Key Points
Patient-reported fatigue, pain, and impaired QOL are associated with decreased survival in newly diagnosed patients with MM.
The prognostic impact of fatigue and decreased QOL on OS is independent of age, advanced disease stage, and transplant status.
Visual Abstract
Patient-reported outcomes (PROs) are associated with treatment outcomes in multiple myeloma (MM) in the clinical trial setting. However, most PRO tools are time consuming, which hinders use in routine practice. Our institution incorporated a “Hematology Patient-Reported Symptom Screen” (HPRSS), a 3-item questionnaire for fatigue, pain, and quality of life (QOL). The main objective of this study was to evaluate the impact of these symptoms on progression-free survival (PFS) and overall survival (OS) in a real-world cohort of patients with newly diagnosed MM. This retrospective study included patients with MM diagnosed between April 2011 and December 2017, seen at Mayo Clinic (Rochester, MN), who completed the HPRSS. Patients rated their symptoms on a scale from 0 to 10. Clinically relevant symptoms were defined as scores ≥5 for pain and fatigue, and ≤5 for QOL. At diagnosis, 735 patients had scores for all domains. The median follow-up was 8.1 years. Age was associated with increased odds of fatigue and decreased QOL. Female sex and comorbidities were associated with fatigue and pain. Higher disease stage, anemia, lytic lesions, and increased plasma cell burden were associated with fatigue, pain, and decreased QOL. Clinically relevant fatigue, pain, and decreased QOL were associated with decreased PFS and OS. On multivariate analysis including age, International Staging System III, high-risk cytogenetics, and postinduction transplantation, fatigue and decreased QOL were independently associated with decreased OS. A composite HPRSS score stratified patients into 3 groups with distinct OS. Patient-reported symptoms are prognostic in patients with newly diagnosed MM. The prognostic values of fatigue and decreased QOL are independent of age, disease stage, and transplant status.
Introduction
The treatment landscape of multiple myeloma (MM) has evolved significantly in the past 2 decades, resulting in a marked improvement in survival outcomes.1 However, there remains considerable variability in prognosis among individual patients. Although disease-related factors, including disease burden and cytogenetic profile, contribute significantly to prognosis, they do not solely account for this variability. The subjective experiences of patients have a significant impact on various aspects of their treatment journey. However, these are commonly overlooked in both research and clinical settings. Patients with MM have the highest symptom burden among all hematologic malignancies, with fatigue and pain being the most commonly endorsed symptoms.2,3 An increase in symptom burden has been shown to be associated with decreased functional status, impaired health-related quality of life (QOL), and increased healthcare use.4-8 Additionally, this accounts for dose reductions and premature treatment discontinuation in a significant proportion of patients, compromising the effectiveness of treatment.9-11 This highlights the importance of assessing symptoms in clinical practice. Given the tendency of providers to underestimate symptoms reported by patients,12 it is essential that those assessments reflect the patient’s perspective. Patient-reported outcomes (PROs) refer to information on symptoms, well-being, and overall health provided directly by the patient.13 A number of PRO tools have been developed in MM and are increasingly being incorporated as secondary clinical trial end points.14,15 However, there are several barriers to their use in clinical practice, including time constraints, absence of a standardized tool, lack of familiarity with existing tools, and limited resources for administering and analyzing the data.16 Recognizing these barriers, our institution incorporated a brief 3-item questionnaire for fatigue, pain, and overall QOL termed the “Hematology Patient-Reported Symptom Screen” (HPRSS).17 Symptom intensity is captured using the numerical linear analogue self-assessment scale, which is a validated tool for assessing patient-reported symptoms and other QOL domains using a single-item linear scale.18 The main objective of this study was to evaluate the prognostic impact of patient-reported fatigue, pain, and overall QOL on overall survival (OS) in a real-world cohort of patients with newly diagnosed MM.
Methods
Patient population
We included all patients with MM seen at Mayo Clinic (Rochester, MN) between 2011 and 2017 who completed the HPRSS at any ≥1 of the following time points: (1) at diagnosis (before starting treatment); (2) after completing 1, 2, 3, and/or 4 cycles of first-line treatment; and (3) at the time of first progression. A total of 1261 patients completed the HPRSS at ≥1 time points of interest and were included in the corresponding analyses; 735 patients were seen at Mayo Clinic at the time of diagnosis and answered all 3 items of the HPRSS. Data for these patients were used for analyses corresponding to baseline HPRSS. The baseline characteristics of all included patients are shown in supplemental Table 1. The study was approved by the Mayo Clinic institutional review board. All included patients authorized the use of their electronic medical record information for research purposes.
HPRSS questionnaire and clinical data
The HPRSS questionnaire is composed of 3 items assessing the domains of fatigue, pain, and overall QOL using the linear analogue self-assessment format. Patients were asked to rate their average level of fatigue, pain, and overall QOL in the past week on a scale from 0 to 10, with 10 considered the worst score for fatigue, and 10 the best score for overall QOL (supplemental Figure 1). Patients completed the questionnaire using paper forms. The scores were then entered into the electronic medical record by a clinical assistant and were available for review by the treating provider during the visit. We abstracted the HPRSS scores at different time points and collected additional laboratory and clinical data including: disease stage according to the International Staging System (ISS)19 and revised ISS,20 cytogenetic risk profile by fluorescence in situ hybridization (FISH), first-line treatment regimen, autologous stem cell transplantation (ASCT), and best response to first-line treatment. Upfront ASCT was defined as ASCT after first-line induction. High-risk FISH was defined by the presence of ≥1 of the following: high-risk immunoglobulin H translocation (t[4;14], t[14;16], or t[14;20] translocation), deletion 17p, and 1q gain/amplification.20,21 Treatment response was defined in accordance with the International Myeloma Working Group criteria.22 We also collected data on comorbidities and calculated the Charlson comorbidity index (CCI) for each patient.23
Statistical analysis
Logistic regression analysis was used to evaluate the association between clinically relevant symptoms and baseline patient- and disease-related characteristics. For fatigue and pain, scores of ≥5 were considered to be clinically relevant; for QOL, a score of ≤5 was considered clinically relevant. The Wilcoxon signed-rank test was used to compare the scores of individual patients at 2 different time points; a change of at least 1 point (± 1 half of the standard deviation) was considered to be clinically significant.18 The total HPRSS score (0-30) was also calculated at different time points by summing the individual scores. For this purpose, the QOL score was reversed so that the 3 scores were reported in the same direction. Patients were divided into 3 groups using tertiles of the total score at the time of diagnosis. The same cutoffs were used to divide patients into 3 groups at different time points. Progression-free survival (PFS) was defined as the time from diagnosis to disease progression or death from any cause. Patients without an event at last follow-up were censored. OS was calculated from each time point until death from any cause, with patients alive at the time of last follow-up censored. PFS and OS were estimated using the Kaplan-Meier method and compared between groups using the log-rank test. The impact of age, ISS, FISH cytogenetic risk, and score group (based on total score tertiles) on PFS and OS were assessed using univariate and multivariate Cox proportional regression models. For all tests, 2-sided P values < .05 were considered statistically significant. Statistical analysis was performed using JMP statistical software.
Results
Baseline HPRSS scores
A total of 752, 745, and 750 patients provided scores for fatigue, pain, and overall QOL, respectively, at the time of diagnosis; 735 patients provided scores for all domains; they were diagnosed with MM between 21 April 2011 and 20 December 2017. The median age was 66 years, and 38% were female. Forty percent (292 patients) underwent transplantation after induction. Induction therapies were: proteasome inhibitor (PI) or immunomodulatory drug–based doublets (27%), PI + immunomodulatory drug triplets (28%), PI + alkylator triplets (38%), quadruplets (4%), and other (3%).
Factors associated with clinically relevant symptoms
At baseline, the median scores for fatigue, pain, and QOL were 4 (interquartile range [IQR], 2-6), 3 (IQR, 1-5), and 7 (IQR, 5-9), respectively; 43%, 34%, and 31% patients had clinically relevant fatigue, pain, and QOL, respectively. There was high concordance between fatigue, pain, and decreased QOL. Age was associated with increased odds of reporting fatigue and decreased QOL. Females and patients with CCI of ≥3 had higher odds or reporting fatigue and pain. ISS/revised ISS III, anemia, ≥60% bone marrow plasma cells, and lytic lesions were associated with higher odds of fatigue, pain, and decreased QOL. Patients who underwent upfront transplantation after first-line induction had lower odds of having reported fatigue or decreased QOL at baseline. There was no association between the cytogenetic risk profile and clinically relevant symptoms in any of the 3 domains. There was no significant difference between patients with and without clinically relevant fatigue/pain/decreased QOL in terms of receiving a doublet vs triplet/quadruplet therapy for induction (Table 1).
Association between baseline characteristics and clinically relevant fatigue, pain, and decreased QOL
| Variable . | Fatigue ≥5, odds ratio (95% CI) . | P value . | Pain ≥5, odds ratio (95% CI) . | P value . | QOL ≤5, odds ratio (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Age | 1.03 (1.01-1.04) | <.001 | 1.01 (1.00-1.02) | .18 | 1.02 (1.00-1.03) | .02 |
| >65 y | 1.4 (1.0-1.8) | .04 | 1.0 (0.8-1.4) | .84 | 1.1 (0.8-1.4) | .51 |
| >75 y | 2.0 (1.4-2.9) | <.001 | 1.3 (0.9-1.8) | .19 | 1.6 (1.1-2.3) | .01 |
| Female | 1.4 (1.0-1.9) | .03 | 1.7 (1.2-2.3) | .001 | 1.0 (0.7-1.3) | .76 |
| CCI ≥3 | 1.4 (1.0-2.0) | .03 | 1.5 (1.1-2.1) | .02 | 1.1 (0.8-1.5) | .67 |
| ISS III (vs I/II) | 2.2 (1.5-3.2) | <.001 | 1.8 (1.3-2.6) | .002 | 1.6 (1.1-2.4) | .02 |
| High-risk FISH∗ | 1.0 (0.8-1.5) | .85 | 1.0 (0.7-1.5) | .99 | 0.9 (0.6-1.4) | .63 |
| R-ISS III | 2.8 (1.5-5.0) | <.001 | 2.3 (1.3-4.1) | .003 | 2.4 (1.4-4.3) | .003 |
| Hb <10 g/dL | 2.3 (1.7-3.3) | <.001 | 1.5 (1.1-2.2) | .02 | 1.7 (1.2-2.4) | .003 |
| Ca >11 mg/dL | 0.9 (0.4-2.0) | .88 | 1.4 (0.6-3.1) | .39 | 2.7 (1.3-5.8) | .01 |
| Cr >2 mg/dL | 1.7 (1.0-2.9) | .05 | 1.3 (0.7-2.3) | .36 | 0.8 (0.5-1.5) | .59 |
| BMPCs ≥60 (%) | 1.7 (1.2-2.3) | .001 | 1.7 (1.2-2.4) | .001 | 1.4 (1.0-1.9) | .06 |
| Lytic lesions | 1.5 (1.1-2.1) | .02 | 2.5 (1.7-3.6) | <.001 | 1.4 (1.0-2.1) | .05 |
| ASCT after induction | 0.7 (0.5-1.0) | .03 | 0.8 (0.6-1.1) | .20 | 0.7 (0.5-1.0) | .04 |
| Fatigue, ≥5 | NA | NA | ||||
| Pain, ≥5 | 9.3 (6.5-13.2) | <.001 | NA | NA | ||
| QOL, ≤5 | 3.8 (2.7-5.2) | <.001 | 4.6 (3.3-6.4) | <.001 | NA | NA |
| Variable . | Fatigue ≥5, odds ratio (95% CI) . | P value . | Pain ≥5, odds ratio (95% CI) . | P value . | QOL ≤5, odds ratio (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Age | 1.03 (1.01-1.04) | <.001 | 1.01 (1.00-1.02) | .18 | 1.02 (1.00-1.03) | .02 |
| >65 y | 1.4 (1.0-1.8) | .04 | 1.0 (0.8-1.4) | .84 | 1.1 (0.8-1.4) | .51 |
| >75 y | 2.0 (1.4-2.9) | <.001 | 1.3 (0.9-1.8) | .19 | 1.6 (1.1-2.3) | .01 |
| Female | 1.4 (1.0-1.9) | .03 | 1.7 (1.2-2.3) | .001 | 1.0 (0.7-1.3) | .76 |
| CCI ≥3 | 1.4 (1.0-2.0) | .03 | 1.5 (1.1-2.1) | .02 | 1.1 (0.8-1.5) | .67 |
| ISS III (vs I/II) | 2.2 (1.5-3.2) | <.001 | 1.8 (1.3-2.6) | .002 | 1.6 (1.1-2.4) | .02 |
| High-risk FISH∗ | 1.0 (0.8-1.5) | .85 | 1.0 (0.7-1.5) | .99 | 0.9 (0.6-1.4) | .63 |
| R-ISS III | 2.8 (1.5-5.0) | <.001 | 2.3 (1.3-4.1) | .003 | 2.4 (1.4-4.3) | .003 |
| Hb <10 g/dL | 2.3 (1.7-3.3) | <.001 | 1.5 (1.1-2.2) | .02 | 1.7 (1.2-2.4) | .003 |
| Ca >11 mg/dL | 0.9 (0.4-2.0) | .88 | 1.4 (0.6-3.1) | .39 | 2.7 (1.3-5.8) | .01 |
| Cr >2 mg/dL | 1.7 (1.0-2.9) | .05 | 1.3 (0.7-2.3) | .36 | 0.8 (0.5-1.5) | .59 |
| BMPCs ≥60 (%) | 1.7 (1.2-2.3) | .001 | 1.7 (1.2-2.4) | .001 | 1.4 (1.0-1.9) | .06 |
| Lytic lesions | 1.5 (1.1-2.1) | .02 | 2.5 (1.7-3.6) | <.001 | 1.4 (1.0-2.1) | .05 |
| ASCT after induction | 0.7 (0.5-1.0) | .03 | 0.8 (0.6-1.1) | .20 | 0.7 (0.5-1.0) | .04 |
| Fatigue, ≥5 | NA | NA | ||||
| Pain, ≥5 | 9.3 (6.5-13.2) | <.001 | NA | NA | ||
| QOL, ≤5 | 3.8 (2.7-5.2) | <.001 | 4.6 (3.3-6.4) | <.001 | NA | NA |
BMPC, bone marrow plasma cells; Ca, calcium; Cr, creatinine; Hb, hemoglobin; R-ISS, revised ISS.
Statistically significant P values are indicated in bold.
Defined as presence of t(4;14), t(14;16), t(16;20), del 17p, and/or 1q gain/amplification.
Prognostic impact of baseline fatigue, pain, and QOL scores
The median follow-up was 8.1 (95% confidence interval [CI], 7.8-8.4) years. There was no association between clinically relevant symptoms and achievement of more than, or equal to, partial response or more than, or equal to, very good partial response to first-line induction.
There was a significant association between clinically relevant symptoms and PFS; PFS was 2.3 (95% CI, 2.0-2.7) vs 3.5 (95% CI, 3.0-3.9) years in patients with and without clinically relevant fatigue, respectively (P < .001); 2.6 (95% CI, 2.1-2.9) vs 3.3 (95% CI, 2.8 -3.6) years in patients with and without clinically relevant pain, respectively (P = .03); and 2.3 (95% CI, 1.9-2.8) vs 3.1 (95% CI, 2.8-3.5) years in patients with and without clinically relevant decreased QOL, respectively (P = .04).
Age, high-risk FISH, and fatigue score of ≥5 were all independently associated with decreased PFS in a multivariate model that included age, ISS III, high-risk FISH, ASCT after induction, fatigue score of ≥5, pain score of ≥5, and QOL score of ≤5 scores (Table 2).
Univariate and multivariate PFS analysis for fatigue, pain, and QOL at diagnosis
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age | 1.03 (1.02-1.03) | <.001 | 1.02 (1.01-1.03) | <.001 |
| ISS III | 1.6 (1.3-2.0) | <.001 | 1.3 (1.0-1.6) | .06 |
| High-risk FISH∗ | 1.4 (1.1-1.7) | .001 | 1.5 (1.2-1.9) | .002 |
| ASCT after induction | 0.7 (0.6-0.8) | <.001 | 0.9 (0.7-1.2) | .62 |
| Fatigue, ≥5 | 1.5 (1.3-1.8) | <.001 | 1.5 (1.2-1.9) | .001 |
| Pain, ≥5 | 1.2 (1.0-1.4) | .03 | 0.9 (0.7-1.2) | .48 |
| QOL, ≤5 | 1.2 (1.0-1.4) | .04 | 1.1 (0.8-1.4) | .58 |
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age | 1.03 (1.02-1.03) | <.001 | 1.02 (1.01-1.03) | <.001 |
| ISS III | 1.6 (1.3-2.0) | <.001 | 1.3 (1.0-1.6) | .06 |
| High-risk FISH∗ | 1.4 (1.1-1.7) | .001 | 1.5 (1.2-1.9) | .002 |
| ASCT after induction | 0.7 (0.6-0.8) | <.001 | 0.9 (0.7-1.2) | .62 |
| Fatigue, ≥5 | 1.5 (1.3-1.8) | <.001 | 1.5 (1.2-1.9) | .001 |
| Pain, ≥5 | 1.2 (1.0-1.4) | .03 | 0.9 (0.7-1.2) | .48 |
| QOL, ≤5 | 1.2 (1.0-1.4) | .04 | 1.1 (0.8-1.4) | .58 |
HR, hazard ratio.
Defined as presence of t(4;14), t(14;16), t(16;20), del 17p, and/or 1q gain/amplification.
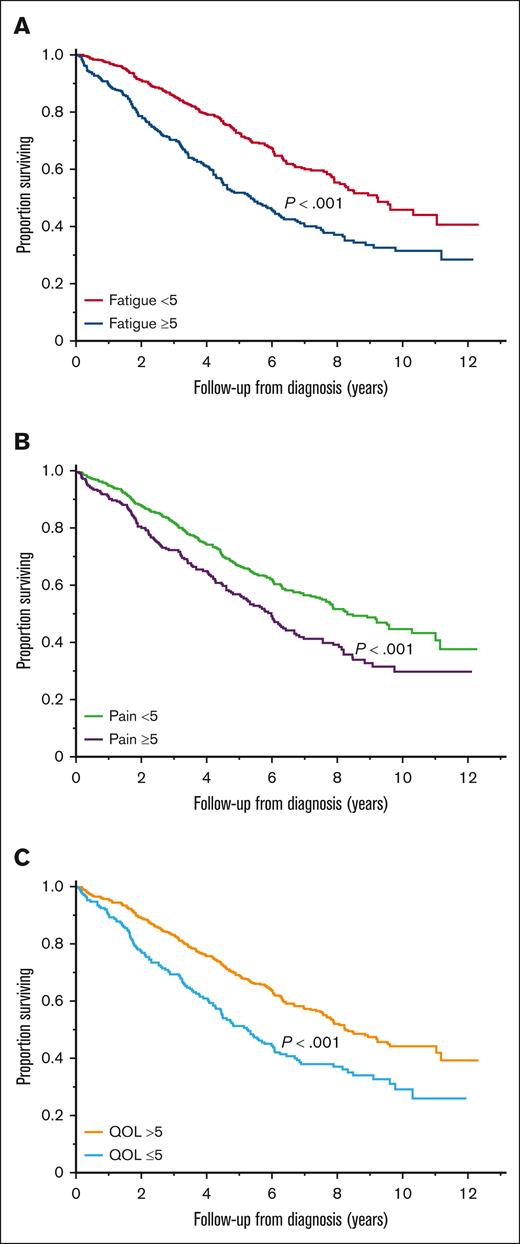
There was a significant association between clinically relevant symptoms and OS; the median OS was 5.4 (95% CI, 4.5-6.2) vs 9.3 (95% CI, 7.9-11.1) years in patients with and without clinically relevant fatigue, respectively (P < .001; Figure 1A); 6.0 (95% CI, 5.1-6.7) vs 8.4 (95% CI, 7.6-10.3) years in patients with and without clinically relevant pain, respectively (P < .001; Figure 1B); and 5.3 (95% CI, 4.4-6.1) vs 8.2 (95% CI, 7.6-9.6) years in patients with and without a clinically relevant decrease in overall QOL, respectively (P < .001; Figure 1C).
Association between baseline symptoms and OS. (A) OS (years) in patients with and without clinically relevant fatigue. (B) OS (years) in patients with and without clinically relevant pain. (C) OS (years) in patients with and without clinically relevant impairment in overall QOL.
Association between baseline symptoms and OS. (A) OS (years) in patients with and without clinically relevant fatigue. (B) OS (years) in patients with and without clinically relevant pain. (C) OS (years) in patients with and without clinically relevant impairment in overall QOL.
On multivariate analysis, all variables were independently associated with decreased OS, except pain score of ≥5 (Table 3).
Univariate and multivariate OS analysis for fatigue, pain, and QOL at diagnosis
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age | 1.05 (1.04-1.06) | <.001 | 1.04 (1.03-1.06) | <.001 |
| ISS III | 2.3 (1.8-2.8) | <.001 | 1.4 (1.0-1.9) | .04 |
| High-risk FISH∗ | 2.0 (1.6-2.4) | <.001 | 2.0 (1.5-2.7) | <.001 |
| ASCT after induction | 0.5 (0.4-0.5) | <.001 | 0.6 (0.4-0.8) | .001 |
| Fatigue, ≥5 | 1.8 (1.4-2.2) | <.001 | 1.7 (1.3-2.4) | <.001 |
| Pain, ≥5 | 1.5 (1.2-1.9) | <.001 | 0.9 (0.7-1.3) | .74 |
| QOL, ≤5 | 1.7 (1.3-2.1) | <.001 | 1.4 (1.0-1.9) | .03 |
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age | 1.05 (1.04-1.06) | <.001 | 1.04 (1.03-1.06) | <.001 |
| ISS III | 2.3 (1.8-2.8) | <.001 | 1.4 (1.0-1.9) | .04 |
| High-risk FISH∗ | 2.0 (1.6-2.4) | <.001 | 2.0 (1.5-2.7) | <.001 |
| ASCT after induction | 0.5 (0.4-0.5) | <.001 | 0.6 (0.4-0.8) | .001 |
| Fatigue, ≥5 | 1.8 (1.4-2.2) | <.001 | 1.7 (1.3-2.4) | <.001 |
| Pain, ≥5 | 1.5 (1.2-1.9) | <.001 | 0.9 (0.7-1.3) | .74 |
| QOL, ≤5 | 1.7 (1.3-2.1) | <.001 | 1.4 (1.0-1.9) | .03 |
Defined as presence of t(4;14), t(14;16), t(16;20), del 17p, and/or 1q gain/amplification.
Prognostic impact of total HPRSS score at baseline
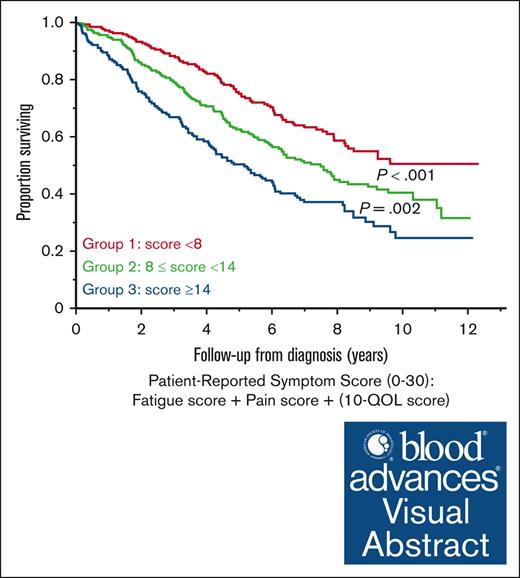
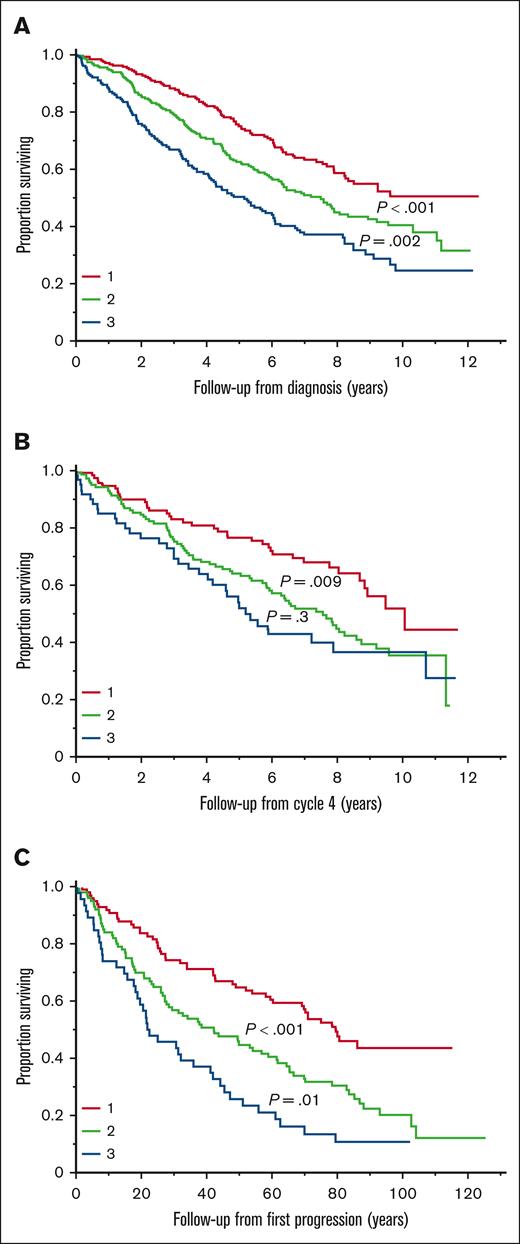
The total HPRSS score was calculated for the 735 patients with available data for all 3 domains at the time of diagnosis. The median total score was 10 (IQR, 6-15). The median OS from diagnosis was 9.6 vs 5.4 years in patients with a total score of ≤10 vs >10, respectively (P < .001). Patients were divided into 3 groups using tertiles of the total score: group 1 (score of <8), group 2 (score of 8-14), and group 3 (score of >14). There was a significant difference in PFS between groups 1 and 2 (3.5 vs 2.8 years; P = .02) and groups 1 and 3 (3.5 vs 2.2 years; P < .001) but not between groups 2 and 3 (P = .18). There was a significant difference in OS between all 3 groups; median OS was not reached (95% CI, 8.2 to not reached) vs 7.5 (95% CI, 6.0-9.0) vs 5.2 (95% CI, 4.1-6.1) years in the 3 groups, respectively (Figure 2A).
Prognostic impact of total HPRSS score at different time points. (A) Median OS in groups 1, 2, and 3 at the time of diagnosis. (B) Median OS in groups 1, 2, and 3 after 4 cycles of induction treatment. (C) Median OS in groups 1, 2, and 3 at the time of first disease progression.
Prognostic impact of total HPRSS score at different time points. (A) Median OS in groups 1, 2, and 3 at the time of diagnosis. (B) Median OS in groups 1, 2, and 3 after 4 cycles of induction treatment. (C) Median OS in groups 1, 2, and 3 at the time of first disease progression.
On multivariate analysis including age, ISS III, high-risk FISH, ASCT after induction, and total HPRSS score group, there was an increased hazard of death for group 2 vs 1 and group 3 vs 1 but not group 3 vs 2 (Table 4).
Univariate and multivariate OS analysis for the total HPRSS score at baseline
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Total score group∗ | ||||
| 2 vs 1 | 1.5 (1.1-2.0) | .003 | 1.5 (1.0-2.1) | .03 |
| 3 vs 1 | 2.2 (1.7-3.0) | <.001 | 1.6 (1.1-2.3) | .02 |
| 3 vs 2 | 1.5 (1.2-1.9) | .002 | 1.1 (0.8-1.5) | .71 |
| Age | 1.05 (1.03-1.07) | <.001 | ||
| High-risk FISH† | 2.0 (1.5-2.7) | <.001 | ||
| ISS III | 1.4 (1.1-2.0) | .02 | ||
| ASCT after induction | 0.6 (0.4-0.8) | .003 | ||
| Variable . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Total score group∗ | ||||
| 2 vs 1 | 1.5 (1.1-2.0) | .003 | 1.5 (1.0-2.1) | .03 |
| 3 vs 1 | 2.2 (1.7-3.0) | <.001 | 1.6 (1.1-2.3) | .02 |
| 3 vs 2 | 1.5 (1.2-1.9) | .002 | 1.1 (0.8-1.5) | .71 |
| Age | 1.05 (1.03-1.07) | <.001 | ||
| High-risk FISH† | 2.0 (1.5-2.7) | <.001 | ||
| ISS III | 1.4 (1.1-2.0) | .02 | ||
| ASCT after induction | 0.6 (0.4-0.8) | .003 | ||
Statistically significant P values are indicated in bold.
Groups are defined using tertiles of the total HPRSS score: group 1 (score of <8), group 2 (score of 8-14), and group 3 (score of >14)
Defined as presence of t(4;14), t(14;16), t(16;20), del 17p, and/or 1q gain/amplification.
Prognostic impact of total HPRSS score at different time points
After 4 cycles of induction treatment, 326 patients had available data to calculate the total HPRSS score. The median score was 10 (IQR, 6-14). Groups 1, 2, and 3 included 118 (36%), 146 (45%), and 62 (19%) patients, respectively. The median OS was 10.1 vs 7.6 vs 5.2 years in groups 1, 2, and 3, respectively (Figure 2B). At the time of first disease progression, 244 had available data for the total score. The median score was 9 (IQR, 5-13). Groups 1, 2, and 3 included 98 (40%), 100 (41%), and 46 (19%) patients, respectively. The median OS was 6.7 vs 3.5 vs 1.9 years in the 3 groups, respectively (Figure 2C).
Trajectory of symptoms
The HPRSS score was available for 400, 451, 339, and 326 patients after completing 1, 2, 3, and 4 cycles of first-line treatment, respectively. Figure 3A illustrates the proportion of patients with clinically relevant fatigue, pain, and decreased QOL at each time point. Ninety-five patients had HPRSS scores for all domains available at baseline and at each of the intervals. Figure 3B illustrates the trajectory of symptoms in this subgroup.
Trajectory of symptoms. Proportion of patients with clinically relevant fatigue, pain, and impaired QOL at baseline and after 1, 2, 3, and 4 cycles of treatment among (A) patients with available data at any time point and (B) patients with available data for all time points.
Trajectory of symptoms. Proportion of patients with clinically relevant fatigue, pain, and impaired QOL at baseline and after 1, 2, 3, and 4 cycles of treatment among (A) patients with available data at any time point and (B) patients with available data for all time points.
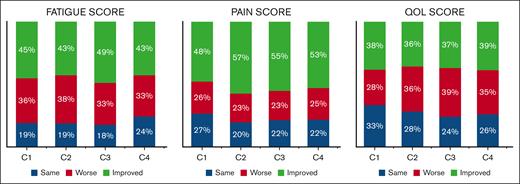
Figure 4 illustrates the proportion of patients with stable, improved, and worse symptoms at each time point compared with baseline. After completing the first treatment cycle, 36% reported worse fatigue scores, whereas 45% had improvement compared with baseline. After 4 cycles, 33% had worse scores compared with baseline, whereas 43% had improved scores, with another 24% reporting the same score as baseline. For pain, 26% reported worse scores after the first cycle, whereas 48% improved. After 4 cycles, 25% had worse pain scores, whereas 53% had improved. For QOL, 38% had worse scores after the first cycle and 28% had improved scores. After 4 cycles, 35% of patients had worse scores compared with baseline, whereas 39% had improved scores. Supplemental Figure 2 shows the corresponding percentages when the threshold for a clinically significant change was set at 2 instead of 1.
Changes in symptom scores. Proportion of patients with changes in symptom scores by at least 1 point compared with baseline.
Changes in symptom scores. Proportion of patients with changes in symptom scores by at least 1 point compared with baseline.
Among all patients, 177, 174, and 176 patients had available scores both at baseline and after 4 cycles for fatigue, pain, and QOL, respectively. After 4 cycles, there was no improvement in median fatigue (median: 4) and QOL scores (median: 7), but there was significant improvement in the pain score (median: 2 vs 3; 2-sided Wilcoxon signed rank, P < .001).
At the time of first progression, pain scores were lower than at diagnosis (median: 2 vs 3; P < .001), but there was no significant difference in fatigue (median: 3 vs 4; P = .07) and QOL scores (median: 8 vs 7; P = .08) between the 2 time points.
Discussion
With the increased use of PRO measures in clinical trials, there have been several reports on their prognostic impact in patients with MM.24 However, older patients, those with multiple comorbidities, and/or reduced performance status are excluded from most clinical trials. This poses limitations on the generalizability of the findings to the real-world population.25,26 Moreover, the length and complexity of most PRO questionnaires hinders their integration in routine practice. In this study, we reported on the longitudinal use of a simple 3-item symptom screen in patients with MM in a real-world setting. We observed that >50% of patients had a clinically relevant symptom in at least 1 of the 3 domains, with fatigue being the most common. We observed a high concordance between the 3 symptoms, which is consistent with findings from a recent pragmatic trial conducted at Mayo Clinic (MN) and its affiliated community-based hospitals, which included >30 000 patients with cancer.27 Not surprisingly, patients with higher stage and disease burden had increased odds of reporting clinically relevant fatigue, pain, and decreased QOL. We found that females had higher odds of reporting clinically relevant fatigue and pain, and that older patients had higher odds of reporting clinically relevant fatigue and decreased QOL. Several cancer studies have similarly reported increased symptom severity in females including fatigue, pain, and psychological symptoms.28-30 However, this observation has not been consistent across studies.27,31 Similarly, the association between age and symptom severity in patients with cancer has been variable across studies. In a large Danish study, older patients with hematological malignancies had increased pain and decreased QOL compared with younger patients. In contrast, several studies have reported increased odds of moderate-to-severe symptoms and impaired QOL in younger patients.2,27,28,30,32,33 In a large population-based study, females had higher odds of reporting moderate-to-severe fatigue, pain, and impaired well-being, and older age was associated with lower odds of moderate-to-severe pain, depression, and nausea; that study included transplant-ineligible patients only. In the same study, a higher CCI (cutoff of 2) was not associated with increased odds of moderate-to-severe symptoms.32 The prognostic impact of comorbidities on OS has been established among patients with MM. However, the association between comorbidities and symptom burden is unclear. In contrast to the aforementioned study, we found that a CCI of ≥3 was associated with higher odds of reporting clinically relevant fatigue and pain. The variability across studies is, at least in part, related to differences in patient populations, symptom assessment tools, timing of assessments, and cutoffs used to define clinically relevant symptoms. Patients with lower socioeconomic status (SES) have been reported to have higher symptom burden in prior studies.30,32 Our population, which is composed of patients seen at an academic center, does not provide an adequate representation of patients with lower SES. In addition, our population is predominantly composed of White individuals. These factors precluded the assessment of the impact of SES and race/ethnicity on symptom burden.
In recent years, there has been increased use of PROs in MM clinical trials, mainly in the form of QOL questionnaires, with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 tool being the most commonly used.34 Multiple studies have reported an association between various QOL domains and mortality in patients with newly diagnosed and relapsed/refractory MM.24 Given limited use in routine practice, the prognostic impact of PROs in the real-world setting has yet to be established. In a population-based Canadian study, a higher symptom score, using the Edmonton Symptom Assessment System, was associated with increased likelihood of emergency department visits and/or hospitalizations within 2 weeks in transplant-ineligible patients with MM.35 Another large study in The Netherlands showed an association between the EORTC QLQ-C30 summary score and all-cause mortality in patients with different malignancies including those with MM. The prognostic value of this score was independent of clinical, demographic, and social characteristics.36 A smaller United States–based study found that a high-distress score, measured using a 7-item PRO tool, was present in a quarter of patients with MM seen in the outpatient setting, and was associated with a trend toward decreased OS.37 In clinical practice, the utility of PROs is highly dependent on their feasibility. Valuable information on a patient’s wellbeing can be captured with quick, simple tools. A single question assessing self-reported health was shown to predict all-cause mortality in patients with a subsequent MM diagnosis.38 In our study, single-item assessments for fatigue, pain, and QOL could stratify patients into 2 groups with distinct PFS and OS. The impact of fatigue on PFS and OS was independent of age and disease characteristics. Similarly, impaired QOL was an independent predictor of inferior OS. We also used the composite symptom score (HPRSS score) of fatigue, pain, and QOL at diagnosis to stratify patients into 3 prognostic groups; this score also demonstrated utility at the time of progression. Although we cannot ascertain the cause of increased mortality using the available data, we hypothesize that increased symptom burden is associated with an increased risk of dose reductions and premature treatment discontinuation, which contributes to increased disease-related mortality. Although we did not observe differences in the proportion of patients who received double vs triplet-based first-line therapy, we cannot draw conclusions about the intensity of treatment in patients with and without clinically relevant symptoms because of the absence of data on dosing, duration, and subsequent treatments. We have previously shown that patients with clinically relevant symptoms are more likely to be frail compared with those without clinically relevant symptoms.39 Therefore, we hypothesize that nonrelapse mortality is also higher in patients with clinically relevant symptoms. In addition to baseline assessments, it is imperative that symptoms and QOL are reassessed throughout the disease course. A few studies have reported longitudinal symptoms collected using the EORTC QLQ-30 tool40; in general, patients reported improvements in fatigue, pain, physical functioning, and QOL scores during first-line treatment. This suggests that symptoms at diagnosis may be, at least in part, disease related.40 Another Canadian population-based study examined the trajectory of symptoms in transplant-ineligible patients during the first year after diagnosis, showing a decrease in the proportion of patients with moderate-to-severe pain, fatigue, and well-being over time. However, >30% of patients still reported moderate-to-severe fatigue and/or pain by the end of the first year.32 In our study, there was a significant improvement in the pain score after 4 treatment cycles but no significant change in fatigue or QOL scores. When looking at the proportion of patients with clinically relevant symptoms at each time point, we observed a decrease in pain as early as 1 cycle, reaching a nadir after 2 cycles. QOL worsened after the first cycle but improved over the next 2 cycles, and was similar to baseline after 4 cycles. Fatigue was overall stable. A similar trend in all symptoms was seen in the subset of patients who had available data at all time points.
At the individual level, experiences during treatment were variable; although some patients experienced improvement in symptoms, others reported stability or worsening, underscoring the importance of an individualized treatment approach. Importantly, the significant proportion of patients with clinically relevant symptoms seen in our study and other studies32,41 highlights the need for more aggressive supportive care strategies. The collection of PROs has the potential to identify patients who would benefit from closer monitoring and supportive care. This can contribute to improvement in symptom control, patient and provider communication, QOL, and treatment outcomes.42,43
Our study has several limitations: the use of an outpatient-based questionnaire excluded hospitalized patients, who may have a higher disease and symptom burden. Additionally, the follow-up assessments may exclude patients with early mortality and/or those with premature treatment discontinuation. As a tertiary care and transplant center, a significant proportion of patients seen at our institution are referred for transplant at different stages of their treatment course, which likely contributes to the variability in the number and timing of HPRSS data available for patients in this study. The absence of data on subsequent lines of treatment and causes of death precludes drawing accurate conclusions regarding the reasons for increased mortality in patients with a higher symptom burden.
Nonetheless, this study is based on a large, real-world cohort, treated with contemporary regimens. The availability of disease-related data and patient-related data allowed us to evaluate the independent prognostic impact of the HPRSS score.
Conclusions
Patient-reported symptom screening provides prognostic information in real-world patients with newly diagnosed MM. The prognostic impact of fatigue and decreased QOL on OS is independent of age, disease stage, and transplant status. The HPRSS is a simple 3-item screening tool that is feasible and can be adapted to various practice settings. Beyond their prognostic value, the assessment of PROs in clinical practice offers opportunities for supportive care intervention and paves the way for a patient-centered, holistic approach to cancer care.
Authorship
Contribution: N.A. and S.K. designed the study, collected and analyzed the data, and wrote the first draft of the manuscript; A.B. and A.M. wrote the first draft of the manuscript; and F.B., P.K., A.D., M.G., S.H., M.E., D.D., J.C., M.B., Y.L., T.K., R.W., C.T., T.M., and S.V.R. provided critical revision and final approval of the manuscript version for publication.
Conflict-of-interest disclosure: A.D. received research funding from Celgene, Millennium Pharmaceuticals, Pfizer, and Janssen, and a travel grant from Pfizer. M.G. served as a consultant for Millennium Pharmaceuticals and received honoraria from Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. S.V.R. has received grants from the National Institutes of Health and research funding from Celgene for clinical trials. S.K. served as a consultant for Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Janssen, and Bristol Myers Squibb, and received research funding from Celgene, Millennium Pharmaceuticals, Novartis, Onyx Pharmaceuticals, AbbVie, Janssen, and Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Shaji Kumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: kumar.shaji@mayo.edu.
References
Author notes
The data generated in this study are available on request from the corresponding author, Shaji Kumar (kumar.shaji@mayo.edu).
The full-text version of this article contains a data supplement.