structure that spans across the vessel lumen. The described platelet-VWF cable structure is a well-organized, hierarchical structure that is shown to be valuable in order to resist arterial pressure and stop flow.1 This further reframes arterial thrombus formation under high shear as a mechanically self-assembled, VWF-driven process, distinct from classical coagulation.
Thrombosis is classically understood through Virchow’s triad, which implicates blood stasis, endothelial injury, and hypercoagulability as the primary contributors to clot formation. These factors drive coagulation and result in fibrin-rich thrombi, stabilized by thrombin activity. However, in the arterial circulation, where shear rates are high, a second mechanism, shear-induced platelet aggregation (SIPA), dominates. SIPA is initiated when vascular injury exposes subendothelial collagen, causing VWF to unfold and bind to platelet glycoprotein Ib (GP1b) under shear rates exceeding 15 000 per second. This interaction activates platelets and promotes the release of ultralarge VWF (ULVWF) from platelet α-granules, amplifying local platelet aggregation. SIPA thrombi form rapidly, within minutes, and are rich in platelets and VWF but poor in fibrin.2,3 In spite of the high clinical relevance of SIPA in arterial thrombosis, such as myocardial infarction, ischemic stroke, and stent thrombosis, the structural design and mechanics underlying SIPA thrombi and their ability to resist arterial pressure had not been fully described until now.
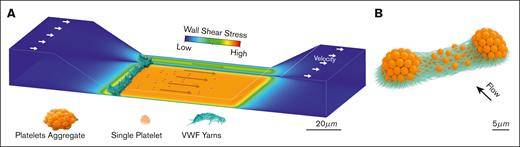
To investigate the structural basis of SIPA occlusion, Machenaud et al employed a microfluidic model of arterial stenosis (see figure panel A), perfused with low dose heparinized human blood. Scanning electron microscopy (SEM) and immunofluorescence imaging revealed that occlusion resulted from a transverse cable composed of platelet strings (1-2 μm in diameter) joined by VWF yarns (∼0.4 μm), themselves made of nanometer-scale VWF fibers (∼4.4 nm). The results showed a clear structural hierarchy: fibers → yarns → strings → cable. Importantly, no fibrin or extracellular DNA was detected, and high-dose heparin did not alter thrombus structure, confirming that formation occurred independently of coagulation and neutrophil extracellular trap formation (NETosis). Mechanical analysis by Machenaud et al identified VWF–VWF bonds (∼4.6 pN) as the weakest link in the structural hierarchy, in contrast to stronger platelet–VWF αIIbβ3 interactions (∼50 pN).4,5 With an estimated rupture force of ∼550 pN per yarn, the data suggest that dozens to hundreds of VWF–VWF interactions work in parallel to sustain the cable’s mechanical stability. White blood cells were seen accumulated on the upstream side of the thrombus, indicating that the cable also functions as a physical filter.
Shear-dependent assembly of a transverse VWF–platelet cable in a stenosed microchannel. (A) Schematic illustrating left-to-right flow through a stenotic channel mimicking an artery, with associated high-shear thrombi. Channel walls are color-mapped by wall shear stress (blue, low; red, high), with peaks and steep gradients at the constriction slopes and throat. At the upstream edge, a large transverse occlusive mass spans the entire cross-section, composed of parallel platelet strings joined by VWF fiber “yarns.” (B) Schematic showing 2 platelet aggregates (orange), at the stenosis entrance, bridged by dense bundles of ULVWF fibers. These fibers form a high surface-area scaffold that tethers platelets and connects aggregates into a continuous transverse cable, resisting hydrodynamic drag and promoting luminal occlusion under high shear.
Shear-dependent assembly of a transverse VWF–platelet cable in a stenosed microchannel. (A) Schematic illustrating left-to-right flow through a stenotic channel mimicking an artery, with associated high-shear thrombi. Channel walls are color-mapped by wall shear stress (blue, low; red, high), with peaks and steep gradients at the constriction slopes and throat. At the upstream edge, a large transverse occlusive mass spans the entire cross-section, composed of parallel platelet strings joined by VWF fiber “yarns.” (B) Schematic showing 2 platelet aggregates (orange), at the stenosis entrance, bridged by dense bundles of ULVWF fibers. These fibers form a high surface-area scaffold that tethers platelets and connects aggregates into a continuous transverse cable, resisting hydrodynamic drag and promoting luminal occlusion under high shear.
The authors propose a “transverse merging” mechanism of growth. Initially, platelet islets form on collagen and are linked by ULVWF when in proximity (∼30 μm). These lateral connections grow into a full-width cable (see figure panel B). Although streamwise growth was observed, only transverse merging produced occlusion. SEM showed activated, degranulated platelets consistent with ULVWF release. These observations build on prior work. Wu et al3 demonstrated that VWF–GP1b binding is essential for thrombus formation under shear. Kim et al6 showed that SIPA clots resist arterial pressures even in the absence of fibrin. Tomaiuolo et al7 described fibrous platelet networks in hemostatic plugs. Machenaud et al now provide structural information that may offer an explanation—a transverse VWF-platelet cable spanning the lumen.
Therapeutically, these findings highlight new targets for arterial thrombosis, including clinically important conditions such as myocardial infarction, ischemic stroke, stent thrombosis, and other high-shear vascular occlusions. Disrupting VWF–VWF interactions, for example, with N-acetylcysteine (NAC) or its dimeric form (DiNAC), may offer a novel approach to clot dissolution without interfering with the coagulation cascade. DiNAC has been shown to reduce disulfide bonding within VWF and promote thrombus lysis and recanalization in vitro.8 Similarly, anti-VWF aptamers, such as BB-031, inhibit VWF-mediated platelet binding and have demonstrated dose-dependent thrombolysis under high shear flow.9 Another interesting therapeutic opportunity is the use of recombinant ADAMTS13, which enzymatically cleaves ULVWF multimers and has shown efficacy in degrading VWF-rich thrombi in microfluidic and animal models.10 However, once VWF is incorporated into densely packed fibrous cables, as observed in the study by Machenaud et al, the accessibility of cleavage sites may be limited. The structural insights presented in this study support a broader therapeutic concept: targeting the mechanical integrity of thrombi, not just coagulation enzymes.
One important limitation of this study is the use of heparinized human blood, which, although necessary to prevent coagulation, suppresses thrombin activity and fibrin formation. As a result, the role of fibrin and the coagulation cascade in stabilizing or remodeling thrombi cannot be assessed in this model. In addition, the study presents only a static snapshot of the thrombus at the point of occlusion, without capturing how structural features evolve over time. The lack of time-resolved data limits understanding of the kinetics of thrombus formation, including the sequence of platelet islet formation, VWF bridging, and cable assembly. A time-lapse experiment could offer critical insight into the dynamics of SIPA. Furthermore, although the microfluidic system effectively models high shear conditions, it does not replicate the size, structural complexity, or flow conditions found in large arteries, which may affect thrombus morphology and resistance to flow in vivo. Lastly, although the authors identify VWF–VWF bonds as a mechanical weak point and propose potential therapeutic targets, no pharmacologic interventions were tested experimentally, leaving translational applicability to be explored in future studies.
In summary, Machenaud et al reveal a robust, noncoagulant mechanism for arterial occlusion based mainly on platelet and VWF interactions, with a unique hierarchical structure. The observation of a mechanically aligned transverse cable challenges classical views and provides a foundation for developing safer, targeted arterial-clot therapeutics.
Conflict-of-interest disclosure: The authors declare no competing financial interests.