Key Points
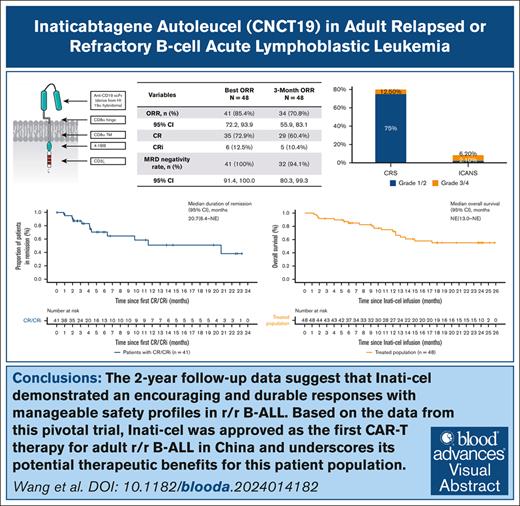
Inati-cel can induce high and durable responses in patients with R/R B-ALL, with a best ORR of 85.4% and a median DOR of 20.7 months.
At a median follow-up of 23.7 months, Inati-cel showed a manageable long-term safety profile and no new safety signal finding.
Visual Abstract
Before November 2023, CD19 chimeric antigen receptor (CAR) T-cell therapies had not been approved in China for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (R/R B-ALL), leaving a significant unmet need. In response, inaticabtagene autoleucel (Inati-cel), a novel CD19 CAR T-cell therapy with a distinct single-chain variable fragment (HI19α), was developed and showed promising efficacy in preliminary clinical research. We conducted a phase 2, single-arm, multicenter study of Inati-cel in adult CD19+ R/R B-ALL in China. The primary end point was the overall remission rate (ORR) at the end of month 3. Forty-eight patients who underwent Inati-cel infusion were evaluated for both efficacy and safety. Among them, 34 patients achieved and maintained remission beyond 3 months, with a 3-month ORR of 70.8% (95% confidence interval [CI], 55.9-83.1). The best ORR was 85.4%, with all responders reaching minimal residual disease negativity. With a median follow-up of 23.7 months, the median duration of remission was 20.7 months (95% CI, 6.4 to not reached), and the median overall survival was not reached (95% CI, 13.0 months to not reached). Additionally, grade ≥3 cytokine release syndrome and neurologic events occurred in 12.5% and 6.2% of patients, respectively. The 2-year follow-up data suggest that Inati-cel demonstrates encouraging and durable responses with manageable safety profiles in R/R B-ALL. Based on the data from this pivotal trial, Inati-cel was approved as the first CAR T-cell therapy for adult R/R B-ALL in China and underscores its potential therapeutic benefits for this patient population. This trial was registered at www.ClinicalTrials.gov as #NCT04684147.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has emerged as a promising therapy for relapsed or refractory B-cell acute lymphoid leukemia (R/R B-ALL).1,2 Promising data from clinical trials, such as ZUMA-3, indicated that 71% of adult patients achieved remission after Brexucabtagene autoleucel (Brexu-cel) infusion.3 Furthermore, the overall remission rate (ORR) reached 81% among children and young adults who received Tisagenlecleucel (Tisa-cel) infusion in ELIANA trial, underscoring the potential of this therapy for R/R B-ALL.4 However, as of November 2023, there remains a significant gap in the evaluative data available for the Chinese population, primarily due to the absence of approval for CAR T-cell therapy for R/R B-ALL in China.
Despite the success of CAR T-cell therapy, questions persist regarding the safety and efficacy of therapies derived from alternative single-chain variable fragments (scFvs) in R/R B-ALL. Therefore, we have developed inaticabtagene autoleucel (Inati-cel; CNCT19), an autologous CD19-specific CAR T-cell product with scFv derived from HI19α clone. This scFv binds to a different but high-affinity epitope on the CD19 compared with FMC63, as demonstrated in preclinical data.5 The HI19α scFv was used to construct the CAR T cells (CNCT19) with 4 other components: CD8 hinge, CD8-α transmembrane, 4-1BB costimulatory domain, and CD3 zeta. Preclinical data showed CNCT19 mediated cytotoxicity, and our prior clinical pilot data demonstrated remarkable clinical efficacy,6,7 prompting the initiation of this phase 2 pivotal study (ClinicalTrials.gov identifier: NCT04684147) to evaluate its safety, effectiveness, and pharmacokinetics in adult patients with R/R B-ALL.
Based on the data from this pivotal trial, Inati-cel was approved as the first CAR T-cell therapy for adult R/R B-ALL in China. Furthermore, this study serves as a crucial step toward enhancing the therapeutic landscape for patients with R/R B-ALL, offering the potential for improved outcomes and extended survival.
Methods
Study design and patients
This phase 2 of single-arm, multicenter, open-label clinical trial enrolled patients at 10 centers across China. Eligible participants were aged 18 to 65 years, with Eastern Cooperative Oncology Group performance status of 0 to 1, diagnosed with R/R B-ALL with ≥5% morphological bone marrow blasts at enrollment. Inclusion criteria included primary refractory, first relapse with the first remission lasting <12 months, R/R after at least 2 previous lines of systemic therapy, or R/R after hematopoietic stem cell transplantation (HSCT). Exclusion criteria included active infection, active central nervous system leukemia, and prior receipt of CAR T-cell therapy.
This study adhered to the Declaration of Helsinki and International Conference of Harmonisation guidelines for Good Clinical Practice. Written informed consent was obtained from all patients, and the protocol was approved by the China Center for Drug Evaluation and the institutional review boards of the participating centers. Detailed clinical protocol is provided in the supplemental Appendix.
Procedures
Eligible patients underwent leukapheresis to obtain T cells for Inati-cel manufacturing. Inati-cel was produced in a Current Good Manufacture Practices (CGMP) facility by Juventas Cell Therapy (Tianjin, China). T cells were isolated using an antibody-affinity method, transduced with the CD19 CAR lentiviral vector, and cultured in a serum-free medium (OpTmizer). Expansion was achieved using a WAVE bioreactor and manually filled in sterile cryobags. The manufacturing process takes a median of 7 days (range, 6-10). The median transduction efficiency was 52.2%, with a median proportion of CD3+CD4+ and CD3+CD8+ cells of 47.7% and 49.1%, respectively.
During manufacturing, bridging therapies were permitted to stabilize the patient’s condition. All patients underwent lymphodepletion with cyclophosphamide (500 mg/m2 daily for 2 days [day 5 and day 4]) and fludarabine (30 mg/m2 daily for 4 days [day 5 to day 2]). A single infusion of Inati-cel at a target dose range of 0.4 × 108 to 0.6 × 108 CAR+ viable T cells was administered. Disease assessment was conducted via bone marrow aspirate.
Outcomes
The primary end point was the 3-month ORR, defined as the rate of complete remission (CR) or CR with incomplete hematologic recovery (CRi) assessed by central bone marrow morphology at the end of month 3. Secondary end points included best ORR (bORR), defined as the rate of CR/CRi achieved anytime, minimal residual disease (MRD) negativity (<0.01% by validated flow cytometry), duration of remission (DOR), relapse-free survival (RFS), overall survival (OS), safety, and pharmacokinetics (PK).
DOR was defined as the time from the first CR/CRi to relapse or death from any cause (whichever occured first) after infusion. Patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) while in remission were censored at the date of allo-HSCT. A supplementary analysis was conducted, in which the DOR in patients who received subsequent allo-HSCT were censored at the date of HSCT. RFS was the time from Inati-cel infusion to disease relapse or death from any cause, whichever occured first. Patients who received new anticancer therapy excluding tyrosine kinase inhibitors while in remission were censored. OS was the time from Inati-cel infusion to death from any cause (more details in supplemental Methods).
AEs
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded based on the criteria of the American Society for Transplantation and Cellular Therapy.8
Statistical analysis
The study had ∼90% power to distinguish the target 3-month ORR of ≥50% from a prespecified historical control rate of 25%, with a 1-sided significance level of .025.
An exact binomial test was used to compare the observed 3-month ORR with the historical control rate. Two-sided 95% confidence intervals (CIs) were calculated using the Clopper-Pearson method. DOR, RFS, and OS were each analyzed using the Kaplan-Meier method.
Additional statistical analysis plan is provided in the supplemental Appendix. The analyses were conducted using SAS software version 9.4, and figures were created with R version 4.3.2.
Results
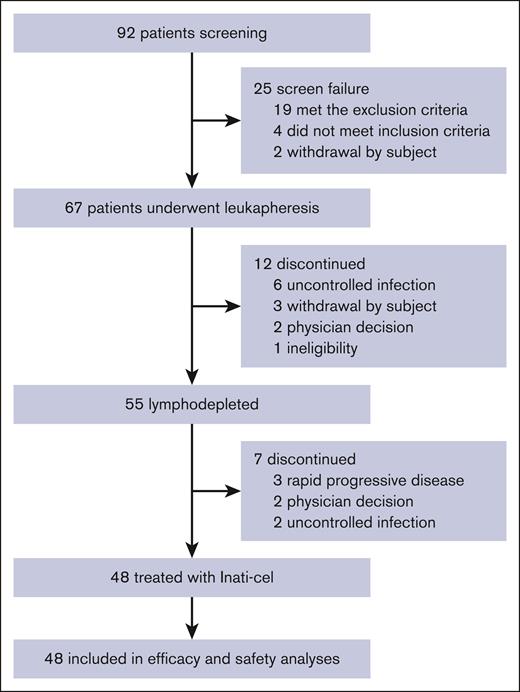
Between 14 January 2021 and 02 April 2024, a total of 92 patients with R/R B-ALL underwent screening, with 67 enrolled in the study representing the intention-to-treat population. Inati-cel was successfully manufactured for 66 patients (1 patient withdrew before manufacture). The median time from leukapheresis to Inati-cel manufacturing release was 20 days (range, 17-33), with a median CAR T-cell product viability of 87.5% (range, 76.8%-93.7%). Notably, the majority of T-cell subtypes in the final products were central memory T (Tcm) cells, whereas the Tcm population was lower in the corresponding leukapheresis products (supplemental Figure 1). A total of 48 patients received a single dose of Inati-cel, and 19 patients did not receive infusion due to active infection (n = 8), disease progression (n = 3), withdrawal (n = 3), ineligibility (n = 1), and physician decision (n = 4; Figure 1).
Flow diagram. The reasons for patients not enrolling in the study after screening included 2 patients withdrawing voluntarily, 4 patients not meeting the inclusion criteria (<5% blasts in the bone marrow at the screening), and 19 patients meeting the exclusion criteria (including hepatitis B infection, hepatitis C infection, Epstein-Barr virus infection, cytomegalovirus infection, or uncontrolled infection in 11 patients; physician decision in 4 patients due to rapid disease progression; ineligible for apheresis procedure in 3 patients, and central nervous system leukemia in 1 patient). All patients who completed screening and whose apheresis product was received by the manufacturing facility were enrolled in the study. Reasons for treatment discontinuation before lymphodepletion included: uncontrolled infection (n = 6), voluntary withdrawal (n = 3), physician decision (n = 2; 1 patient remitted after bridging chemotherapy, and 1 patient was diagnosed as mixed-phenotype acute leukemia), and ineligibility (n = 1). After lymphodepletion, 7 patients did not proceed to Inati-cel infusion due to physician decision (n = 2; 1 patient accompanied with teratoma and 1 patient remitted), uncontrolled infection (n = 2), and rapid disease progression (n = 3).
Flow diagram. The reasons for patients not enrolling in the study after screening included 2 patients withdrawing voluntarily, 4 patients not meeting the inclusion criteria (<5% blasts in the bone marrow at the screening), and 19 patients meeting the exclusion criteria (including hepatitis B infection, hepatitis C infection, Epstein-Barr virus infection, cytomegalovirus infection, or uncontrolled infection in 11 patients; physician decision in 4 patients due to rapid disease progression; ineligible for apheresis procedure in 3 patients, and central nervous system leukemia in 1 patient). All patients who completed screening and whose apheresis product was received by the manufacturing facility were enrolled in the study. Reasons for treatment discontinuation before lymphodepletion included: uncontrolled infection (n = 6), voluntary withdrawal (n = 3), physician decision (n = 2; 1 patient remitted after bridging chemotherapy, and 1 patient was diagnosed as mixed-phenotype acute leukemia), and ineligibility (n = 1). After lymphodepletion, 7 patients did not proceed to Inati-cel infusion due to physician decision (n = 2; 1 patient accompanied with teratoma and 1 patient remitted), uncontrolled infection (n = 2), and rapid disease progression (n = 3).
Patients
Among the 48 patients who were treated with Inati-cel, the median age was 32 years (range, 18-58). At enrollment, these patients had a median marrow blast percentage of 62.5% (range, 5%-96.5%), with 60.4% having >50% blasts in the bone marrow. Additionally, 35 patients (72.9%) underwent CAR T-cell therapy as a third or subsequent salvage treatment, with 8 patients (16.7%) having previously undergone HSCT. Up to 60.4% of patients had at least 1 genetic alteration associated with a poor prognosis (Table 1). After lymphodepletion, these patients received a median total dose of 0.53 × 108 (range, 0.4 × 108 to 0.6 × 108 cells) transduced viable T cells, and the median time from completion of lymphodepletion to Inati-cel infusion was 1 day (range, 1-17).
Demographic and baseline clinical characteristics of the patients
| Variables . | Enrolled patients n = 67 . | Treated patients n = 48 . |
|---|---|---|
| Age, median (range), y | 33 (18-59) | 32 (18-58) |
| Sex | ||
| Female | 33 (49.3%) | 22 (45.8%) |
| Male | 34 (50.7%) | 26 (54.2%) |
| ECOG grade, n (%) | ||
| 0 | 29 (43.3%) | 20 (41.7%) |
| 1 | 38 (56.7%) | 28 (58.3%) |
| Disease status at screening, n (%) | ||
| Relapse (first relapse with the first remission lasting <12 mo) | 12 (17.9%) | 9 (18.8%) |
| Refractory | 55 (82.1%) | 39 (81.2%) |
| Refractory subcategories | ||
| Primary refractory | 13 (19.4%) | 12 (25.0%) |
| Relapse after ≥2 CRs | 11 (16.4%) | 8 (16.7%) |
| First relapse, no remission after at least 1 salvage therapy | 20 (29.9%) | 11 (22.9%) |
| Relapsed or refractory after HSCT | 11 (16.4%) | 8 (16.7%) |
| Type of HSCT | ||
| Autologous | 4 (6.0%) | 3 (6.2%) |
| Allogeneic | 7 (10.4%) | 5 (10.4%) |
| Maximum prior lines of therapy, n (%) | ||
| Median (range) | 2.0 (1-7) | 2 (1–4) |
| 1 line | 15 (22.4%) | 13 (27.1%) |
| 2 lines | 35 (52.2%) | 25 (52.1%) |
| 3 lines | 10 (14.9%) | 7 (14.6%) |
| 4 lines | 5 (7.5%) | 3 (6.2%) |
| 5 lines | 1 (1.5%) | 0 |
| 7 lines | 1 (1.5%) | 0 |
| CD19+, n (%) | ||
| Yes | 67 (100%) | 48 (100%) |
| No | 0 | 0 |
| Blast in bone marrow at screening, % | ||
| Mean (SD) | 57.1 (29.3) | 53.8 (29.5) |
| Median | 65.0 | 62.5 |
| Min, Max | 5.0, 97.0 | 5.0, 96.5 |
| Blast in bone marrow at screening, n (%) | ||
| ≥5% and ≤25% | 13 (19.4%) | 11 (22.9%) |
| >25% and ≤50% | 11 (16.4%) | 8 (16.7%) |
| >50% and ≤75% | 18 (26.9%) | 13 (27.1%) |
| >75% and ≤100% | 25 (37.3%) | 16 (33.3%) |
| Extramedullary disease at screening, n (%) | ||
| Yes | 2 (3.0%) | 2 (4.2%) |
| No | 65 (97.0%) | 46 (95.8%) |
| Cytogenetic alterations, n (%) | ||
| Alterations of IKZF1 | 15 (22.4%) | 11 (22.9%) |
| Ph+ | 14 (20.9%) | 10 (20.8%) |
| MLL rearrangements | 10 (14.9%) | 7 (14.6%) |
| TP53 gene deletion/mutation | 10 (14.9%) | 7 (14.6%) |
| E2A::PBX1 fusion gene | 2 (3.0%) | 2 (4.2%) |
| Ph-like | 4 (6.0%) | 2 (4.2%) |
| Variables . | Enrolled patients n = 67 . | Treated patients n = 48 . |
|---|---|---|
| Age, median (range), y | 33 (18-59) | 32 (18-58) |
| Sex | ||
| Female | 33 (49.3%) | 22 (45.8%) |
| Male | 34 (50.7%) | 26 (54.2%) |
| ECOG grade, n (%) | ||
| 0 | 29 (43.3%) | 20 (41.7%) |
| 1 | 38 (56.7%) | 28 (58.3%) |
| Disease status at screening, n (%) | ||
| Relapse (first relapse with the first remission lasting <12 mo) | 12 (17.9%) | 9 (18.8%) |
| Refractory | 55 (82.1%) | 39 (81.2%) |
| Refractory subcategories | ||
| Primary refractory | 13 (19.4%) | 12 (25.0%) |
| Relapse after ≥2 CRs | 11 (16.4%) | 8 (16.7%) |
| First relapse, no remission after at least 1 salvage therapy | 20 (29.9%) | 11 (22.9%) |
| Relapsed or refractory after HSCT | 11 (16.4%) | 8 (16.7%) |
| Type of HSCT | ||
| Autologous | 4 (6.0%) | 3 (6.2%) |
| Allogeneic | 7 (10.4%) | 5 (10.4%) |
| Maximum prior lines of therapy, n (%) | ||
| Median (range) | 2.0 (1-7) | 2 (1–4) |
| 1 line | 15 (22.4%) | 13 (27.1%) |
| 2 lines | 35 (52.2%) | 25 (52.1%) |
| 3 lines | 10 (14.9%) | 7 (14.6%) |
| 4 lines | 5 (7.5%) | 3 (6.2%) |
| 5 lines | 1 (1.5%) | 0 |
| 7 lines | 1 (1.5%) | 0 |
| CD19+, n (%) | ||
| Yes | 67 (100%) | 48 (100%) |
| No | 0 | 0 |
| Blast in bone marrow at screening, % | ||
| Mean (SD) | 57.1 (29.3) | 53.8 (29.5) |
| Median | 65.0 | 62.5 |
| Min, Max | 5.0, 97.0 | 5.0, 96.5 |
| Blast in bone marrow at screening, n (%) | ||
| ≥5% and ≤25% | 13 (19.4%) | 11 (22.9%) |
| >25% and ≤50% | 11 (16.4%) | 8 (16.7%) |
| >50% and ≤75% | 18 (26.9%) | 13 (27.1%) |
| >75% and ≤100% | 25 (37.3%) | 16 (33.3%) |
| Extramedullary disease at screening, n (%) | ||
| Yes | 2 (3.0%) | 2 (4.2%) |
| No | 65 (97.0%) | 46 (95.8%) |
| Cytogenetic alterations, n (%) | ||
| Alterations of IKZF1 | 15 (22.4%) | 11 (22.9%) |
| Ph+ | 14 (20.9%) | 10 (20.8%) |
| MLL rearrangements | 10 (14.9%) | 7 (14.6%) |
| TP53 gene deletion/mutation | 10 (14.9%) | 7 (14.6%) |
| E2A::PBX1 fusion gene | 2 (3.0%) | 2 (4.2%) |
| Ph-like | 4 (6.0%) | 2 (4.2%) |
ECOG, Eastern Cooperative Oncology Group; Max, maximum; Min, minimum; MLL, mixed-lineage leukemia; Ph, Philadelphia chromosome; SD, standard deviation.
Response rates
As of the cutoff date of 02 April 2024, all 48 patients had completed the 3-month efficacy assessment. The 3-month ORR was 70.8% (34 cases; 95% CI, 55.9-83.1; P < .0001), compared with a historical control rate of 25%, meeting the primary end point of the study (Table 2). The bORR reached 85.4% (35 achieved CR, and 6 were in CRi), and all patients who responded to treatment were tested negative for MRD. Among the 41 responders, 12 (29.3%) underwent consolidative transplant while in remission. In an intention-to-treat population (n = 67), the 3-month ORR was 50.7% (95% CI, 38.2-63.2), and the bORR was 61.2% (95% CI, 48.5-72.9).
ORR and MRD negativity rate
| Variables . | BORR n = 48 . | 3-mo ORR n = 48 . |
|---|---|---|
| ORR, n (%) | 41 (85.4%) | 34 (70.8%) |
| 95% CI | (72.2-93.9) | (55.9-83.1) |
| CR | 35 (72.9%) | 29 (60.4%) |
| CRi | 6 (12.5%) | 5 (10.4%) |
| MRD negativity rate in responders, n (%) | 41 (100%) | 32 (94.1%) |
| 95% CI | (91.4-100.0) | (80.3-99.3) |
| Variables . | BORR n = 48 . | 3-mo ORR n = 48 . |
|---|---|---|
| ORR, n (%) | 41 (85.4%) | 34 (70.8%) |
| 95% CI | (72.2-93.9) | (55.9-83.1) |
| CR | 35 (72.9%) | 29 (60.4%) |
| CRi | 6 (12.5%) | 5 (10.4%) |
| MRD negativity rate in responders, n (%) | 41 (100%) | 32 (94.1%) |
| 95% CI | (91.4-100.0) | (80.3-99.3) |
Additionally, bORR was summarized by baseline and clinical covariates, including sex, age, Eastern Cooperative Oncology Group score, prior lines of therapy, type of R/R, blast percentage in bone marrow during the screening period, and high-risk cytogenetic alteration (supplemental Figure 2). The results indicated that all patients with primary refractory disease, those who had received 4 lines of pre–CAR T-cell therapy, or those who relapsed after HSCT achieved CR/CRi after Inati-cel administration. Additionally, 9 Philadelphia chromosome-positive patients achieved CR/CRi after Inati-cel infusion, with only 1 patient categorized as a nonresponder due to concomitant anticancer therapies with ponatinib, vindesine, and dexamethasone. More than 80% of patients with a high disease burden and specific genetic abnormalities associated with poor prognoses, such as alteration of IKZF1 and MLL rearrangements, experienced favorable outcomes.
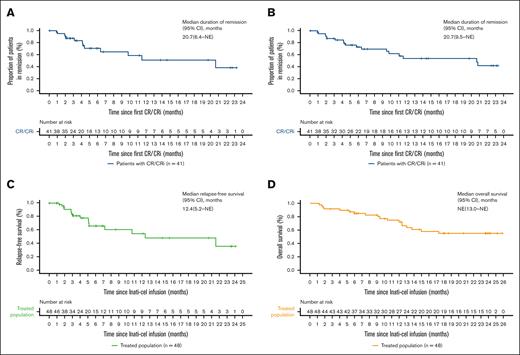
DOR, RFS, and OS
After a median follow-up of 23.7 months (interquartile range, 6.2-23.7), the median DOR both with and without censoring patients at subsequent allo-HSCT was 20.7 months (95% CI, 6.4 to not reached with censoring; 9.5 to not reached without censoring; Figure 2A-B). The median RFS was 12.4 months (95% CI, 5.2 to not reached), with an estimated RFS rate of 54.5% at 12 months and 35.8% at 24 months (Figure 2C). Of 11 patients with available data at relapse, 4 patients showed CD19+ recurrence, and 7 patients showed CD19– recurrence (2 with concomitant CD19+ blasts). The median OS was not reached (95% CI, 13.0 months to not reached), with estimated OS rates of 72.1% at 12 months and 55.2% at 24 months (Figure 2D).
DOR, RFS, and OS. (A-B) Kaplan-Meier estimates the DOR, with censoring patients at subsequent allogeneic stem cell transplant (A) and without censoring (B). (C) Kaplan-Meier estimate of RFS by investigator assessment, with patients censored at subsequent allogeneic stem cell transplant. (D) Kaplan-Meier estimate of OS. NE, not estimable.
DOR, RFS, and OS. (A-B) Kaplan-Meier estimates the DOR, with censoring patients at subsequent allogeneic stem cell transplant (A) and without censoring (B). (C) Kaplan-Meier estimate of RFS by investigator assessment, with patients censored at subsequent allogeneic stem cell transplant. (D) Kaplan-Meier estimate of OS. NE, not estimable.
Safety analysis
All patients experienced at least 1 treatment-emergent AE, with 97.9% encountering grade ≥3 treatment-emergent AEs, primarily within the initial 3 months after infusion (supplemental Table 1). Hematologic AEs were the most frequent grade ≥3 AEs, with most patients able to recover well after remission from their primary disease. The most common nonhematologic AEs of any grade suspected to be related to Inati-cel were CRS (87.5%), infection (68.8%), hypogammaglobulinemia (62.5%), increased alanine aminotransferase (45.8%), and decreased globulins (41.7%). The incidence of other Inati-cel-related AEs of special interest such as ICANS and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) were 8.3% and 6.2%, respectively (Table 3).
Treatment-emergent AEs suspected to be related to Inati-cel, occurring in at least 20% of patients, and AEs of special interest
| Inati-cel adverse reactions . | n = 48 . | ||
|---|---|---|---|
| All grades, n (%) . | Grade 3/4, n (%) . | Grade 5, n (%) . | |
| AEs of special interest | |||
| CRS | 42 (87.5%) | 6 (12.5%) | 0 |
| ICANS | 4 (8.3%) | 3 (6.2%) | 0 |
| Hypogammaglobulinemia | 30 (62.5%) | 0 | 0 |
| Infection | 33 (68.8%) | 21 (43.8%) | 2 (4.2%) |
| HLH/MAS | 3 (6.2%) | 1 (2.1%) | 0 |
| Hematologic | |||
| Neutropenia | 46 (95.8%) | 46 (95.8%) | 0 |
| Leukopenia | 46 (95.8%) | 46 (95.8%) | 0 |
| Anemia | 44 (91.7%) | 38 (79.2%) | 0 |
| Thrombocytopenia | 43 (89.6%) | 37 (77.1%) | 1 (2.1%) |
| Lymphopenia | 42 (87.5%) | 42 (87.5%) | 0 |
| Hyperfibrinolysis | 15 (31.2%) | 1 (2.1%) | |
| Coagulopathy | 10 (20.8%) | 3 (6.2%) | 0 |
| Investigations | |||
| Alanine aminotransferase increased | 22 (45.8%) | 0 | 0 |
| Globulins decreased | 20 (41.7%) | 0 | 0 |
| Blood fibrinogen decreased | 19 (39.6%) | 10 (20.8%) | 0 |
| Aspartate aminotransferase increased | 16 (33.3%) | 2 (4.2%) | 0 |
| γ-glutamyltransferase increased | 15 (31.2%) | 4 (8.3%) | 0 |
| Blood bilirubin increased | 10 (20.8%) | 2 (4.2%) | 0 |
| Metabolism and nutritional disorders | |||
| Hypokalemia | 24 (50.0%) | 4 (8.3%) | 0 |
| Hypocalcemia | 13 (27.1%) | 1 (2.1%) | 0 |
| Hypertriglyceridemia | 12 (25.0%) | 1 (2.1%) | 0 |
| Hypoalbuminemia | 11 (22.9%) | 1 (2.1%) | 0 |
| Inati-cel adverse reactions . | n = 48 . | ||
|---|---|---|---|
| All grades, n (%) . | Grade 3/4, n (%) . | Grade 5, n (%) . | |
| AEs of special interest | |||
| CRS | 42 (87.5%) | 6 (12.5%) | 0 |
| ICANS | 4 (8.3%) | 3 (6.2%) | 0 |
| Hypogammaglobulinemia | 30 (62.5%) | 0 | 0 |
| Infection | 33 (68.8%) | 21 (43.8%) | 2 (4.2%) |
| HLH/MAS | 3 (6.2%) | 1 (2.1%) | 0 |
| Hematologic | |||
| Neutropenia | 46 (95.8%) | 46 (95.8%) | 0 |
| Leukopenia | 46 (95.8%) | 46 (95.8%) | 0 |
| Anemia | 44 (91.7%) | 38 (79.2%) | 0 |
| Thrombocytopenia | 43 (89.6%) | 37 (77.1%) | 1 (2.1%) |
| Lymphopenia | 42 (87.5%) | 42 (87.5%) | 0 |
| Hyperfibrinolysis | 15 (31.2%) | 1 (2.1%) | |
| Coagulopathy | 10 (20.8%) | 3 (6.2%) | 0 |
| Investigations | |||
| Alanine aminotransferase increased | 22 (45.8%) | 0 | 0 |
| Globulins decreased | 20 (41.7%) | 0 | 0 |
| Blood fibrinogen decreased | 19 (39.6%) | 10 (20.8%) | 0 |
| Aspartate aminotransferase increased | 16 (33.3%) | 2 (4.2%) | 0 |
| γ-glutamyltransferase increased | 15 (31.2%) | 4 (8.3%) | 0 |
| Blood bilirubin increased | 10 (20.8%) | 2 (4.2%) | 0 |
| Metabolism and nutritional disorders | |||
| Hypokalemia | 24 (50.0%) | 4 (8.3%) | 0 |
| Hypocalcemia | 13 (27.1%) | 1 (2.1%) | 0 |
| Hypertriglyceridemia | 12 (25.0%) | 1 (2.1%) | 0 |
| Hypoalbuminemia | 11 (22.9%) | 1 (2.1%) | 0 |
Overall, CRS was detected in 42 of 48 patients (87.5%), with only 6 cases (12.5%) classified as severe (6 of grade 3 and no grade 4 or 5). The median time for CRS onset was 4.5 days (range, 1-10), and the median duration was 9 days (range, 3-34). The most common CRS symptoms included fever (42/42 [100%]), hypoxia (17/42 [35.4%]), and hypotension (17/42 [35.4%]). Among the 42 patients with CRS, 32 (76.2%) received tocilizumab, and 30 (71.4%) were treated with steroids. Four patients (8.3%) developed ICANS, occurring 5 to 7 days after the onset of CRS, with 3 patients (6.2%) experiencing grade ≥3 ICANS, characterized by epileptic seizures. HLH/MAS occurred in 3 patients (6.2%) after grade 2 CRS, with 2 cases graded as grade 2 and 1 as grade 3. The median onset of HLH/MAS was on day 14 after infusion (range, 10-33), with a duration of 10 days. All patients recovered without sequelae. Additionally, 30 patients (62.5%) developed hypogammaglobulinemia, with grades 1 to 2. The median onset time for hypogammaglobulinemia was on day 15 after infusion (range, 4-90), with a median duration of 194.5 days (range, 7-272). Of note, 33 patients (68.8%) experienced infections, with 23 (47.9%) of them experiencing infections classified as grade ≥3.
Eighteen patients died, none within 30 days after Inati-cel infusion. Among the 7 nonresponders, 3 died from disease progression, and 1 died from an AE (pseudomonal sepsis on day 37). Of the 29 responders not receiving consolidated allo-HSCT, 7 died from relapse, 1 from an AE (soft tissue infection with thrombocytopenia on day 49), and 2 from unknown causes. Of the 12 responders with subsequent consolidated allo-HSCT after Inati-cel infusion, 2 died from HSCT-mediated complications, and 2 died from relapses after transplant (supplemental Table 3).
Clinical pharmacology
The presence of CAR gene copies of Inati-cel in peripheral blood were assessed using qualitative polymerase chain reaction. After Inati-cel infusion, expansion of Inati-cel was observed, peaking around day 11 (range, 7-21) after infusion, with a maximal concentration (Cmax) of 1.75 × 105 copies per μg Genomic dna Deoxyribonucleic acid (gDNA) (supplemental Figure 3; supplemental Table 4). The median duration of Inati-cel persistence in blood was 92 days (range, 14.0-733.7), with detectability remaining at 24 months after infusion in a patient maintaining CR status. Other pharmacokinetics parameters are detailed in supplemental Table 4. The expansion, as measured by median Cmax and the area under the concentration-time curve in blood (AUC0-28d) of Inati-cel in responders was not significantly different from nonresponders (n = 7; supplemental Table 5). The pharmacokinetics (PK) parameters (Cmax and AUC0-28d) values in patients with CRS or ICANS occurrence were numerically higher than in patients without CRS or ICANS occurrence, although this difference was not statistically significant. (supplemental Figure 4).
Pharmacodynamics biomarkers, including interleukin-6 (IL-6), IL-8, IL-10, interferon gamma, ferritin, and C-reactive protein (CRP), peaked within the first 10 days after Inati-cel infusion and returned to baseline levels within 28 days (supplemental Table 6). Elevated serum levels of IL-6, IL-8, IL-10, and interferon gamma were associated with any grade CRS or ICANS (P < .05; supplemental Figures 5 and 6). Elevated CRP and ferritin levels were only associated with any grade CRS (P < .05).
Discussion
In this prospective, single-arm, multicenter, phase 2 clinical trial, we investigated the efficacy and safety of Inati-cel, a CD19-specific CAR T-cell therapy, in adult patients with R/R B-ALL. Our data revealed that 85.4% of patients achieved CR/CRi after Inati-cel administration, with all responders testing negative for MRD. Furthermore, 70.8% of patients who received infusion remained in remission after 3 months without any anticancer therapy including HSCT, and the median OS was not reached with a median follow-up of 23.7 months, indicating a profound and durable response to treatment. These efficacy results are comparable with those observed in the pivotal ZUMA-3 trials, which reported a 71% ORR rate within 3 months in adults. They also align with the 81% ORR noted in children and young adult patients in the ELIANA study, although direct comparisons are limited by differing patient characteristics, such as disease stage and prior lines of therapy.3,4 Particularly noteworthy was the efficacy seen in patients with high disease burden, heavily pretreated patients, and those harboring specific genetic abnormalities associated with poor prognoses, such as alterations of IKZF1 and MLL rearrangements.9-11 Furthermore, the safety profile of Inati-cel appears to be more tolerant than those observed in the referenced trials, which is an encouraging finding despite the limitations in direct comparisons. These findings underscore the potential of Inati-cel as a promising therapeutic option for patients with R/R B-ALL, particularly in achieving deep and sustained remissions.
Regarding the long-term response, the median DOR and RFS were 20.7 months and 12.4 months, respectively. The median OS was not reached, and the estimated rates of OS showed favorable outcomes up to 24 months after infusion, indicating long-term outcomes. These findings reinforce the potential of Inati-cel to maintain deep and durable remissions in patients with R/R B-ALL.
Despite the favorable efficacy profile of Inati-cel, the safety analysis revealed notable treatment-related AEs, with the most frequent AEs being CRS and ICANS. However, severe CRS and ICANS (grade ≥3) were infrequent, and most patients experienced resolution without long-term sequelae with suitable management.12-15 In this study, severe CRS affected only 12.5% of patients, a significantly lower rate than the 45% reported in the ELIANA study and 24% in the ZUMA-3 trials. Consistent results were observed in severe neurologic events, 6.2% of patients receiving Inati-cel experienced severe ICANS, whereas previous data in adult patients reported rates ranging from 25% to 50%.3,4,16 Importantly, the study's safety profile remained acceptable even in patients with a high disease burden and extensive prior treatment, further indicating the reliable safety of Inati-cel.
Pharmacological characterization of Inati-cel cells revealed notable dynamics in their expansion, persistence, and immunological effects after infusion. Comparing these findings with previous studies, such as ELIANA study and ZUMA-3 trials, reveals similarities in the kinetics of CAR T-cell expansion and immunological responses, highlighting the consistent trends across different CAR T-cell therapies. Previous research has demonstrated that individuals who do not respond typically exhibit limited CAR T-cell expansion.17
Nonetheless, there was no discernible variance in Inati-cel cells between the 2 patient cohorts, possibly due to differences in antigen affinity stemming from diverse scFVs. The high affinity of HI19α-derived scFv to the CD19 antigen may mitigate suboptimal binding by rapid tumor cell proliferation or sparse CD19 antigen expression, thereby promoting robust CAR T-cell expansion in most patients. Additionally, the significant proportion of Tcm cells in the final products, enriched during the manufacturing process, may contribute to the durable remission and low toxicity profile of Inati-cel,18 which is in agreement with previous reports.19-22 However, such enrichment of Tcm cell subsets was not seen in Tecartus,3 which could attribute to the differences in the CAR structure and the manufacturing process between these 2 products. Further study is warranted. A notable case involved a patient whose leukemia cell count exceeded 100 × 109/L cells shortly after the initial CAR T-cell infusion, requiring the addition of low-dose chemotherapy for control. During this period, CAR T cells underwent rapid proliferation and promptly eradicated the tumor, further emphasizing the robust expansion and killing capabilities of Inati-cel cells. Additionally, these findings suggest that additional crucial factors beyond CAR T-cell expansion continue to play a role in the effectiveness of CAR T-cell therapy.
The strengths of this study encompass its prospective design, multicenter collaboration, and comprehensive evaluation of efficacy and safety outcomes. Additionally, it stands as the inaugural prospective, single-arm, multicenter, phase 2 clinical trial of a CD19-specific CAR T cell conducted to endorse a New Drug Application submission in China for adult patients with R/R B-ALL. However, several limitations should be acknowledged. As a single-arm trial with a relatively small sample size, the potential for selection bias and confounding factors cannot be entirely ruled out. Additionally, the lack of a control arm limits the ability to directly compare the outcomes with alternative treatment modalities. However, ongoing follow-up and future large-scale studies will provide further insights into the long-term efficacy and safety of Inati-cel in this patient population. Furthermore, considering that the scFV of Inati-cel cells targets a distinct CD19 binding site compared with approved CD19 CAR T-cell therapies,6 it would be valuable to assess whether it could provide an alternative option for patients who have relapsed after treatment with CD19 CAR T-cell therapies based on the FMC63 clone. Additionally, exploring the integration of Inati-cel into the early phases of B-ALL treatment through combination with other agents (ie, blinatumomab/inotuzumab) or immunomodulatory strategies should be explored to enhance its efficacy and broaden its applicability across different patient populations.
The findings of this study have significant implications for clinical practice, offering a promising therapeutic option for adult patients with R/R B-ALL who have exhausted standard treatments. Inati-cel's high remission rates and manageable safety profile suggest its potential to become a cornerstone in B-ALL management, particularly when conventional therapies have failed.
In conclusion, the findings from this phase 2 study support the potential of Inati-cel as a promising therapeutic option for adult patients with R/R B-ALL, demonstrating high rates of 85.4% MRD-negative ORR and durable responses with manageable safety profiles. The success of Inati-cel highlights the potential of CAR T-cell therapy in addressing unmet medical needs in leukemia management and underscores the importance of continued research and development in this field to advance patient care and treatment strategies. Further research and ongoing clinical trials will continue to refine our understanding of the optimal use of CAR T-cell therapies in the management of hematologic malignancies, paving the way for improved outcomes and personalized treatment approaches in the future.
Acknowledgments
The authors thank Ying Yuan (The University of Texas MD Anderson Cancer Center) for advice and assistance in the statistical analysis and result discussion. The authors sincerely thank Zheng Ye (Shanghai Daotian Evidence-based Technology Co, Shanghai, China) for assisting with language and medical editing. The authors sincerely thank inaticabtagene autoleucel development team members, patients, and their families for their contributions to this clinical trial (HY001201).
This study is supported by funding from Juventas Cell Therapy Ltd.
The sponsor collaborated with authors in study design, data collection, analysis, interpretation, and report writing.
Authorship
Contribution: Y.W. designed research, performed research, interpreted data, and contributed to writing, including review and editing, data supervision, and validation (directly accessed and verified the underlying data reported in the manuscript); L.L. designed research, performed research, and contributed to writing, including review and editing (directly accessed and verified the underlying data reported in the manuscript); Y.S. and A.L. designed research and contributed to data interpretation and writing, including review and editing; X.W. performed research and contributed to data interpretation and writing, including review and editing; H.Z., Q.L., K.X., D.Y., C.Z., S.L., J.J., H.M., and T.N. performed research and contributed to data interpretation and writing, including review and editing; R.G. contributed to literature search and writing, including review and editing; J.R. designed research, performed research, and contributed to data interpretation and writing, including review and editing (directly accessed and verified the underlying data reported in the manuscript); Y.F. designed research, performed research, and contributed to data interpretation, writing, including review and editing, and literature search; W.J. designed research and contributed to data analysis and figures (directly accessed and verified the underlying data reported in the manuscript); Y.Z. performed research and contributed to data collection and project administration; Y.D. contributed to data interpretation and writing, including review and editing; and J.W. designed and performed research and contributed to data interpretation, data supervision, and writing the original draft.
Conflict-of-interest disclosure: J.W. reports adviser fees from AbbVie. L.L., J.R., Y.F., Y.Z., and Y.D. own stock option of Juventas Cell Therapy Ltd. The remaining authors declare no competing financial interests.
Correspondence: Jianxiang Wang, Leukemia Center,State Key Laboratory of Experimental Hematology, National Clinical Research Center of Blood Diseases, Haihe Laboratory of Cell Ecosystem, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 288 Nanjing Rd, Tianjin, 300020, China; email: wangjx@ihcams.ac.cn.
References
Author notes
Y.W. and L.L. contributed equally to this study.
Data are available on request from the corresponding author, Jianxiang Wang (wangjx@ihcams.ac.cn).
The full-text version of this article contains a data supplement.