Key Points
In APL, frontline oral- or IV-ATO reduced early deaths and improved RFS and OS, independent of risk category.
Without frontline ATO, inferior RFS could still be mitigated by using an oral-ATO–based regimen in maintenance.
Visual Abstract
The Acute Promyelocytic Leukemia (APL) Asian Consortium analyzed a contemporaneous cohort of newly diagnosed patients with APL treated with and without frontline arsenic trioxide (ATO) in 6 centers. The objectives were to define the impact of ATO on early deaths and relapses and its optimal positioning in the overall treatment strategy. In a 21.5-year period, 324 males and 323 females at a median age of 45.5 years (range, 18.1-91.8; low/intermediate risk, n = 448; high risk, n = 199) were treated. Regimens included frontline all-trans retinoic acid (ATRA)/chemotherapy and maintenance with/without ATO (n = 436), ATRA/IV-ATO/chemotherapy (ATRA/IV-ATO; n = 61), and ATRA/oral-ATO/ascorbic acid with ATO maintenance (oral-AAA; n = 150). The ATRA/chemotherapy group had significantly more frequent early deaths within 60 days (8.3% vs 3.3%; P = .05), inferior 60-day survival (91.7% vs 98.4%/96%; P < .001), inferior 5-year relapse-free survival (RFS; 76.9% vs 92.8%/97.8%; P < .001), and inferior 5-year overall survival (OS; 84.6% vs 91.4%/92.3%; P = .03) than ATO-containing groups (ATRA/IV-ATO and oral-AAA). The addition of oral-ATO maintenance partly mitigated the inferior 5-year RFS resulting from the omission of ATO during induction (ATRA/chemotherapy/non-ATO maintenance vs ATRA/chemotherapy/ATO maintenance vs ATRA/IV-ATO vs oral-AAA, 71.1% vs 87.9% vs 92.8% vs 97.8%; P < .001). The favorable survival impacts of ATO were observed in all risk groups. In conclusion, ATO decreased early deaths, improved 60-day survival, and resulted in significantly superior RFS and OS. This trial was registered at www.clinicaltrials.gov as #NCT04251754.
Introduction
Acute promyelocytic leukemia (APL), arising from t(15;17)(q24.1;q21.2) that results in PML::RARA fusion, is an uncommon but unique acute myeloid leukemia.1,2 With treatment regimens tested in clinical trials, durable remission potentially translating into cure can be accomplished in >90% of newly diagnosed patients.3-11
Such results, however, cannot be achieved in routine practice. Patients with APL typically present with profound thrombocytopenia and coagulopathy, which without timely treatment can be rapidly fatal.12 Furthermore, all-trans retinoic acid (ATRA), a key component of standard induction regimens, may only be available to a minority of treatment centers, even in localities with advanced medical systems.13 Lastly, in regions with resource constraints, standard treatment algorithms often cannot be implemented.14 These factors reduce the survival of patients with APL, which otherwise ought to be highly curable.
Arsenic trioxide (ATO) is a crucial component in the treatment of APL. Used initially in relapsed disease, ATO has now been moved to the treatment of newly diagnosed patients.15 In practically all countries, ATO is only available as an IV preparation at a high cost.16 An oral formulation of ATO has been used in Hong Kong for >2 decades in relapsed and newly diagnosed APL,17-20 giving results comparable with those of IV-ATO. Advantages of oral-ATO include improved cardiac safety17-19,21 and significant health cost savings because of its outpatient administration and low unit cost.22 Finally, a maintenance strategy that contains ATO is only feasible with the oral formulation.
Favorable results have been reported for frontline ATO in clinical trials.7-11 However, real-world data of ATO in induction and consolidation/maintenance treatment of APL are very scarce. Furthermore, there are currently still uncertainties related to ATO, including impacts of its omission in frontline therapy, its relative benefits in low/intermediate-risk and high-risk cases, its optimal positioning in the overall treatment algorithm, and prognostic indicators relevant to its use.
The APL Asian Consortium (APLAC)23 was established in 2020 to determine unmet needs and improve the management of APL in Asia, a vast continent with widely contrasting socioeconomic backgrounds. Because of dissimilarities in health economics, ATO is not available for the treatment of newly diagnosed APL in many Asian countries. These disparities provide the APLAC a unique opportunity to compare contemporaneous cohorts of newly diagnosed patients with APL managed with and without ATO.
In this study, the APLAC conducted a retrospective multicenter analysis of newly diagnosed patients with APL treated with different regimens in 5 regions to specifically address the impact of ATO on outcome and prognosis in actual clinical practice.
Materials and methods
Patients
Adult patients (aged ≥18 years) with newly diagnosed APL treated in 6 Asian academic centers (Hong Kong, n = 1; Malaysia, n = 2; Singapore, n = 1; Taipei, n = 1; Thailand, n = 1) from 1 January 2001 to 31 July 2022 were retrospectively analyzed. The inclusion criterion was pathologically confirmed APL based on standard morphology, karyotype, and demonstration of PML::RARA on reverse transcription polymerase chain reaction.2,24 All consecutively diagnosed patients were included. The study was approved by the institutional review boards/ethics committees of the participating centers (University of Hong Kong; Universiti Malaya and Sunway Medical Center; National University Singapore; the National Taiwan University Hospital; and Chiang Mai University) and registered as part of the APLAC project (www.clinicaltrials.gov identifier: NCT04251754).
Treatment regimens
Three regimens were used during the study period (details of schedules and drug doses are given in supplemental Table 1 and the supplemental File). The first regimen was ATRA/chemotherapy, comprising induction (ATRA + idarubicin), consolidation (ATRA + mitoxantrone + idarubicin + cytarabine), and 2 different maintenance strategies. One maintenance did not contain ATO (non-ATO; ATRA + methotrexate + 6-mercaptopurine), whereas the other maintenance contained oral-ATO (ATRA + oral-ATO + ascorbic acid [AAA]). Maintenance was selected according to the discretion of the treating physicians.25 The second regimen was ATRA/IV-ATO, comprising induction (ATRA + idarubicin/IV-ATO), consolidation (ATRA + IV-ATO), and maintenance (ATRA + methotrexate + 6-mercaptopurine).8,9 The third regimen was oral-AAA, comprising induction (AAA ± hydroxyurea/daunorubicin), consolidation (AAA + daunorubicin + cytarabine), and maintenance (AAA).18,26 All regimens were risk adapted, according to presentation leukocyte count (standard risk, ≤10 × 109/L; high risk, >10 × 109/L) or presentation leukocyte and platelet counts (Sanz risk score; low risk, leukocyte ≤10 × 109/L and platelet >40 × 109/L; intermediate risk, leukocyte ≤10 × 109/L and platelet ≤40 × 109/L; high risk, leukocyte >10 × 109/L).25,27
Remission and relapse
Complete remission (CR) was defined according to standard criteria24 based on a postinduction bone marrow examination on day 28. Morphologic relapse was defined as the recurrence of abnormal promyelocytes in the peripheral blood or bone marrow. After CR1, molecular monitoring was performed on the peripheral blood every 8 to 12 weeks for 2 years. Molecular relapse was defined as 2 consecutively positive reverse transcription polymerase chain reaction results for PML::RARA fusion transcript on the peripheral blood or bone marrow, performed at least 2 weeks apart.18
Treatment of relapses
In Hong Kong, first relapse (R1) was treated with AAA (for 42 days) + idarubicin (6 mg/m2 per day for 2 days).17 On achieving CR2, consolidation with AAA (for 7 days) + idarubicin (6 mg/m2 per day for 2 days) every 4 weeks for 2 cycles were administered, along with central nervous system (CNS) prophylaxis (intrathecal methotrexate 12 mg and cytarabine 50 mg weekly ×4). After consolidation, maintenance with AAA was given (14 days every 8 weeks for 12 cycles). Hematopoietic stem cell transplantation (HSCT) was not performed.17 Outside Hong Kong, depending on the accessibility or affordability of IV-ATO, R1 was treated with either IV-ATO (0.15 mg/kg per day) + ATRA until CR2 or ATRA + chemotherapy (3 days of idarubicin 12 mg/m2 per day or mitoxantrone 10 mg/m2 per day). On achieving morphologic CR2, patients with undetectable PML::RARA and aged <65 years received autologous HSCT, whereas patients with detectable PML::RARA, aged <60 years, and with a suitable donor received allogeneic HSCT. Patients with confirmed molecular relapse received the same treatment as those with morphologic relapse.
Definitions and end points
Early deaths were defined as deaths from presentation to day 60, which included mortalities due to leukemia (intracranial hemorrhage [ICH]) and treatment (APL differentiation syndrome [APL-DS] and infection).28,29 Sixty-day survival was defined as the time from presentation to death (event), censoring at day 60.28 Post–60-day overall survival (OS) was defined as the time from day 61 to death (event) or latest follow-up (censor). Relapse-free survival (RFS) was defined as the time from CR1 to hematologic or molecular relapse (event), death (event), or latest follow-up (censor). OS was defined as the time from presentation to death (event) or latest follow-up (censor). Data were censored on 1 October 2022.
Statistical analyses
Categorical variables were analyzed with χ2 test and continuous variables with nonparametric tests. Survivals (60-day survival, post–60-day OS, RFS, and OS) were analyzed with the Kaplan-Meier method, with differences between groups being determined by the log-rank test and Cox proportional hazard model. Prognostic impacts on survivals were evaluated for the following parameters: sex (male vs female); age (≤50 vs >50 years); presentation leukocyte counts (≤10 × 109/L vs >10 × 109/L); presentation platelet count (≤40 × 109/L vs >40 × 109/L); Sanz risk scores (low vs intermediate vs high); APL morphology (classical vs microgranular); APL type (de novo vs therapy related); CNS involvement on presentation (present vs absent); APL-DS (present vs absent); and peak leukocyte counts during induction (≤10 × 109/L vs >10 × 109/L). For evaluating the impact of treatment on survivals, regimen groupings differed slightly. For OS and 60-day survival, grouping was according to initial therapies: non-ATO regimen (ATRA/chemotherapy) vs ATO-regimens (ATRA/IV-ATO and oral-AAA); and ATRA/chemotherapy vs ATRA/IV-ATO vs oral-AAA. For post–60-day survival and RFS, the ATRA/chemotherapy group was further split into non-ATO and AAA maintenance subgroups. Hence, besides the analyses performed for 60-day survival and OS, additional analyses were also conducted for ATRA/chemotherapy/non-ATO maintenance vs ATRA/chemotherapy/AAA maintenance vs ATRA/IV-ATO vs oral-AAA. Prognostic factors with P value <.10 on univariate analysis were then examined by multivariate analyses. Two-tailed P values < .05 were regarded as significant. Statistical analyses were performed with SPSS version 26.0 (Chicago, IL).
Results
Patients
During the 21.5-year study period, 647 consecutive patients with newly diagnosed APL were treated (supplemental Table 2). There were 324 males and 323 females with a median age of 45.5 years (range, 18.1-91.8), of whom 199 patients (31.5%) belonged to the high-risk group. Notably, 22 patients (3.4%) had therapy-related APL (prior malignancies requiring chemotherapy: breast cancer, n = 11; colorectal cancer, n = 3; nasopharyngeal cancer, n = 2; testicular cancer, n = 1; hypopharyngeal cancer, n = 1; endometrial cancer, n = 1; cervical cancer, n = 1; lung cancer, n = 1; follicular lymphoma, n = 1). Patient disposition is shown in Figure 1.
Patient disposition, treatment characteristics and complications during induction. (A) Disposition of 647 newly diagnosed patients with APL treated with ATO-containing and non–ATO-containing regimens. Refer to the supplemental File 1 for details of treatment regimens used. (B) Trend in the distribution and number of patients treated with 4 different treatment regimens from 2001 to 2011. Refer to the supplemental File 1 for details of treatment regimens used. (C) Trends in the number and causes of early or induction deaths from 2001 to 2022. (D) Trends in the number of patients with APL-DS from 2001 to 2022. ICH, intracranial hemorrhage.
Patient disposition, treatment characteristics and complications during induction. (A) Disposition of 647 newly diagnosed patients with APL treated with ATO-containing and non–ATO-containing regimens. Refer to the supplemental File 1 for details of treatment regimens used. (B) Trend in the distribution and number of patients treated with 4 different treatment regimens from 2001 to 2011. Refer to the supplemental File 1 for details of treatment regimens used. (C) Trends in the number and causes of early or induction deaths from 2001 to 2022. (D) Trends in the number of patients with APL-DS from 2001 to 2022. ICH, intracranial hemorrhage.
Clinicopathologic features of different treatment groups
Table 1 shows the clinicopathologic features of the different treatment groups. The oral-AAA group had significantly more females and a higher median age. The ATRA/IV-ATO group had significantly higher presentation platelet count and proportion of APL with classical morphology. Otherwise, the 3 treatment groups were comparable in the analyzed parameters.
Clinicopathologic features and outcome of 647 patients with newly diagnosed APL treated with 3 different induction regimens
| Parameters . | ATRA/chemotherapy . | ATRA/IV-ATO . | Oral-AAA . | P value∗ . |
|---|---|---|---|---|
| Total number | 436 | 61 | 150 | |
| Sex | ||||
| Male | 228 (52%) | 36 (59%) | 60 (40%) | .01 |
| Female | 208 (48%) | 25 (41%) | 90 (60%) | |
| Age, y | ||||
| Median (IQR) | 44 (33.7-54.7) | 45 (33.7-62.9) | 52 (40.5-63.7) | <.001 |
| ≤50 | 287 (66%) | 37 (61%) | 72 (48%) | .001 |
| >50 | 149 (34%) | 24 (39%) | 78 (52%) | |
| Presentation leukocyte†, × 109/L | ||||
| Median (IQR) | 3.8 (1.5-13.4) | 2.6 (1.31-13.4) | 3.2 (1.3-12.7) | .43 |
| ≤10 | 285 (65%) | 43 (71%) | 104 (69%) | .88 |
| >10 | 135 (31%) | 18 (30%) | 46 (31%) | |
| Presentation platelet‡, × 109/L | ||||
| Median (IQR) | 28 (15-49) | 42 (20-65) | 24 (14-39) | .003 |
| ≤40 | 273 (66%) | 29 (48%) | 114 (76%) | .001 |
| >40 | 143 (34%) | 31 (52%) | 36 (24%) | |
| Sanz risk score§ | ||||
| Low | 93 (22%) | 21 (34%) | 28 (19%) | .13 |
| Intermediate | 191 (46%) | 22 (36%) | 76 (51%) | |
| High | 135 (32%) | 18 (30%) | 46 (31%) | |
| APL morphology | ||||
| Classical | 408 (94%) | 61 (100%) | 135 (90%) | .03 |
| Microgranular variant | 28 (6%) | 0 | 15 (10%) | |
| Therapy-related APL | 15 (3%) | 2 (3%) | 5 (3%) | .99 |
| Additional karyotypic abnormalities | 96 (22%) | 14 (23%) | 42 (28%) | .41 |
| PML::RARA isoform|| | ||||
| bcr1 | 80 (66%) | 4 (40%) | 62 (63%) | .49 |
| bcr2 | 4 (3%) | 1 (10%) | 3 (3%) | |
| bcr3 | 37 (31%) | 5 (50%) | 34 (34%) | |
| CNS involvement | 22 (5%) | 3 (5%) | 11 (7%) | .56 |
| Peak leukocyte count¶, × 109/L | ||||
| Median (IQR) | 13.7 (4.6-31.9) | 19.6 (7.4-40.6) | 16.1 (6.7-30.1) | .13 |
| ≤10 | 162 (41%) | 21 (34%) | 49 (33%) | .17 |
| >10 | 230 (59%) | 40 (66%) | 99 (67%) | |
| APL-DS | 129 (30%) | 21 (34%) | 79 (53%) | <.001 |
| Early deaths | 36 (8%) | 1 (2%) | 6 (4%) | .05 |
| Intracranial hemorrhage | 13 (36%) | 0 | 4 (67%) | |
| APL-DS | 15 (42%) | 0 | 2 (33%) | |
| Infection | 8 (22%) | 1 (100%) | 0 | |
| First CR | 399 (92%) | 59 (97%) | 144 (96%) | .05 |
| Median follow-up (IQR), mo | 102 (42-160) | 37 (21.5-66) | 46.5 (21-78) | <.001 |
| Relapse | 101 (25%) | 3 (5%) | 2 (1%) | <.001 |
| Second cancers | 28 (6%) | 3 (5%) | 2 (1%) | .05 |
| Cause of deaths | ||||
| All causes | 92 (21%) | 7 (12%) | 12 (8%) | .001 |
| Early/induction deaths | 36 (39%) | 1 (14%) | 6 (50%) | |
| Refractory APL | 23 (25%) | 2 (29%) | 1 (8%) | |
| Treatment-related complications | 3 (3%) | 0 | 1 (8%) | |
| Infection | 12 (13%) | 1 (14%) | 4 (33%) | |
| Cardiovascular events | 2 (2%) | 0 | 0 | |
| Second primary cancers | 11 (12%) | 3 (43%) | 0 | |
| Unknown | 5 (5%) | 0 | 0 |
| Parameters . | ATRA/chemotherapy . | ATRA/IV-ATO . | Oral-AAA . | P value∗ . |
|---|---|---|---|---|
| Total number | 436 | 61 | 150 | |
| Sex | ||||
| Male | 228 (52%) | 36 (59%) | 60 (40%) | .01 |
| Female | 208 (48%) | 25 (41%) | 90 (60%) | |
| Age, y | ||||
| Median (IQR) | 44 (33.7-54.7) | 45 (33.7-62.9) | 52 (40.5-63.7) | <.001 |
| ≤50 | 287 (66%) | 37 (61%) | 72 (48%) | .001 |
| >50 | 149 (34%) | 24 (39%) | 78 (52%) | |
| Presentation leukocyte†, × 109/L | ||||
| Median (IQR) | 3.8 (1.5-13.4) | 2.6 (1.31-13.4) | 3.2 (1.3-12.7) | .43 |
| ≤10 | 285 (65%) | 43 (71%) | 104 (69%) | .88 |
| >10 | 135 (31%) | 18 (30%) | 46 (31%) | |
| Presentation platelet‡, × 109/L | ||||
| Median (IQR) | 28 (15-49) | 42 (20-65) | 24 (14-39) | .003 |
| ≤40 | 273 (66%) | 29 (48%) | 114 (76%) | .001 |
| >40 | 143 (34%) | 31 (52%) | 36 (24%) | |
| Sanz risk score§ | ||||
| Low | 93 (22%) | 21 (34%) | 28 (19%) | .13 |
| Intermediate | 191 (46%) | 22 (36%) | 76 (51%) | |
| High | 135 (32%) | 18 (30%) | 46 (31%) | |
| APL morphology | ||||
| Classical | 408 (94%) | 61 (100%) | 135 (90%) | .03 |
| Microgranular variant | 28 (6%) | 0 | 15 (10%) | |
| Therapy-related APL | 15 (3%) | 2 (3%) | 5 (3%) | .99 |
| Additional karyotypic abnormalities | 96 (22%) | 14 (23%) | 42 (28%) | .41 |
| PML::RARA isoform|| | ||||
| bcr1 | 80 (66%) | 4 (40%) | 62 (63%) | .49 |
| bcr2 | 4 (3%) | 1 (10%) | 3 (3%) | |
| bcr3 | 37 (31%) | 5 (50%) | 34 (34%) | |
| CNS involvement | 22 (5%) | 3 (5%) | 11 (7%) | .56 |
| Peak leukocyte count¶, × 109/L | ||||
| Median (IQR) | 13.7 (4.6-31.9) | 19.6 (7.4-40.6) | 16.1 (6.7-30.1) | .13 |
| ≤10 | 162 (41%) | 21 (34%) | 49 (33%) | .17 |
| >10 | 230 (59%) | 40 (66%) | 99 (67%) | |
| APL-DS | 129 (30%) | 21 (34%) | 79 (53%) | <.001 |
| Early deaths | 36 (8%) | 1 (2%) | 6 (4%) | .05 |
| Intracranial hemorrhage | 13 (36%) | 0 | 4 (67%) | |
| APL-DS | 15 (42%) | 0 | 2 (33%) | |
| Infection | 8 (22%) | 1 (100%) | 0 | |
| First CR | 399 (92%) | 59 (97%) | 144 (96%) | .05 |
| Median follow-up (IQR), mo | 102 (42-160) | 37 (21.5-66) | 46.5 (21-78) | <.001 |
| Relapse | 101 (25%) | 3 (5%) | 2 (1%) | <.001 |
| Second cancers | 28 (6%) | 3 (5%) | 2 (1%) | .05 |
| Cause of deaths | ||||
| All causes | 92 (21%) | 7 (12%) | 12 (8%) | .001 |
| Early/induction deaths | 36 (39%) | 1 (14%) | 6 (50%) | |
| Refractory APL | 23 (25%) | 2 (29%) | 1 (8%) | |
| Treatment-related complications | 3 (3%) | 0 | 1 (8%) | |
| Infection | 12 (13%) | 1 (14%) | 4 (33%) | |
| Cardiovascular events | 2 (2%) | 0 | 0 | |
| Second primary cancers | 11 (12%) | 3 (43%) | 0 | |
| Unknown | 5 (5%) | 0 | 0 |
χ2 for categorical variables, and independent sample Kruskal-Wallis test for continuous variables.
Missing data in 16 patients (2.5%).
Missing data in 21 patients (3.2%).
Missing data in 17 patients (2.6%).
PML::RARA transcript subtype was not determined in 417 patients (64.5%).
Missing data in 46 patients (7.1%).
The ATRA/chemotherapy group
Four hundred and thirty-six patients were treated (Table 1). Early deaths occurred in 36 patients (8.3%), and 1 patient could not attain remission. The remaining 399 patients (91.5%) all achieved CR1. Non-ATO maintenance was used in 273 cases (68.4%; Figure 1), predominantly in centers with no access to ATO. AAA maintenance was used in 126 cases (31.5%). After CR1, 7 patients defaulted. The median follow-up was 102 months (interquartile range [IQR], 42-160). In the non-ATO maintenance group, 83 patients (30.3%) relapsed, and 37 patients (13.5%) died. In the AAA maintenance group, 18 patients (14.3%) relapsed, and 19 patients (15.1%) died.
The ATRA/IV-ATO group
Sixty-one patients were treated. Early deaths occurred in 1 patient (1.6%), and 1 patient could not attain a remission (Table 1; Figure 1). Hence, 59 patients (96.7%) achieved CR1. After CR1, 1 patient defaulted. After a median follow-up of 37.0 months (IQR, 21.5-66.0), 3 patients (5.1%) relapsed, and 6 patients (10.2%) died.
The oral-AAA group
One hundred and fifty patients were treated. Early deaths occurred in 6 patients (4.0%). The remaining 144 patients (96.0%) all achieved CR1 (Table 1; Figure 1). Post-CR1, 1 patient defaulted. At a median follow-up of 46.5 months (IQR, 21.0-27.0), 2 patients (1.4%) relapsed, and 6 patients (4.2%) died.
Early deaths and 60-day survival
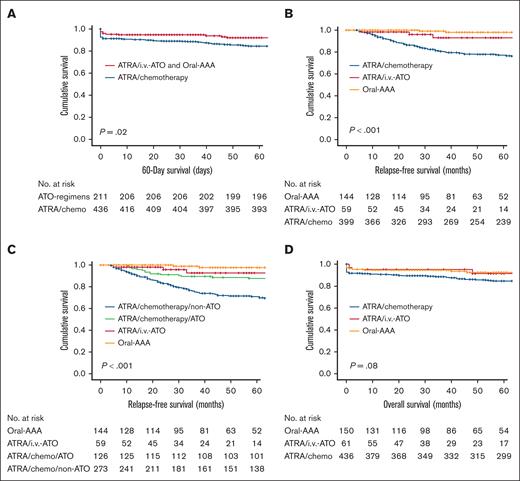
There were 43 early deaths, occurring significantly more often in the ATRA/chemotherapy group (36/43 [84%]; P = .05; Table 1). ICH and APL-DS each accounted for ∼40% of the early deaths. The 60-day survival for the entire cohort was 91.6% (supplemental Figure 1; supplemental File). On univariate analysis, inferior 60-day survival was associated with age >50 years (P = .01); presenting leukocyte count >10 × 109/L (P = .001); high-risk Sanz score (P = .003); CNS involvement at presentation (P < .001); APL-DS (P < .001); peak leukocyte count during induction >10 × 109/L (P = .002); and non-ATO induction regimen (P = .02; supplemental Table 3; supplemental Figure 1). On multivariate analysis, inferior 60-day survival was associated with non-ATO regimen (P < .001; Figure 2A; Table 2) and CNS involvement at diagnosis (P < .001; Figure 3A). Notably, with individual treatment groups, the 60-day survival still remained significantly inferior for ATRA/chemotherapy (91.7%), compared with ATRA/IV-ATO (98.4%) or oral-AAA (96%; P < .001; Table 2; supplemental Figure 1).
Impact of ATO on the outcome of newly diagnosed APL. (A) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on 60-day survival compared with ATRA/chemotherapy induction. P values were .02 on univariate analysis and <.001 on multivariate analysis. (B) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS, compared with ATRA/chemotherapy induction. P values on both univariate and multivariate analysis were <.001. (C) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance. P values on both univariate and multivariate analysis were <.001. (D) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on OS, compared with the ATRA/chemotherapy induction. P value was .08 on univariate analysis and .03 on multivariate analysis.
Impact of ATO on the outcome of newly diagnosed APL. (A) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on 60-day survival compared with ATRA/chemotherapy induction. P values were .02 on univariate analysis and <.001 on multivariate analysis. (B) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS, compared with ATRA/chemotherapy induction. P values on both univariate and multivariate analysis were <.001. (C) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance. P values on both univariate and multivariate analysis were <.001. (D) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on OS, compared with the ATRA/chemotherapy induction. P value was .08 on univariate analysis and .03 on multivariate analysis.
Significant prognostic factors on multivariate analysis for survivals in patients with newly diagnosed APL
| Prognostic indicators . | P value . | HR for inferior survival . | 95% CI . |
|---|---|---|---|
| 60-d survival | |||
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.06 | 0.03-0.13 |
| Present | 15.95 | 7.56-33.63 | |
| Induction regimens | |||
| Non-ATO containing | <.001 | 5.33 | 2.30-12.35 |
| ATO containing | 0.19 | 0.08-0.44 | |
| Treatment groups | |||
| ATRA/chemotherapy | <.001 | 5.53 | 2.24-13.68 |
| IV-ATO/ATRA | 0.22 | 0.03-1.68 | |
| Oral-AAA | 0.18 | 0.07-0.45 | |
| Post–60-d OS | |||
| Sex | |||
| Male | .02 | 1.87 | 1.12-3.11 |
| Female | 0.54 | 0.32-0.90 | |
| Age | |||
| ≤50 y | .04 | 0.60 | 0.36-0.97 |
| >50 y | 1.68 | 1.03-2.75 | |
| Therapy-related APL | |||
| No | .002 | 0.28 | 0.12-0.64 |
| Yes | 3.59 | 1.57-8.20 | |
| CNS involvement at diagnosis | |||
| Absent | .04 | 0.35 | 0.13-0.96 |
| Present | 2.89 | 1.05-7.99 | |
| RFS | |||
| Sex | |||
| Male | .03 | 1.53 | 1.04-2.25 |
| Female | 0.66 | 0.45-0.97 | |
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.20 | 0.10-0.42 |
| Present | 4.94 | 2.63-11.1 | |
| Treatment groups | |||
| ATRA/chemotherapy | <.001 | 13.59 | 3.33-55.45 |
| IV-ATO/ATRA | 0.25 | 0.08-0.79 | |
| Oral-AAA | 0.07 | 0.02-0.30 | |
| Year of diagnosis | |||
| 2001-2011 | <.001 | 3.48 | 1.97-6.12 |
| 2012-2022 | 0.29 | 0.16-0.51 | |
| OS | |||
| Age | |||
| ≤50 y | .002 | 0.52 | 0.35-0.78 |
| >50 y | 1.92 | 1.28-2.89 | |
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.15 | 0.09-0.26 |
| Present | 6.55 | 3.80-11.30 | |
| APL-DS | |||
| Absent | <.001 | 0.31 | 0.20-0.50 |
| Present | 3.14 | 2.02-4.90 | |
| Induction regimen | |||
| ATRA/chemotherapy | .03 | 2.33 | 1.23-4.42 |
| IV-ATO/ATRA | 0.72 | 0.33-1.60 | |
| Oral-AAA | 0.43 | 0.22-0.80 |
| Prognostic indicators . | P value . | HR for inferior survival . | 95% CI . |
|---|---|---|---|
| 60-d survival | |||
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.06 | 0.03-0.13 |
| Present | 15.95 | 7.56-33.63 | |
| Induction regimens | |||
| Non-ATO containing | <.001 | 5.33 | 2.30-12.35 |
| ATO containing | 0.19 | 0.08-0.44 | |
| Treatment groups | |||
| ATRA/chemotherapy | <.001 | 5.53 | 2.24-13.68 |
| IV-ATO/ATRA | 0.22 | 0.03-1.68 | |
| Oral-AAA | 0.18 | 0.07-0.45 | |
| Post–60-d OS | |||
| Sex | |||
| Male | .02 | 1.87 | 1.12-3.11 |
| Female | 0.54 | 0.32-0.90 | |
| Age | |||
| ≤50 y | .04 | 0.60 | 0.36-0.97 |
| >50 y | 1.68 | 1.03-2.75 | |
| Therapy-related APL | |||
| No | .002 | 0.28 | 0.12-0.64 |
| Yes | 3.59 | 1.57-8.20 | |
| CNS involvement at diagnosis | |||
| Absent | .04 | 0.35 | 0.13-0.96 |
| Present | 2.89 | 1.05-7.99 | |
| RFS | |||
| Sex | |||
| Male | .03 | 1.53 | 1.04-2.25 |
| Female | 0.66 | 0.45-0.97 | |
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.20 | 0.10-0.42 |
| Present | 4.94 | 2.63-11.1 | |
| Treatment groups | |||
| ATRA/chemotherapy | <.001 | 13.59 | 3.33-55.45 |
| IV-ATO/ATRA | 0.25 | 0.08-0.79 | |
| Oral-AAA | 0.07 | 0.02-0.30 | |
| Year of diagnosis | |||
| 2001-2011 | <.001 | 3.48 | 1.97-6.12 |
| 2012-2022 | 0.29 | 0.16-0.51 | |
| OS | |||
| Age | |||
| ≤50 y | .002 | 0.52 | 0.35-0.78 |
| >50 y | 1.92 | 1.28-2.89 | |
| CNS involvement at diagnosis | |||
| Absent | <.001 | 0.15 | 0.09-0.26 |
| Present | 6.55 | 3.80-11.30 | |
| APL-DS | |||
| Absent | <.001 | 0.31 | 0.20-0.50 |
| Present | 3.14 | 2.02-4.90 | |
| Induction regimen | |||
| ATRA/chemotherapy | .03 | 2.33 | 1.23-4.42 |
| IV-ATO/ATRA | 0.72 | 0.33-1.60 | |
| Oral-AAA | 0.43 | 0.22-0.80 |
CI, confidence interval; HR, hazard ratio.
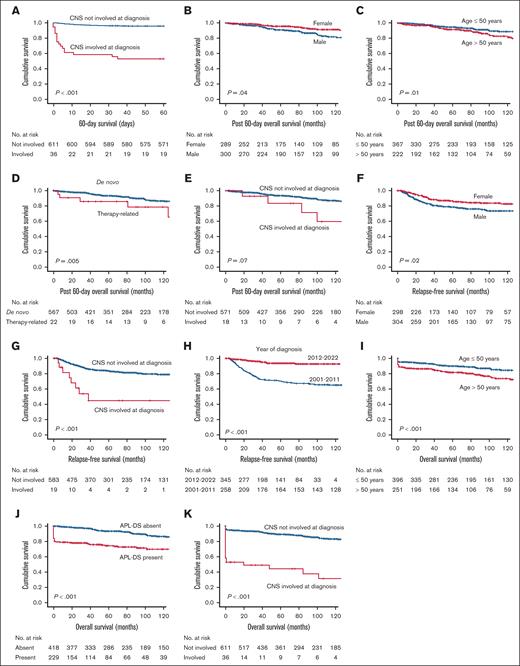
Other significant factors affecting outcome in newly diagnosed APL.P values shown were those obtained from univariate log-rank test. Table 2 gives the corresponding P values on multivariate Cox regression analysis. (A) Impact of CNS involvement at presentation on 60-day survival. (B) Impact of sex on post–60-day OS. (C) Impact of age on post–60-day OS. (D) Impact of therapy-related APL on post–60-day OS. (E) Impact of CNS involvement at presentation on post–60-day OS. (F) Impact of sex on RFS. (G) Impact of CNS involvement at presentation on RFS. (H) Impact of year of diagnosis on RFS. (I) Impact of age on OS. (J) Impact of APL-DS on OS. (K) Impact of CNS involvement at presentation on OS.
Other significant factors affecting outcome in newly diagnosed APL.P values shown were those obtained from univariate log-rank test. Table 2 gives the corresponding P values on multivariate Cox regression analysis. (A) Impact of CNS involvement at presentation on 60-day survival. (B) Impact of sex on post–60-day OS. (C) Impact of age on post–60-day OS. (D) Impact of therapy-related APL on post–60-day OS. (E) Impact of CNS involvement at presentation on post–60-day OS. (F) Impact of sex on RFS. (G) Impact of CNS involvement at presentation on RFS. (H) Impact of year of diagnosis on RFS. (I) Impact of age on OS. (J) Impact of APL-DS on OS. (K) Impact of CNS involvement at presentation on OS.
Post–60-day OS
The 5-year post–60-day OS for the whole cohort was 93.2% (supplemental Figure 2). On univariate analysis, inferior post–60-day OS was associated with male sex (P = .04); age >50 years (P = .01); and therapy-related APL (P = .005; supplemental Table 4; supplemental Figure 2). On multivariate analysis, inferior post–60-day survival was associated with male sex (P = .02; Figure 3B); age >50 years (P = .04; Figure 3C); therapy-related APL (P = .002; Figure 3D); and CNS involvement at diagnosis (P = .04; Figure 3E; Table 2). The period of diagnosis did not have a statistically significant impact on post–60-day OS on multivariate analysis (P = .07; supplemental Table 4; supplemental Figure 2).
CR1 and R1
The CR rate was significantly inferior in the ATRA/chemotherapy group, compared with the ATRA/IV-ATO and oral-AAA groups (P = .05; Table 1). The median follow-up for the entire cohort was 75 months (IQR, 29-133), with a significantly longer follow-up for the ATRA/chemotherapy group at 102 months (IQR: 42-160; P < .001). There were 106 R1s (morphologic relapse, n = 101; molecular relapse, n = 5), arising at a median time of 23.5 months (range, 12.8-38.0) after CR1 (supplemental Table 5). Remarkably, the ATRA/chemotherapy group accounted for almost all (95.2%) of the relapses (n = 101), followed by the ATRA/IV-ATO group (n = 3) and the oral-AAA group (n = 2). The ATRA/chemotherapy/non-ATO maintenance subgroup had the highest number of relapses (83/273 [30.4%]; Figure 1; supplemental Table 5). The addition of ATO maintenance to the ATRA/chemotherapy/oral-AAA maintenance subgroup partly decreased relapses (18/126 [14.3%]) but failed to fully bring the relapses down to the levels of the frontline ATO groups ATRA/IV-ATO (3/59 [5.1%]) and oral-AAA (2/143 [1.4%]).
RFS
The 5-year RFS was 82.5% (supplemental Figure 3). On univariate analysis, inferior RFS was associated with male sex (P = .02), age >50 years (P = .01), CNS involvement at diagnosis (P < .001), absence of APL-DS (P = .01), non-ATO induction regimen (ATRA/chemotherapy; P < .001), ATRA/chemotherapy/non-ATO maintenance (P < .001), and diagnosis between 2001 and 2011 (P < .01; supplemental Table 6; supplemental Figure 3). On multivariate analysis, inferior RFS was associated with male sex (P = .03; Figure 3F; Table 2); CNS involvement at diagnosis (P < .001; Figure 3G); ATRA/chemotherapy (P < .001; Table 2; Figure 2B); and diagnosis between 2001 and 2011 (P < .001; Table 2; Figure 3H). Notably, ATO use at any point significantly improved 5-year RFS, which was 97.8% for the oral-AAA group, 92.8% for the ATRA/IV-ATO group, and 87.9% for the ATRA/chemotherapy/oral-AAA maintenance subgroup, compared with 71.1% for the ATRA/chemotherapy/non-ATO maintenance subgroup (P < .001, both on univariate and multivariate analysis; Figure 2C; supplemental Table 6).
Deaths and OS
There were 101 deaths, occurring significantly more often in the ATRA/chemotherapy group (92/101; P = .001; Table 1). The 5-year OS was 86.8% (supplemental Figure 4). On univariate analysis, inferior OS was associated with male sex (P = .02); age >50 years (P < .001); presenting leukocyte count >10 × 109/L (P = .02); high-risk Sanz score (P = .03); CNS involvement at presentation (P < .001); APL-DS (P < .001); and peak leukocyte count during induction >10 × 109/L (P = .01; supplemental Table 7; supplemental Figure 4). On multivariate analysis, inferior OS was associated with age >50 years (P = .002; Figure 3H), APL-DS (P < .001; Figure 3I), CNS involvement at presentation (P < .001; Figure 3J), and the ATRA/chemotherapy regimen (P = .03; Figure 2D; Table 2).
Impact of frontline ATO on low/intermediate-risk and high-risk patients
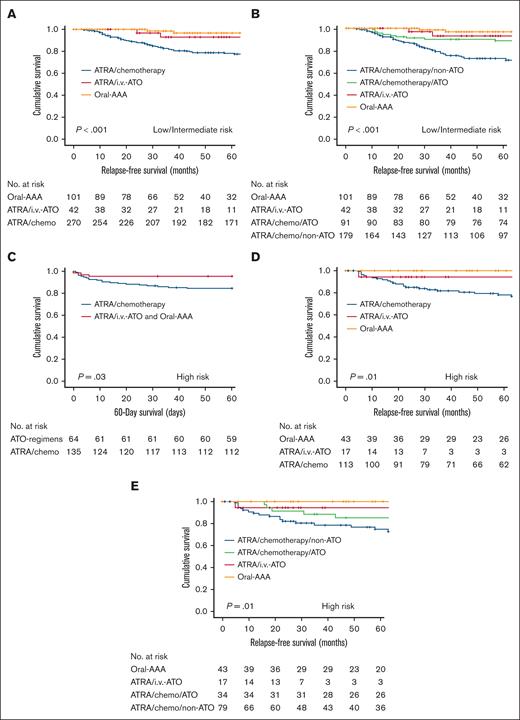
In our cohort, conventional risks (presentation leukocyte and platelet counts) were no longer prognostically relevant. Whether these risks were mitigated in low/intermediate-risk or high-risk patients was further examined. For low/intermediate-risk disease (presentation leukocyte count ≤10 × 109/L and platelet count >40 × 109/L or ≤40 × 109/L), the ATRA/chemotherapy group had a significantly inferior 5-year RFS at 78.6%, compared with that of the ATRA/IV-ATO and oral-AAA groups at 95.7% (P < .001; supplemental Figure 5). The inferior RFS was mainly contributed by the ATRA/chemotherapy/non-ATO maintenance subgroup (5-year RFS, 72.8%), because the addition of oral-ATO to the ATRA/chemotherapy/oral-AAA maintenance subgroup successfully restored the 5-year RFS to 88.8%, which was comparable with those of the ATRA/IV-ATO and oral-AAA groups (at 93.1% and 96.8%, respectively; Figure 4A-B; Table 3). For high-risk disease (presentation leukocyte count >10 × 109/L), induction with ATRA/chemotherapy led to a significantly inferior 60-day survival at 84.4%, compared with that of the ATRA/IV-ATO and oral-AAA groups at 95.3% (P = .03; Figure 4C). Furthermore, the ATRA/chemotherapy group had a significantly inferior 5-year RFS at 78.0%, compared with that of ATRA/IV-ATO and oral-AAA groups at 98.3% (P = .002; supplemental Figure 6; Table 3). Similarly, the inferior outcome in the ATRA/chemotherapy group was predominantly contributed by the ATRA/chemotherapy/non-ATO maintenance group (5-year RFS, 74.5%); and the addition of oral-ATO to the ATRA/chemotherapy/oral-AAA maintenance group successfully restored the 5-year RFS to 85.1%, comparable with those of the ATRA/IV-ATO and oral-AAA groups (at 94.1% and 100%, respectively; Figure 4D; Figure 4E). Notably, once ATO (IV or oral) was included in the treatment algorithm (induction or consolidation or maintenance), low/intermediate-risk and high-risk patients had comparable survivals.
Impact of ATO on the outcome of newly diagnosed APL with different conventional risks. (A) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS compared with ATRA/chemotherapy induction in patients with low/intermediate-risk disease. (B) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance in patients with low/intermediate-risk disease. (C) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on 60-day survival compared with the ATRA/chemotherapy induction in patients with high-risk disease. (D) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS compared with ATRA/chemotherapy induction in patients with high-risk disease. (E) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance in patients with high-risk disease.
Impact of ATO on the outcome of newly diagnosed APL with different conventional risks. (A) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS compared with ATRA/chemotherapy induction in patients with low/intermediate-risk disease. (B) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance in patients with low/intermediate-risk disease. (C) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on 60-day survival compared with the ATRA/chemotherapy induction in patients with high-risk disease. (D) Impact of ATO-containing induction (ATRA/IV-ATO and oral-AAA) on RFS compared with ATRA/chemotherapy induction in patients with high-risk disease. (E) Impact of ATRA/chemotherapy/ATO maintenance, ATRA/IV-ATO, and frontline oral-AAA groups on RFS compared with ATRA/chemotherapy/non-ATO maintenance in patients with high-risk disease.
Impact of ATO on outcome in low/intermediate-risk and high-risk patients with newly diagnosed APL
| Regimens . | Number . | Survivals . | P value . | HR for inferior survival . | 95% CI . |
|---|---|---|---|---|---|
| Low/intermediate risk | 432 | ||||
| 60-d survival | |||||
| ATRA/chemotherapy | 285 | 94.7% | .22 | 1.97 | 0.65-5.93 |
| ATRA/IV-ATO and oral-AAA | 147 | 97.3% | 0.51 | 0.17-1.53 | |
| Post–60-d OS (5 y) | |||||
| ATRA/chemotherapy | 265 | 91.4% | .99 | 1.0 | 0.45-2.23 |
| ATRA/IV-ATO and oral-AAA | 137 | 95.2% | 1.0 | 0.45-2.23 | |
| ATRA/chemotherapy/non-ATO maintenance | 174 | 90.4% | .94 | 1.28 | 0.37-4.37 |
| ATRA/chemotherapy/oral-AAA maintenance | 91 | 93.2% | 0.92 | 0.46-1.81 | |
| ATRA/IV-ATO | 40 | 95.5% | 0.92 | 0.46-1.81 | |
| Oral-AAA | 97 | 95.3% | 0.85 | 0.32-2.29 | |
| RFS (5 y) | |||||
| ATRA/chemotherapy | 270 | 78.6% | <.001 | 6.54 | 2.37-18.01 |
| ATRA/IV-ATO and oral-AAA | 143 | 95.7% | 0.15 | 0.06-0.42 | |
| ATRA/chemotherapy/non-ATO maintenance | 179 | 72.8% | <.001 | 7.54 | 3.39-19.20 |
| ATRA/chemotherapy/oral-AAA maintenance | 91 | 88.8% | 0.39 | 0.21-0.73 | |
| ATRA/IV-ATO | 42 | 93.1% | 0.20 | 0.05-0.85 | |
| Oral-AAA | 101 | 96.8% | 0.08 | 0.02-0.34 | |
| OS (5 y) | |||||
| ATRA/chemotherapy | 285 | 86.3% | .50 | 1.24 | 0.66-2.32 |
| ATRA/IV-ATO and oral-AAA | 147 | 92.0% | 0.81 | 0.43-1.52 | |
| High risk | 199 | ||||
| 60-d survival | |||||
| ATRA/chemotherapy | 135 | 84.4% | .03 | 3.45 | 1.03-11.55 |
| ATRA/IV-ATO and oral-AAA | 64 | 95.3% | 0.29 | 0.09-0.97 | |
| Post–60-d survival (5 y) | |||||
| ATRA/chemotherapy | 112 | 94.9% | .85 | 1.13 | 0.31-4.16 |
| ATRA/IV-ATO and oral-AAA | 59 | 96.6% | 0.88 | 0.24-3.23 | |
| ATRA/chemotherapy/non-ATO maintenance | 78 | 95.5% | .47 | 2.38 | 0.50-11.49 |
| ATRA/chemotherapy/oral-AAA maintenance | 34 | 94.1% | 0.40 | 0.05-3.23 | |
| ATRA/IV-ATO | 18 | 94.4% | 1.05 | 0.38-2.92 | |
| Oral-AAA | 41 | 97.6% | 0.40 | 0.05-3.23 | |
| RFS (5 y) | |||||
| ATRA/chemotherapy | 113 | 78.0% | .002 | 11.48 | 1.55-85.00 |
| ATRA/IV-ATO and oral-AAA | 60 | 98.3% | 0.09 | 0.01-0.65 | |
| ATRA/chemotherapy/non-ATO maintenance | 79 | 74.5% | .01 | 1.00 | |
| ATRA/chemotherapy/oral-AAA maintenance | 34 | 85.1% | 0.47 | 0.18-1.26 | |
| ATRA/IV-ATO | 17 | 94.1% | 0.29 | 0.04-2.18 | |
| Oral-AAA | 43 | 100% | 0.00 | NE | |
| OS (5 y) | |||||
| ATRA/chemotherapy | 135 | 80.1% | .06 | 2.23 | 0.93-5.36 |
| ATRA/IV-ATO and oral-AAA | 64 | 92.1% | 0.45 | 0.19-1.08 |
| Regimens . | Number . | Survivals . | P value . | HR for inferior survival . | 95% CI . |
|---|---|---|---|---|---|
| Low/intermediate risk | 432 | ||||
| 60-d survival | |||||
| ATRA/chemotherapy | 285 | 94.7% | .22 | 1.97 | 0.65-5.93 |
| ATRA/IV-ATO and oral-AAA | 147 | 97.3% | 0.51 | 0.17-1.53 | |
| Post–60-d OS (5 y) | |||||
| ATRA/chemotherapy | 265 | 91.4% | .99 | 1.0 | 0.45-2.23 |
| ATRA/IV-ATO and oral-AAA | 137 | 95.2% | 1.0 | 0.45-2.23 | |
| ATRA/chemotherapy/non-ATO maintenance | 174 | 90.4% | .94 | 1.28 | 0.37-4.37 |
| ATRA/chemotherapy/oral-AAA maintenance | 91 | 93.2% | 0.92 | 0.46-1.81 | |
| ATRA/IV-ATO | 40 | 95.5% | 0.92 | 0.46-1.81 | |
| Oral-AAA | 97 | 95.3% | 0.85 | 0.32-2.29 | |
| RFS (5 y) | |||||
| ATRA/chemotherapy | 270 | 78.6% | <.001 | 6.54 | 2.37-18.01 |
| ATRA/IV-ATO and oral-AAA | 143 | 95.7% | 0.15 | 0.06-0.42 | |
| ATRA/chemotherapy/non-ATO maintenance | 179 | 72.8% | <.001 | 7.54 | 3.39-19.20 |
| ATRA/chemotherapy/oral-AAA maintenance | 91 | 88.8% | 0.39 | 0.21-0.73 | |
| ATRA/IV-ATO | 42 | 93.1% | 0.20 | 0.05-0.85 | |
| Oral-AAA | 101 | 96.8% | 0.08 | 0.02-0.34 | |
| OS (5 y) | |||||
| ATRA/chemotherapy | 285 | 86.3% | .50 | 1.24 | 0.66-2.32 |
| ATRA/IV-ATO and oral-AAA | 147 | 92.0% | 0.81 | 0.43-1.52 | |
| High risk | 199 | ||||
| 60-d survival | |||||
| ATRA/chemotherapy | 135 | 84.4% | .03 | 3.45 | 1.03-11.55 |
| ATRA/IV-ATO and oral-AAA | 64 | 95.3% | 0.29 | 0.09-0.97 | |
| Post–60-d survival (5 y) | |||||
| ATRA/chemotherapy | 112 | 94.9% | .85 | 1.13 | 0.31-4.16 |
| ATRA/IV-ATO and oral-AAA | 59 | 96.6% | 0.88 | 0.24-3.23 | |
| ATRA/chemotherapy/non-ATO maintenance | 78 | 95.5% | .47 | 2.38 | 0.50-11.49 |
| ATRA/chemotherapy/oral-AAA maintenance | 34 | 94.1% | 0.40 | 0.05-3.23 | |
| ATRA/IV-ATO | 18 | 94.4% | 1.05 | 0.38-2.92 | |
| Oral-AAA | 41 | 97.6% | 0.40 | 0.05-3.23 | |
| RFS (5 y) | |||||
| ATRA/chemotherapy | 113 | 78.0% | .002 | 11.48 | 1.55-85.00 |
| ATRA/IV-ATO and oral-AAA | 60 | 98.3% | 0.09 | 0.01-0.65 | |
| ATRA/chemotherapy/non-ATO maintenance | 79 | 74.5% | .01 | 1.00 | |
| ATRA/chemotherapy/oral-AAA maintenance | 34 | 85.1% | 0.47 | 0.18-1.26 | |
| ATRA/IV-ATO | 17 | 94.1% | 0.29 | 0.04-2.18 | |
| Oral-AAA | 43 | 100% | 0.00 | NE | |
| OS (5 y) | |||||
| ATRA/chemotherapy | 135 | 80.1% | .06 | 2.23 | 0.93-5.36 |
| ATRA/IV-ATO and oral-AAA | 64 | 92.1% | 0.45 | 0.19-1.08 |
NE, not evaluable.
Outcome and prognostic indicators after R1
Of 106 R1s, CR2 was achieved in 103 cases (97.2%). Ninety-five patients received ATO-based reinduction (oral-AAA, n = 78; IV-ATO/ATRA, n = 17). The 5-year RFS after CR2 was 58.6% (supplemental Figure 7). RFS after CR2 was not significantly related to any of the clinicopathologic features (supplemental Table 8). Notably, the 5-year RFS after CR2 for patients receiving oral-AAA without HSCT at 59.9% was comparable with those of patients receiving ATRA/chemotherapy or IV-ATO/ATRA followed by autologous HSCT (20.8% and 69.8%, respectively; P = .30; supplemental Figure 7). The 5-year OS after R1 was 69.2%. None of the clinicopathologic features examined were related to the 5-year OS after R1 (supplemental Table 9). Notably, the 5-year OS after R1 for patients undergoing or not undergoing HSCT was comparable (61.8% vs 71.0%; P = .14; supplemental Figure 7).
Discussion
This analysis represented, to our knowledge, the first multinational study of APL in Asia and included one of the largest cohorts of newly diagnosed patients treated in the ATO era.30 Our observations provided important information on the management of newly diagnosed APL. Specifically, 2 areas crucial to curing APL were addressed: avoidance of early death and prevention of relapses.
Early death is the most important challenge in the initial management of patients with APL. Risks involve patient factors (advanced age, poor performance, and coexisting infections); leukemia factors (high white blood cell counts, low platelet counts, and coagulopathy); and treatment factors (delay in ATRA administration and APL-DS). Hemorrhage, including ICH, is a culmination of these risks and is the leading cause of early deaths.31-36
In the ATRA era, early deaths were partly ameliorated but still significant. Population-based studies of unselected patients with APL reported early deaths from 9.5% to a staggering 61.5% of cases.37-44 The development of international treatment recommendations had led to an improvement in early death rates from 28% in the 1990s to ∼15% in the last 2 decades.24,44,45 Despite these efforts, delay in ATRA administration still remained an important cause of early deaths.46,47 A cohort of 806 South-American patients with APL treated from 2005 to 2020 had recently been reported.29 With international networking that entailed standardized protocols for initial diagnosis, supportive care, and treatment, early death rates gradually fell from 32% to 14.6% over 15 years.29,37,48 In clinical trials of ATRA-containing regimens in which preselected patients were treated in highly controlled settings, early deaths still occurred in 3% to 10% of patients.9,11,25,27,49 Therefore, further reduction of early deaths remains a priority in the post-ATRA era.
Our patients were treated after the advent of ATO, although not all cases received frontline ATO owing to issues of drug accessibility. For the ATRA/chemotherapy group without access to ATO, the early death rate was 8%. In the frontline ATO (ATRA/IV-ATO and oral-AAA) groups, the early death rate fell to 3.3%, which resulted in a correspondingly superior 60-day survival. The low early death rate was remarkable, given that these were patients treated in routine practice. A higher frequency of APL-DS was observed in the frontline ATO groups than the ATRA/chemotherapy group, which was consistent with findings from previous studies.9,11 Early cytoreduction with chemotherapy, especially anthracyclines in the ATRA/chemotherapy group, prevented leukocytosis and hence decreased APL-DS.9,11,50 Conversely, the reduced use of chemotherapy in the ATO groups, particularly the oral-AAA group, resulted in a higher frequency of leukocytosis and, consequently, APL-DS. However, deaths due to APL-DS were much more prevalent in the ATRA/chemotherapy group and exceptionally rare in the ATO groups. During differentiation, leukemic leukocytes secrete cytokines including interleukin-6 and tumor necrosis factor alpha, which mediate a systemic inflammatory response syndrome characteristic of APL-DS. Secretion of cytokines and inflammation is suppressed by ATO51,52 but not chemotherapy, which may explain why, despite more APL-DS, ATO groups had fewer early deaths. Furthermore, ATO acts synergistically with ATRA to reduce the expression of annexin A2 and S110A1, both key mediators of primary fibrinolysis,53,54 thereby decreasing deaths due to bleeding. Reduction of early deaths to <5% had also been observed in reported trials of ATO.8-11,49 The period of diagnosis did have a significant impact on 60-day survival (supplemental Figure 1; supplemental Table 3). That fact that all patients were managed in academic centers with expertise in APL may explain the relatively small changes in the incidence of early deaths over the 2 decades studied (Figure 1C).
In our cohort, relapses in the ATRA/chemotherapy/non-ATO maintenance group were most frequent, with a 5-year RFS of merely 71.1%. In clinical trials of ATRA combined with chemotherapy, cumulative relapse rates over 4 to 6 years ranged from 9% to 14%.5,25,55 Deferral of ATO to maintenance partly decreased relapses (5-year RFS, ATRA/chemotherapy/oral-AAA group, 87.9%), although frontline ATO was clearly superior in preventing relapses (5-year RFS, ATRA/IV-ATO group, 92.8%; oral-AAA group, 97.8%). The year of diagnosis and treatment between 2001 and 2011 had a significant adverse impact on RFS due to the higher proportion of patients treated with ATO-based regimens.
Therefore, frontline ATO decreased early deaths and reduced relapses, leading to significantly superior 60-day survival and 5-year RFS, which combined to result in superior OS. The trend toward a lower incidence of second primary cancers (Table 1; supplemental Table 10) in patients treated with frontline ATO may also explain the long-term survival benefit. There were 4 patients with myeloid neoplasm after cytotoxic therapy, all of whom received ATRA/chemotherapy induction and consolidation.
For the entire cohort, conventional risk factors (presentation leukocyte count and Sanz score) were no longer prognostic. Previous studies have shown that frontline ATO improves outcomes in low/intermediate-risk APL.9,10,49 In our cohort, when frontline ATO was used, low/intermediate-risk and high-risk patients had comparable 60-day survival and 5-year PFS, with these survival rates at >90% in both groups. Therefore, frontline ATO resulted in excellent results in both low/intermediate-risk and high-risk patients. On the contrary, in patients treated with frontline ATRA/chemotherapy, the 60-day survival and 5-year RFS were inferior in both the low/intermediate-risk and high-risk groups. However, partial improvement in 5-year RFS was still possible if ATO was added to the maintenance phase for both low/intermediate-risk and high-risk patients. Therefore, ATO effectively negated the significance of conventional risk factors, best at the time of induction and partially during maintenance.
This study also defined other risks that must be surmounted. The most important risk was CNS involvement on presentation, negatively affecting all analyzed survival rates (60-day survival, post–60-day survival, RFS, and OS). In patients treated with ATRA-containing regimens without ATO, CNS relapse had already become an important problem, occurring in 1% to 5% of cases.56,57 During oral-ATO therapy, arsenic concentration in the cerebrospinal fluid was shown to be ∼18% of that of plasma, suggesting that, other than intrathecal chemotherapy, ATO could represent another way of overcoming this risk.58 Age >50 years was associated with inferior post–60-day survival and OS, likely due to the impact of age-related comorbidities. Male sex was associated with inferior post–60-day survival and RFS, the biological reasons of which are yet unclear. Therapy-related APL portended inferior post–60-day OS, partly related to the negative impact of the underlying primary cancers on long-term survival.
In Hong Kong, relapsed APL was treated with an oral-AAA–based strategy without HSCT.17 In this cohort, HSCT after relapse did not improve survival. In fact, in patients achieving CR2 with ATO-based treatment, few studies addressed the optimal postremission strategy. In one study, the 5-year failure-free survival and OS were 59% and 77% for patients receiving HSCT, respectively.59 In another study evaluating allogeneic HSCT in patients with APL in CR2, the 4-year OS and relapse rate were 62% and 32%, respectively.60 A more recent registry study that evaluated 140 patients with APL in CR2 undergoing autologous HSCT showed a 5-year OS of 78%.61 Patients in this study receiving oral-AAA had a 5-year RFS after CR2 of 59.9% and a 5-year OS after R1 of 71%, which were comparable with those of patients receiving HSCT previously reported.59-61 Therefore, oral-AAA maintenance is an effective postremission strategy for CR2, reducing the risk of HSCT-related morbidity and mortality.
In conclusion, this study showed that ATO significantly improved all outcomes in every risk category of newly diagnosed APL. The observation that ATO given only during maintenance still improved outcome suggests that this may be a remedial strategy for patients who cannot receive ATO during induction. ATO effectively negated the significance of conventional risk factors. Access to ATO, owing to socioeconomic reasons, is the most important obstacle in Asia, significantly curtailing patient survival. In Hong Kong, oral-ATO is available free of cost to patients, having been incorporated into the drug formulary of all public hospitals. In addition to improved patient outcomes, low cost and simplicity of administration without hospitalization are key advantages in Asia,22 where health cost is a major consideration. This issue is being addressed in the phase 2 APL003 trial (NCT04687176) conducted by the APLAC, with a view to supply oral-ATO free of cost to all newly diagnosed patients with APL of any risk category in participating Asian centers.
Acknowledgments
This work was funded by the Health and Medical Research Fund, Health Bureau (project number 08191946) and the Innovation and Technology Fund, Innovation and Technology Commission (project code PRP/029/22FX) of the Government of the Hong Kong Special Administrative Region.
Authorship
Contribution: H.G. conceived the study, treated the patients, analyzed the data, and wrote and approved the manuscript; R.R. collected and analyzed the data and reviewed and approved the manuscript; H.-A.H., X.C.-H.T., A.T., M.G.O., G.-G.G., and C.-L.W. treated the patients, collected the data, and reviewed and approved the manuscript; L.C., R.Y., P.L., L.A., V.W.K.L., and Q.Z. collected and analyzed the data and reviewed and approved the manuscript; G.M.K.L., T.K.Y.W., C.Y.Y.L., W.-J.C., and H.-F.T. treated the patients, collected the data, and reviewed and approved the manuscript; C.R.K. invented the oral-ATO and reviewed and approved the manuscript; and Y.-L.K. invented the oral-ATO, conceived the study, treated the patients, and wrote and approved the manuscript.
Conflict-of-interest disclosure: H.G. and Y.-L.K. are employees of the University of Hong Kong, which holds 2 US patents (7521071 B2 and 8906422 B2), 1 Japan patent (4786341), and 1 European patent (1562616 B1) for the use of oral-ATO in the treatment of leukemias and lymphomas. The remaining authors declare no competing financial interests.
Correspondence: Harinder Gill, Department of Medicine, Professorial Block, Queen Mary Hospital, Pokfulam Rd, Hong Kong, China; email: gillhsh@hku.hk.
References
Author notes
Deidentified data are available on reasonable request from the corresponding author, Harinder Gill (gillhsh@hku.hk).
The full-text version of this article contains a data supplement.