TO THE EDITOR:
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder caused by severe deficiency of ADAMTS13—a von Willebrand factor-cleaving protease—leading to accumulation of ultra-large von Willebrand factor multimers and platelet-rich thrombi. Traditionally described by the pentad of thrombocytopenia, microangiopathic hemolytic anemia, neurologic symptoms, renal dysfunction, and fever, this full constellation is now rarely seen.1 Microangiopathic hemolytic anemia and thrombocytopenia (typically severe; median platelet count, 10 × 109/L to 17 × 109/L) are the most common presenting features.2
In response to a case of TTP presenting with stroke and near-normal platelets, we implemented a single-center screening initiative for ADAMTS13 deficiency in cryptogenic stroke.
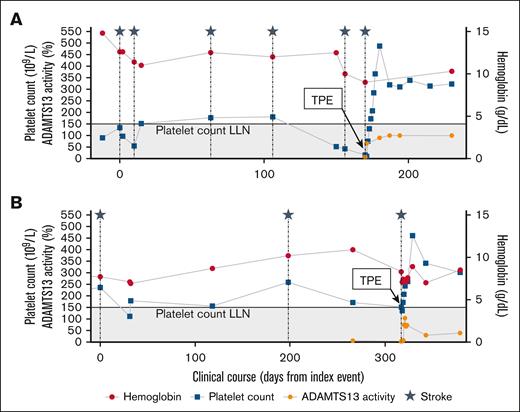
The index case, a 34-year-old woman with iron deficiency anemia, type 2 diabetes, taking an estrogen-containing oral contraceptive, presented with right-sided weakness following a laparoscopic cholecystectomy. Brain imaging revealed bilateral infarcts. Complete blood count revealed mild thrombocytopenia (white blood cells 9 × 109/L, hemoglobin 13.8 g/dL, and platelets 134 × 109/L). Peripheral blood smear showed no schistocytes, creatinine was normal (0.7 mg/dL), and total bilirubin was within reference range (1.1 mg/dL). Evaluation, including prolonged rhythm monitoring, echocardiography, vascular imaging, and laboratory studies for hypercoagulable/inflammatory conditions, was unrevealing. Aspirin was started, and she was instructed to discontinue estrogen. Over the next 5 months, she had 4 additional strokes despite anticoagulation. At presentation for these events, laboratory studies showed platelet counts of 43 × 109/L to 181 × 109/L, low ferritin, and normal bilirubin and lactate dehydrogenase (LDH) values (available for 1 of 4 presentations); haptoglobin was not obtained. On her sixth presentation with acute stroke, the patient was found to have laboratory abnormalities concerning for TTP (platelets 16 × 109/L; hemoglobin 8.9 g/dL; 1-4 schistocytes per high-power field; elevated LDH [325 U/L]; undetectable haptoglobin). ADAMTS13 activity was 8% (fluorescence resonance transfer assay), with a negative inhibitor assay and low-positive antibody (15 U; upper limit of normal, 12 U). She was treated with 5 sessions of therapeutic plasma exchange (TPE), corticosteroids, and rituximab. Genetic testing for congenital TTP was negative, and she has remained in clinical and ADAMTS13 remission (ADAMTS13 >lower limit of normal3) without additional strokes for 18 months (Figure 1A).
Clinical courses of index (A) and screen-positive (B) patients. The gray shaded region indicates a platelet count of <150 × 109/L. Initiation of plasma exchange is noted. Both patients were also treated with corticosteroids and rituximab when the diagnosis of TTP was established. ECG, electrocardiogram; ECHO, echocardiogram; LLN, lower limit of normal; TPE, therapeutic plasma exchange.
Clinical courses of index (A) and screen-positive (B) patients. The gray shaded region indicates a platelet count of <150 × 109/L. Initiation of plasma exchange is noted. Both patients were also treated with corticosteroids and rituximab when the diagnosis of TTP was established. ECG, electrocardiogram; ECHO, echocardiogram; LLN, lower limit of normal; TPE, therapeutic plasma exchange.
This case contributes to an emerging recognition that TTP may present as stroke with absent or only subtle hematologic abnormalities.4-7 Matthews et al reported on 4 similar cases and identified 8 additional cases in the literature.6 All patients in this series, like our patient, were 50 years or younger (22-50) and had a platelet count <300 × 109/L (125 × 109/L to 256 × 109/L).6 Current guidelines for cryptogenic stroke emphasize evaluation for cardioembolic and large-vessel sources through brain imaging, vascular imaging, echocardiography, prolonged rhythm monitoring, and laboratory studies for hypercoagulable or inflammatory conditions.8 ADAMTS13 deficiency is not routinely considered, and hematologists are rarely involved in these workups. Given ADAMTS13 testing is now widely available, reasonably priced (∼$390 at our center), and that TTP is a treatable condition with high morbidity if missed, we partnered with our neurology colleagues to implement a screening strategy.
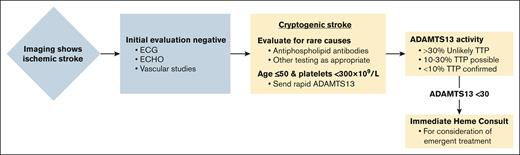
We advised ADAMTS13 testing in the evaluation of stroke after negative cardiac (electrocardiagrams and echocardiograms) and vascular imaging for individuals ≤50 years with platelet counts <300 × 109/L (Figure 2). We suggested hematology consultation for ADAMTS13 activities <30% as levels in the intermediate range (10%-30%) are common in other disorders, including sepsis, advanced liver disease, or disseminated intravascular coagulation, but do not fully exclude TTP.3,9,10 Interpretation and treatment were left to the consulting hematologist.
Algorithm for incorporating ADAMTS13 testing in the workup of cryptogenic stroke.
Algorithm for incorporating ADAMTS13 testing in the workup of cryptogenic stroke.
Institutional review board approval was obtained for retrospective review of screening results. Between August 2024 and April 2025, 6 patients (3/6 female) with cryptogenic stroke were screened for ADAMTS13 deficiency. They were between 39 to 55 years old and had platelet counts between 171 × 109/L to 407 × 109/L at presentation. One case of TTP was uncovered (Figure 1B). All other screened patients had ADAMTS13 activities >100%.
The screen-positive case, a 49-year-old woman with a history of renal artery thrombosis and myocardial infarction, presented with neurologic symptoms. Brain imaging showed multifocal cerebral infarcts. Initial evaluation was unrevealing for arrhythmia, structural cardiac disease, vascular abnormalities, or antiphospholipid antibodies. Given her prior thrombotic history, she was started on anticoagulation. Despite this, she experienced another stroke. Aspirin was added, and she was referred to our center. Her most recent complete blood count was notable for anemia (white blood cells 7.44 × 109/L, hemoglobin 8.8 g/dL, platelets 175 × 109/L). A recent ferritin was 12 ng/mL. Per our screening algorithm (cryptogenic stroke, age <50 years, platelet count <300 × 109/L), ADAMTS13 testing was recommended. Testing, delayed by challenges with local coordination, revealed severely reduced ADAMTS13 activity (6.6% by liquid chromatography–tandem mass spectrometry). Before treatment was initiated, she had another stroke. Platelets were 152 × 109/L, and hemolysis markers were equivocal (haptoglobin 77 mg/dL, bilirubin 1.1 mg/dL, LDH 167 U/L, 1-4 schistocytes/high-power-field), and creatinine 0.91 mg/dL. Given the lack of an alternative cause for severe ADAMTS13 deficiency and recurrent strokes, treatment was initiated for presumed immune TTP with TPE, corticosteroids, and rituximab. Repeat ADAMTS13 activity before TPE was 3.1%. ADAMTS13 inhibitor and antibody testing were negative. She underwent 5 sessions of TPE, resulting in a prompt rise in platelet count (152 × 109/L to 461 × 109/L). ADAMTS13 activity has remained above 30% on monthly monitoring (30.2% and 39.5% on day 20 and 56 after TPE completion), with no further ischemic events for over 3 months (Figure 1B). Genetic testing showed a variant of undetermined significance in ADAMTS13 (NM139025.4:c.3853C>T, p.Arg1285Trp). A single variant is not sufficient to cause congenital TTP but may reduce baseline ADAMTS13 activity.11
Although both our cases had negative inhibitor testing and only 1 had a detectable anti-ADAMTS13 antibody, their clinical courses and severe ADAMTS13 deficiency (specificity for TTP 97%-100%) were consistent with TTP.12 The ADAMTS13 inhibitor and antibody findings may reflect the presence of a low-titer or non-IgG antibody, or one that promotes enzyme clearance without inhibiting in vitro activity.13 Three of 12 patients in the Matthews et al6 series had negative inhibitor testing. Both of our patients have demonstrated sustained recovery of ADAMTS13 activity following immunosuppressive therapy, making congenital TTP less likely. Moreover, in both cases, genetic testing excluded biallelic pathogenic ADAMTS13 mutations. In the screen-positive patient, testing identified a heterozygous variant of uncertain significance. Whether this variant could reduce baseline ADAMTS13 activity and account for this patient’s posttreatment ADAMTS13 activity of 30.2% to 39.5% remains speculative.
TPE for TTP is typically continued until the platelet count exceeds 150 × 109/L for 2 consecutive days.14 In cases with normal or mildly reduced platelets at presentation, pending further research, we think it reasonable to complete at least 5 sessions regardless of platelet count or ADAMTS13 normalization, based on estimates of cumulative inhibitor clearance.15 Other aspects of TTP management, including immunosuppression and caplacizumab use, should follow standard guidelines.16 We advise regular monitoring of ADAMTS13 activity during remission to inform management and identify early relapse.17
Although there has been a recent call to consider ADAMTS13 screening in patients with cryptogenic stroke, to our knowledge, this is the first reported application of a screening algorithm for occult TTP in patients with stroke.4
The addition of ADAMTS13 testing in patients with cryptogenic stroke is feasible and may identify occult TTP, preventing harmful diagnostic delays. Notably, in our screening strategy, ADAMTS13 testing is incorporated after the initial cryptogenic stroke workup was unrevealing. Given the consequences of missing a TTP diagnosis, there may be a subset of patients for whom upfront testing is warranted. Newer rapid assays, which result in <1 hour, may be particularly valuable in this setting.18 A multicenter prospective study is needed to validate this strategy and assess the prevalence, clinical impact, and cost-effectiveness of identifying severe ADAMTS13 deficiency in cryptogenic stroke.
This study was approved by the University of Washington Institutional Review Board.
Contributions: A.B. led the development of the ADAMTS13 screening strategy in cryptogenic stroke, conducted data collection, case review, analysis, and wrote the manuscript; J.N.R.L., L.H., and D.T. contributed to screening strategy development and manuscript revisions; R.C., D.M., S.H., G.T., and B.T. reviewed data and revised the manuscript; and S.K. contributed to strategy development and manuscript revision.
Conflict-of-interest disclosure: A.B. and J.N.R.L. were supported by an institutional training grant from the National Institutes of Health, the National Heart, Lung, and Blood Institute (T32 HL007093). The remaining authors declare no competing financial interests.
Correspondence: Aaron Boothby, University of Washington, 825 Eastlake Ave E. Mail stop LG-700, PO Box 19023, Seattle, WA 98109; email: boothby1@uw.edu.
References
Author notes
Patient data will not be shared. Please contact the corresponding author, Aaron Boothby (boothby1@uw.edu) with any questions.