Key Points
TBL1X plays a multifaceted role in promoting the proliferation and survival of MCL cells.
Targeting TBL1X promotes destabilization of cyclin D1 and RAD51, resulting in cell cycle arrest, DNA damage, and MCL cell death.
Visual Abstract
Mantle cell lymphoma (MCL) is an incurable B-cell lymphoma characterized by significant genomic instability. Patients with MCL who progress on targeted therapies have a short survival; thus, novel therapeutic strategies are urgently needed. Overexpression of transducin β-like protein 1 X-linked (TBL1X) has been documented in several types of cancer and associated with poor prognosis. TBL1X is a critical regulator of multiple oncogenic networks; however, its function in MCL has not been explored. Our data show that, unlike normal B cells, MCL cells express abundant levels of TBL1X and that genetic knockdown of TBL1X and treatment with tegavivint (Iterion), a first-in-class small molecule targeting TBL1X, promote MCL cell death in vitro and in vivo. Moreover, TBL1X controls the stability of key MCL oncogenic drivers, cyclin D1 and RAD51; and targeting TBL1X results in significant DNA damage, cell cycle arrest, and ultimately cell death. Combining tegavivint with poly(adenosine 5′-diphosphate-ribose) polymerase-1/2 inhibitor talazoparib results in synergistic MCL cell death in vitro, and in vivo this combination significantly prolongs the survival of a patient-derived MCL xenograft. Together, our results define the role of TBL1X in maintaining genomic stability in MCL and establish targeting TBL1X as a novel therapeutic strategy for patients with this incurable disease.
Introduction
Mantle cell lymphoma (MCL) is a rare, aggressive B-cell non-Hodgkin lymphoma. Although significant progress has been made in the MCL treatment landscape over the past decade, if patients progress on targeted therapies (eg, ibrutinib) or CD19 chimeric antigen receptor T-cell therapy, median overall survival (OS) is a dismal 3 to 8 months.1-4 Thus, there is an urgent need for novel therapeutic strategies for this disease.
MCL is characterized by a high degree of genomic instability, with >90% of the MCL cases having highly altered genomes. Several studies have identified frequent gains/amplifications and homozygous/heterozygous losses in MCL.5,6 Additionally, many of the individual gene mutations identified in MCL contribute further to genetic instability via interference with DNA damage response (DDR) pathways.7,8 The cytogenetic hallmark of this disease, chromosomal translocation t(11;14)(q13;q32), leads to overexpression of cyclin D1 (encoded by CCND1), a key cell cycle regulator and mediator of the DDR.9-13 Direct functional links have been identified between cyclin D1 and several DDR proteins (summarized in Table 1), including radiation sensitive protein 51 (RAD51), a key mediator of homologous recombination (HR) and DNA double-stranded break (DSB) repair. In addition to CCND1, other genetic alterations implicated in MCL pathogenesis involve key mediators of the cell cycle, DDR, and apoptosis, and include TP53, ataxia telangiectasia mutated (ATM), and checkpoint kinase 1/2 (CHK1/2), which are mutated in up to 50% of MCL cases.14-19 Because normal B-cell development is reliant upon DDR mechanisms, dysregulation of these processes represents not only a key driver of B-cell lymphomagenesis but also a therapeutic opportunity.7,20 Targeting remaining, functional DDR mechanisms has been proven an effective approach for cancer therapy development, and the concept of “synthetic lethality” has been validated clinically with poly(adenosine 5′-diphosphate-ribose) polymerase (PARP) inhibitor treatment for BRCA1/2-mutated cancers.21-23
Summary of functional interactions between cyclin D1 and DNA damage proteins
| DDR protein . | Functional link with cyclin D1 . | References . |
|---|---|---|
| RAD51 | Cyclin D1 tethered to chromatin → recruits RAD51 recombinase → HR | 24,25 |
| BRCA1 | Cyclin D1–CDK4 kinase phosphorylates BRCA1 (Ser632) → BRCA1 not recruited to target promoters Cyclin D1 competes with BRCA1 for ERα binding → reduced BRCA1-mediated repression of ERα transcriptional activity | 26 27 |
| BRCA2 | Physical interaction of cyclin D1/BRCA2/RAD51 with Sp1 transcription factor → complexes with γH2AX and members of the MRN complex → DSB repair | 28 |
| PCNA | Physical interaction between cyclin D1 and PCNA → interaction with BRCA1 during UV-induced DNA damage repair | 29 |
| RFC | Physical interaction between cyclin D1 and RFC → interaction with BRCA1 during UV-induced DNA damage repair | 29 |
| DDR protein . | Functional link with cyclin D1 . | References . |
|---|---|---|
| RAD51 | Cyclin D1 tethered to chromatin → recruits RAD51 recombinase → HR | 24,25 |
| BRCA1 | Cyclin D1–CDK4 kinase phosphorylates BRCA1 (Ser632) → BRCA1 not recruited to target promoters Cyclin D1 competes with BRCA1 for ERα binding → reduced BRCA1-mediated repression of ERα transcriptional activity | 26 27 |
| BRCA2 | Physical interaction of cyclin D1/BRCA2/RAD51 with Sp1 transcription factor → complexes with γH2AX and members of the MRN complex → DSB repair | 28 |
| PCNA | Physical interaction between cyclin D1 and PCNA → interaction with BRCA1 during UV-induced DNA damage repair | 29 |
| RFC | Physical interaction between cyclin D1 and RFC → interaction with BRCA1 during UV-induced DNA damage repair | 29 |
ERα, estrogen receptor α; MRN, Mre11, Rad50, and Nbs1; PCNA, proliferating cell nuclear antigen; RFC, replication factor C.
Upregulation of adaptor protein transducin β-like protein 1 X-linked (TBL1X) has been demonstrated in multiple types of cancer and its overexpression shown to be associated with poor clinical outcomes.30-33 Our group has shown that TBL1X is overexpressed in diffuse large B-cell lymphoma (DLBCL) and that targeting TBL1X pharmacologically with tegavivint (BC2059, Iterion Therapeutics) is highly effective in preclinical models of this disease.33 Via interaction with a SKP1, CUL1, F-box protein (SCF) supercomplex, we demonstrated the role of TBL1X in maintaining the stability of key oncoproteins (eg cMyc and polo-like kinase 1 or PLK1) that are necessary for DLBCL cell survival. A first-in-class small molecule targeting TBL1X’s N-terminal domain, tegavivint is currently being evaluated in several clinical trials, including 2 at The Ohio State University, as a single agent for patients with relapsed/refractory cMyc overexpressing LBCL (ClinicalTrials.gov identifier: NCT05755087) and as first-line therapy in combination with osimertinib for patients with metastatic epidermal growth factor receptor (EGFR)-mutant non–small cell lung cancer (ClinicalTrials.gov identifier: NCT04780568).
Our characterization of TBL1X in DLBCL led us to explore this promising therapeutic target in other B-cell malignancies. In this study, we mechanistically characterize the oncogenic role of TBL1X in MCL and establish targeting TBL1X as a novel therapeutic strategy for this disease. Our findings demonstrate abundant expression of TBL1X in MCL, and, using a dual approach pairing pharmacologic targeting of TBL1X with genetic knockdown (KD) studies, this study details the biological role and therapeutic potential of targeting TBL1X in MCL. We demonstrate the multifaceted role of TBL1X in driving MCL pathogenesis through stabilization of cyclin D1 and RAD51, thus supporting cell survival, cell cycle progression, and HR. Moreover, we leverage these mechanistic findings to develop a novel therapeutic combination, targeting several key DDR pathways and demonstrate that combining tegavivint with the orally bioavailable PARP-1/2 inhibitor talazoparib results in synergistic MCL cell death. Together, these studies demonstrate the oncogenic function and therapeutic potential of targeting TBL1X in this aggressive, incurable disease.
Materials and methods
Cell culture and reagents
MCL cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA), Leibniz-Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany), or MilliporeSigma (Burlington, MA). Normal B cells and MCL cells from primary patient samples (patients with nodal MCL) were obtained after written, informed consent in accordance with the Declaration of Helsinki under protocols approved by The Ohio State University or Central Drug Research Institute institutional review board. See supplemental Tables 1 and 2 for patient characteristics.
Drug and cytotoxicity assays
The TBL1X-targeting small molecule, tegavivint, was provided by Iterion Therapeutics (Houston, TX). Combenefit platform (Cancer Research UK Cambridge Institute, Cambridge, United Kingdom) and the Loewe model of additivity were used for synergy analyses.34
Statistical analyses
Unless stated otherwise in figure legends, results are presented as mean ± standard deviation, and data were collected from at least 3 independent experiments. The 2-sample t test and analysis of variance were used for 2-group comparisons and for multiple-group comparisons, respectively. Survival data are displayed as Kaplan-Meier analyses, with significance determined by the log-rank (Mantel-Cox) test. Relative risks are expressed as log-rank hazard ratios with 95% confidence intervals (CI). A P value of <.05 for 2-group comparisons or after adjustment for multiple comparisons was considered statistically significant. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Detailed experimental materials can be found in the supplemental Methods.
Results
TBL1X is highly expressed in MCL
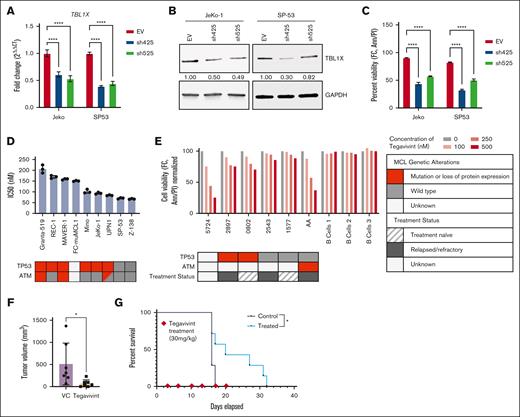
TBL1X was found to be highly expressed in MCL cell lines (n = 9; Figure 1A) and primary MCL patient samples (n = 10; Figure 1B) of variable genetic backgrounds; based on our group’s earlier work defining TBL1X expression in DLBCL,33 we included 2 DLBCL cell lines as positive controls. At the transcript level, compared with normal B cells from healthy donors (n = 10), TBL1X is expressed abundantly in samples from patients with MCL (n = 36; Figure 1C). To analyze the association of TBL1X expression with clinical outcome, a cohort of patients with MCL (n = 33) was separated into high or low TBL1X-expressing groups, in which the top 33% and bottom 33% of relative, normalized expression values (ΔΔ threshold cycle (Ct) or ddCT) were considered high (n = 14) or low (n = 19), respectively. A retrospective Kaplan-Meier analysis generated from these groups demonstrates that median OS was significantly shorter for patients with MCL with high TBL1X expression (21.37 months) than for those with low expression (31.38 months, P = .0405; hazard ratio = 2.8; Figure 1D). Taken together, these results indicate that TBL1X is expressed in MCL and that high TBL1X expression is associated with poorer prognosis in this disease.
TBL1X is highly expressed in MCL, and high TBL1X expression is associated with poorer prognosis in patients with MCL. Immunoblot analyses for TBL1X expression in (A) MCL cell lines (n = 9) and (B) primary MCL patient samples (n = 10) of variable genetic backgrounds, as compared with normal B cells. (C) Comparison of relative TBL1X transcript levels (ΔΔCT) in normal B cells (n = 10) compared with MCL samples from patients with MCL (n = 36). (D) OS for patients with MCL (n = 33) having the highest (top 33%) and lowest (bottom 33%) levels of TBL1X expression (TBL1XHigh [n = 19] and TBL1XLow [n = 14], respectively) depicted by Kaplan-Meier plot and analyzed with the log-rank Mantel-Cox test. Median OS for patients with MCL with high TBL1X expression was 21.37 vs 31.38 months for those with low expression (P = .0405; hazard ratio = 2.8). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
TBL1X is highly expressed in MCL, and high TBL1X expression is associated with poorer prognosis in patients with MCL. Immunoblot analyses for TBL1X expression in (A) MCL cell lines (n = 9) and (B) primary MCL patient samples (n = 10) of variable genetic backgrounds, as compared with normal B cells. (C) Comparison of relative TBL1X transcript levels (ΔΔCT) in normal B cells (n = 10) compared with MCL samples from patients with MCL (n = 36). (D) OS for patients with MCL (n = 33) having the highest (top 33%) and lowest (bottom 33%) levels of TBL1X expression (TBL1XHigh [n = 19] and TBL1XLow [n = 14], respectively) depicted by Kaplan-Meier plot and analyzed with the log-rank Mantel-Cox test. Median OS for patients with MCL with high TBL1X expression was 21.37 vs 31.38 months for those with low expression (P = .0405; hazard ratio = 2.8). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
TBL1X is essential for MCL cell survival
Given the abundant expression of TBL1X and the association of expression with prognosis in MCL, we sought to examine the importance of TBL1X to MCL cell survival. Short hairpin RNA (shRNA) genetic KD studies with 2 TBL1X-specific constructs were performed in 2 MCL cells lines, including a cell line with wild-type TP53 (SP-53) and a cell line with mutated TP53 (JeKo-1). Compared with an empty vector control, efficient TBL1X KD, verified at the transcript (Figure 2A) and protein level (Figure 2B), resulted in significant cell death in both cell lines (Figure 2C). Given the relevance of TBL1X’s closely related homolog TBL1XR1 in lymphoma biology,35 we assessed the TBL1X specificity of the KD and did not find a reduction in TBL1XR1 (supplemental Figure 1). Treatment of MCL cell lines (n = 9) with tegavivint for 24 hours resulted in dose-dependent MCL cell death with a 50% inhibitory concentration (IC50) of <250 nM (range, 67-206) in all cell lines (Figure 2D). Cytotoxicity was also observed in primary MCL patient samples (n = 6) incubated with increasing concentrations of tegavivint for 18 hours (Figure 2E). Consistent with our previous work, which showed minimal cytotoxicity in normal immune cell subsets treated with tegavivint,33 the viability of activated normal human B cells (n = 3) was unaffected by tegavivint treatment under the same experimental conditions. These results demonstrate that TBL1X is essential for MCL cell survival.
Targeting TBL1X is cytotoxic to MCL cells.TBL1X-specific shRNA KD in MCL cell lines (n = 2) using 2 constructs (sh425 and sh525) compared with an empty vector (EV) control: (A) TBL1X transcript levels (quantitative reverse transcription polymerase chain reaction, 1 day after transduction), (B) TBL1X protein levels (immunoblot, 4 days after transduction), and (C) cell viability. (D) Tegavivint sensitivity (IC50 at 24 hours) of 9 MCL cell lines (n = 9). (E) Cytotoxicity in samples from patients with MCL (n = 6) treated with tegavivint (18 hour). Subcutaneous murine MCL cell line model receiving tegavivint (n = 7; 30 mg/kg, IV, twice weekly) or VC (n = 7); (F) tumor volume at day 13 after engraftment and (G) Kaplan-Meier plot with log-rank Mantel-Cox analysis. Median OS was 20 days (range, 16-32) for drug-treated animals vs 16 days (range, 16-17) for VC-treated animals (P = .0230, hazard ratio = 2.213). Cell viability was determined for all experiments by annexin-V/PI staining and flow cytometry. Ann, annexin-V; FC, flow cytometry; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PI, propidium iodide. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Targeting TBL1X is cytotoxic to MCL cells.TBL1X-specific shRNA KD in MCL cell lines (n = 2) using 2 constructs (sh425 and sh525) compared with an empty vector (EV) control: (A) TBL1X transcript levels (quantitative reverse transcription polymerase chain reaction, 1 day after transduction), (B) TBL1X protein levels (immunoblot, 4 days after transduction), and (C) cell viability. (D) Tegavivint sensitivity (IC50 at 24 hours) of 9 MCL cell lines (n = 9). (E) Cytotoxicity in samples from patients with MCL (n = 6) treated with tegavivint (18 hour). Subcutaneous murine MCL cell line model receiving tegavivint (n = 7; 30 mg/kg, IV, twice weekly) or VC (n = 7); (F) tumor volume at day 13 after engraftment and (G) Kaplan-Meier plot with log-rank Mantel-Cox analysis. Median OS was 20 days (range, 16-32) for drug-treated animals vs 16 days (range, 16-17) for VC-treated animals (P = .0230, hazard ratio = 2.213). Cell viability was determined for all experiments by annexin-V/PI staining and flow cytometry. Ann, annexin-V; FC, flow cytometry; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PI, propidium iodide. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To assess the anti-MCL activity of targeting TBL1X in vivo, murine MCL cells (FC-muMCL1)36 were engrafted (lateral flank, subcutaneously, 5 million cells per mouse) in C57/Bl6 mice. Animals were randomized into treatment groups to receive vehicle control (VC; n = 7) or tegavivint (30 mg/kg; n = 7) IV twice weekly starting 3 days after engraftment. At 13 days after engraftment, significantly attenuated tumor growth was observed in mice receiving tegavivint compared with VC (mean volume = 73 mm3 vs 512 mm3, respectively; P = .032; Figure 2F), which translated into a survival advantage for the drug-treated cohort vs VC (median OS = 20 days [range, 16-32] vs 16 days [range, 16-17], respectively; P = .023, hazard ratio = 2.213; Figure 2G). The results of this in vivo study reinforce our in vitro data in demonstrating that TBL1X plays an essential role in MCL cell survival and represents a potential novel therapeutic target in this disease.
TBL1X maintains genomic stability in MCL
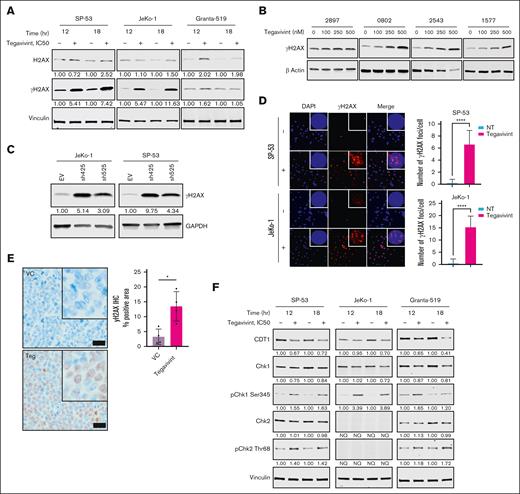
Given that genomic instability is a defining feature of this disease, the effects of targeting TBL1X on the genomic integrity of MCL cells were tested across several MCL cell lines, including TP53 wild-type SP-53 and TP53 mutant JeKo-1. As shown in Figure 3A, treatment with tegavivint resulted in robust serine-139 phosphorylation of histone variant H2AX (gamma-H2AX or γH2AX), an established marker of DSBs.37 This also occurred in the ATM-deficient cell line (Granta-519), although to a lesser degree (Figure 3A). Confirming the presence of damaged, fragmented DNA, incubation of Jeko-1 cells with tegavivint resulted in a concentration-dependent DNA damage assessed by the Comet assay (supplemental Figure 2). Concordantly, pronounced γH2AX accumulation was also observed in tegavivint-treated primary MCL patient samples (Figure 3B) and MCL cell lines subjected to TBL1X shRNA KD (Figure 3C). Additionally, nuclear γH2AX foci quantified by immunofluorescence were significantly more abundant in JeKo-1 and SP53 cells treated with tegavivint (IC50, 12 hour, mean number of foci per cell = 15.39 and 6.65, respectively) compared with untreated controls (0.6087 and 0.1739, respectively, P < .0001 for both cell lines; Figure 3D). Of note, tegavivint treatment (up to 1 μM) of MCL cell lines failed to induce generation of reactive oxygen species, thus ruling out a tegavivint-induced reactive oxygen species–mediated DNA damage effect (supplemental Figure 3). As validation, using our subcutaneous FC-muMCL1 model (as described earlier), a subset of mice (n = 8) were euthanized 2 weeks after engraftment after 4 doses of tegavivint, and tumors were subjected to γH2AX immunohistochemistry. Positive γH2AX immunostaining was significantly elevated in the tumors of tegavivint-treated animals (n = 4) compared with VC (n = 4; mean of 13.48% of the total tissue section area vs 3.257%, respectively, P = .0106; Figure 3E). In tegavivint-treated MCL cell lines, JeKo-1 and SP53, γH2AX formation was accompanied by additional changes associated with an activated DDR, including loss of CDT1 and activating phosphorylation of key DDR kinases, Chk1 (serine 345) and Chk2 (threonine 68; Figure 3F). Together, these results demonstrate that targeting TBL1X results in significant DNA damage in MCL cells in vitro and in vivo.
Targeting TBL1X induces DNA damage in MCL cells. (A) Immunoblot analyses of MCL cell lines (JeKo-1, SP-53, and Granta-519) treated with tegavivint (IC50, 12-18 hours) compared with dimethyl sulfoxide (DMSO) control for quantification of γH2AX formation (Ser-139 phosphorylation). Immunoblot analyses for γH2AX formation in (B) tegavivint-treated (18-hour, 0-100 nM) primary MCL patient samples (n = 4) and (C) MCL cell lines (n = 2) subjected to TBL1X shRNA KD with specific constructs (n = 2; sh425 and sh525) or EV control. (D) Immunofluorescence for γH2AX (red) foci with quantification (number of γH2AX per cell) in tegavivint-treated (IC50, 12 hour) MCL cell lines (n = 2) compared with DMSO control; nuclei are stained with DAPI (blue). Photomicrographs were taken at original magnification ×200 or ×600 (inset). (E) γH2AX immunohistochemistry with quantification (digital analysis for positive pixels) in the tumors of a subset of mice from the FC-muMCL1 study (Figure 2D) euthanized at a predetermined time point (day 14 after engraftment, after n = 4 tegavivint treatments). Brown chromogen (3,3'-diaminobenzidine or DAB) indicates positive γH2AX immunostaining; nuclei were counterstained blue-purple with Harris hematoxylin. Photomicrographs were taken at original magnification ×600; black scale bars, 20 μm. (F) Immunoblot analyses of MCL cell lines (JeKo-1, SP-53, and Granta-519) treated with tegavivint (IC50, 12-18 hours) compared with DMSO control for quantification of DDR Chk1 and Chk2 kinase activation (phosphorylation) and CDT1 levels. Tegavivint IC50 concentrations = 95 nM for JeKo-1, 65 nM for SP-53, and 200 nM for Granta-519. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; NT, no treatment; NQ, not quantifiable. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Targeting TBL1X induces DNA damage in MCL cells. (A) Immunoblot analyses of MCL cell lines (JeKo-1, SP-53, and Granta-519) treated with tegavivint (IC50, 12-18 hours) compared with dimethyl sulfoxide (DMSO) control for quantification of γH2AX formation (Ser-139 phosphorylation). Immunoblot analyses for γH2AX formation in (B) tegavivint-treated (18-hour, 0-100 nM) primary MCL patient samples (n = 4) and (C) MCL cell lines (n = 2) subjected to TBL1X shRNA KD with specific constructs (n = 2; sh425 and sh525) or EV control. (D) Immunofluorescence for γH2AX (red) foci with quantification (number of γH2AX per cell) in tegavivint-treated (IC50, 12 hour) MCL cell lines (n = 2) compared with DMSO control; nuclei are stained with DAPI (blue). Photomicrographs were taken at original magnification ×200 or ×600 (inset). (E) γH2AX immunohistochemistry with quantification (digital analysis for positive pixels) in the tumors of a subset of mice from the FC-muMCL1 study (Figure 2D) euthanized at a predetermined time point (day 14 after engraftment, after n = 4 tegavivint treatments). Brown chromogen (3,3'-diaminobenzidine or DAB) indicates positive γH2AX immunostaining; nuclei were counterstained blue-purple with Harris hematoxylin. Photomicrographs were taken at original magnification ×600; black scale bars, 20 μm. (F) Immunoblot analyses of MCL cell lines (JeKo-1, SP-53, and Granta-519) treated with tegavivint (IC50, 12-18 hours) compared with DMSO control for quantification of DDR Chk1 and Chk2 kinase activation (phosphorylation) and CDT1 levels. Tegavivint IC50 concentrations = 95 nM for JeKo-1, 65 nM for SP-53, and 200 nM for Granta-519. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; NT, no treatment; NQ, not quantifiable. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
TBL1X regulates cell cycle progression in MCL through stabilization of cyclin D1
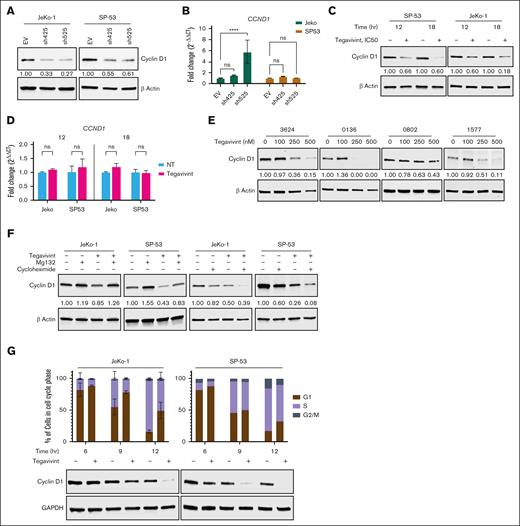
Given the essential role that cyclin D1 plays in MCL pathogenesis and previous work showing that cyclin D1 is intimately involved in several DDR mechanisms, the effects of targeting TBL1X on cyclin D1 were investigated. TBL1X shRNA KD (Figure 4A-B) and treatment of MCL cell lines with tegavivint (IC50; Figure 4C-D) resulted in reduced levels of cyclin D1 protein, despite unchanged or increased CCND1 RNA levels without compensatory upregulation of cyclin D2 or D3 (supplemental Figure 4). Similarly, treatment of primary samples from MCL patients (n = 4) with tegavivint (0-500 nM, 18 hours) resulted in dose-dependent depletion of cyclin D1 (Figure 4E). Because TBL1X is known to directly bind β-catenin and protect it from proteasomal degradation,38,39 we therefore hypothesized that TBL1X may similarly bind to and stabilize cyclin D1. However, there was no direct interaction between TBL1X and cyclin D1 by mass spectrometry (data not shown) nor by immunoprecipitation (IP)/co-IP (supplemental Figure 5A-B). To further examine the mechanism by which tegavivint/TBL1X KD-induced cyclin D1 depletion occurs, translation inhibition and protein rescue experiments were performed. Treatment of MCL cell lines with tegavivint (IC50) and proteasome inhibitor MG132 (10 μM) resulted in near complete “rescue” of cyclin D1 levels compared with tegavivint alone (Figure 4F). Moreover, cotreatment with cycloheximide (10 μg/mL) resulted in further depletion of cyclin D1, compared with cyclin D1 levels in cells treated with either single agent alone (Figure 4F). Taken together, these data indicate that cyclin D1 loss in the context of targeting TBL1X is a mechanism of protein destabilization/enhanced turnover.
Targeting TBL1X results in cyclin D1 depletion and cell cycle arrest in MCL cells. Cyclin D1 protein and CCND1 RNA levels in MCL cell lines (n = 2) after (A-B) TBL1X shRNA KD with specific constructs (n = 2; sh425 and sh525) compared with EV control or (C-D) tegavivint treatment (IC50, 12-18 hours) compared with DMSO control. (E) Cyclin D1 protein levels in primary MCL patient samples (n = 3) treated with tegavivint (18 hours, 0-500 nM). (F) Cyclin D1 protein levels in MCL cell lines (n = 2) treated with proteasome inhibitor Mg132 or translation inhibitor cycloheximide combined with tegavivint compared with tegavivint-only treated cells. Cells were first treated with tegavivint (IC50), then MG132 (10 μM) or cycloheximide (10 μg/mL) was added for the last 1.5 or 1 hour(s), respectively, for a total incubation time of 18 hours. (G) Cell cycle phase analyses (propidium iodide staining and flow cytometry) and cyclin D1 protein levels (immunoblot) in pharmacologically synchronized (500 nM palbociclib) MCL cell lines (n = 2) treated with tegavivint (IC50, 6-12 hours). Tegavivint IC50 concentrations = 95 nM for JeKo-1 and 65 nM for SP-53. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; G1, gap 1 phase; G2/M, gap 2 phase/mitosis; hr, hour; ns, not significant; S, synthesis phase. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Targeting TBL1X results in cyclin D1 depletion and cell cycle arrest in MCL cells. Cyclin D1 protein and CCND1 RNA levels in MCL cell lines (n = 2) after (A-B) TBL1X shRNA KD with specific constructs (n = 2; sh425 and sh525) compared with EV control or (C-D) tegavivint treatment (IC50, 12-18 hours) compared with DMSO control. (E) Cyclin D1 protein levels in primary MCL patient samples (n = 3) treated with tegavivint (18 hours, 0-500 nM). (F) Cyclin D1 protein levels in MCL cell lines (n = 2) treated with proteasome inhibitor Mg132 or translation inhibitor cycloheximide combined with tegavivint compared with tegavivint-only treated cells. Cells were first treated with tegavivint (IC50), then MG132 (10 μM) or cycloheximide (10 μg/mL) was added for the last 1.5 or 1 hour(s), respectively, for a total incubation time of 18 hours. (G) Cell cycle phase analyses (propidium iodide staining and flow cytometry) and cyclin D1 protein levels (immunoblot) in pharmacologically synchronized (500 nM palbociclib) MCL cell lines (n = 2) treated with tegavivint (IC50, 6-12 hours). Tegavivint IC50 concentrations = 95 nM for JeKo-1 and 65 nM for SP-53. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; G1, gap 1 phase; G2/M, gap 2 phase/mitosis; hr, hour; ns, not significant; S, synthesis phase. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To verify the biologic relevance of the cyclin D1 loss observed with targeting TBL1X in MCL cells, MCL cell lines (JeKo-1 and SP-53) cells were synchronized in the G1 phase of the cell cycle with palbociclib (500 nM, 24 hours), a CDK4/6 inhibitor,40 followed by cell cycle reentry (palbociclib washout), and immediate treatment with tegavivint (IC50) or dimethyl sulfoxide. In comparing cell cycle phase analysis data with protein data, we found that as cyclin D1 levels are depleted in tegavivint-treated cells, cell cycle arrest occurs with accumulation of cells in G1 phase (Figure 4G). Consistent with the known role of cyclin D1 in cell cycle regulation and previously published data,41 genetic KD (shRNA) of CCND1 in MCL cell line JeKo-1 resulted in G1 phase cell cycle arrest and a concordant reduction in proliferation without significant cytotoxicity (supplemental Figure 6A-D). In this experiment, a DNA damage phenotype (eg, γH2AX formation by immunoblot) was not observed with cyclin D1 depletion (supplemental Figure 6A). These data demonstrate that TBL1X is necessary to maintain cyclin D1 levels, cell cycle progression, and proliferation of MCL cells.
The TBL1X-RAD51 axis is essential to the DDR and cell survival in MCL
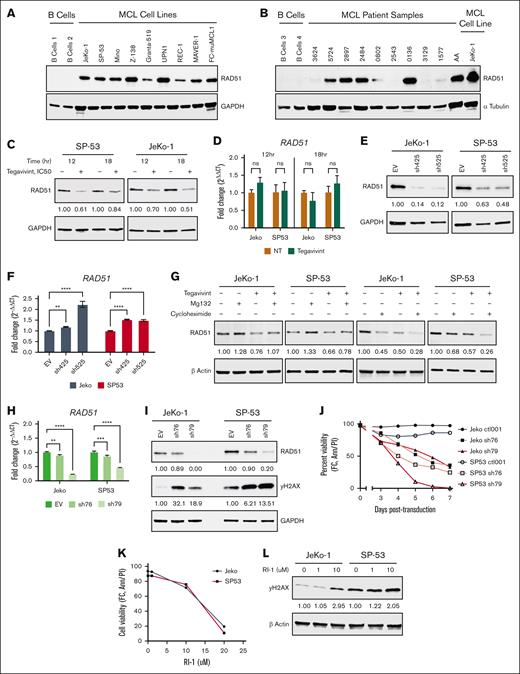
Previous work has shown a direct, functional interaction between cyclin D1 and RAD51, and that cyclin D1 depletion in human cancer cells, including MCL, impairs RAD51 recruitment to sites of damaged DNA, suggesting that cyclin D1 functions as a RAD51 chaperone.24 This work prompted our investigation into the cyclin D1–RAD51-TBL1X axis. As shown in Figure 5A-B, RAD51 is highly expressed in all MCL cell lines (n = 9) and in most primary MCL patient samples(n = 10), compared with no detectable expression in normal donor B cells. Interestingly, in the context of pharmacologic (Figure 5C-D) and genetic (Figure 5E-F) TBL1X targeting, RAD51 protein levels were reduced in MCL cell lines, whereas, concurrently, RAD51 transcript levels were unchanged or increased. In our experimental conditions, cyclin D1, RAD51, and TBL1X did not directly interact, including IP/co-IP experiments in the presence or absence of doxorubicin-induced DNA damage (supplemental Figure 5A-E) and mass spectrometry analysis of cyclin D1, RAD51, and TBL1X IP samples (data not shown). In protein rescue and depletion studies in MCL cell lines, treatment with MG132 restored RAD51 protein levels when combined with tegavivint compared with tegavivint alone, and combining cycloheximide with tegavivint more significantly depleted RAD51 levels compared with either agent alone (Figure 5G). Together, these results demonstrate that TBL1X controls RAD51 protein stability in MCL cells in a posttranscriptional and posttranslational manner.
Targeting TBL1X results in the degradation of RAD51, DNA damage, and MCL cell death. Immunoblot analyses for RAD51 protein expression in (A) MCL cell lines (n = 9) and (B) primary MCL patient samples (n = 10) as compared with normal B cells. RAD51 protein levels by immunoblot and RAD51 transcript levels by qPCR in MCL cell lines (n = 2) (C-D) treated with tegavivint (IC50, 12-18 hours) vs DMSO control or (E-F) subjected to TBL1X KD with specific constructs (n = 2; sh425 and sh525) vs EV control. (G) RAD51 protein levels in MCL cell lines (n = 2) treated with proteasome inhibitor Mg132 or translation inhibitor cycloheximide combined with tegavivint compared with tegavivint-only treated cells. Cells were first treated with tegavivint (IC50), then MG132 (10 μM) or cycloheximide (10 μg/mL) was added for the last 1.5 or 3 hours, respectively, for a total incubation time of 18 hours. RAD51 shRNA KD in MCL cell lines (n = 2) with specific constructs (sh77 and sh79) compared with an EV control: (H) RAD51 transcript levels by qPCR, (I) RAD51/γH2AX protein levels by immunoblot, and (J) cell viability. (K) Cell viability and (L) γH2AX levels by immunoblot in MCL cell lines (n = 2) with targeting RAD51 pharmacologically with small molecule inhibitor RI-1 (72-hour incubation). Cell viability was determined for all experiments by annexin-V/PI staining and flow cytometry. Tegavivint IC50 concentrations = 95 nM for JeKo-1 and 65 nM for SP-53. Ann, annexin-V; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; ns, not significant; NT, no treatment; FC, flow cytometry; PI, propidium iodide; qPCR, quantitative polymerase chain reaction. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Targeting TBL1X results in the degradation of RAD51, DNA damage, and MCL cell death. Immunoblot analyses for RAD51 protein expression in (A) MCL cell lines (n = 9) and (B) primary MCL patient samples (n = 10) as compared with normal B cells. RAD51 protein levels by immunoblot and RAD51 transcript levels by qPCR in MCL cell lines (n = 2) (C-D) treated with tegavivint (IC50, 12-18 hours) vs DMSO control or (E-F) subjected to TBL1X KD with specific constructs (n = 2; sh425 and sh525) vs EV control. (G) RAD51 protein levels in MCL cell lines (n = 2) treated with proteasome inhibitor Mg132 or translation inhibitor cycloheximide combined with tegavivint compared with tegavivint-only treated cells. Cells were first treated with tegavivint (IC50), then MG132 (10 μM) or cycloheximide (10 μg/mL) was added for the last 1.5 or 3 hours, respectively, for a total incubation time of 18 hours. RAD51 shRNA KD in MCL cell lines (n = 2) with specific constructs (sh77 and sh79) compared with an EV control: (H) RAD51 transcript levels by qPCR, (I) RAD51/γH2AX protein levels by immunoblot, and (J) cell viability. (K) Cell viability and (L) γH2AX levels by immunoblot in MCL cell lines (n = 2) with targeting RAD51 pharmacologically with small molecule inhibitor RI-1 (72-hour incubation). Cell viability was determined for all experiments by annexin-V/PI staining and flow cytometry. Tegavivint IC50 concentrations = 95 nM for JeKo-1 and 65 nM for SP-53. Ann, annexin-V; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; ns, not significant; NT, no treatment; FC, flow cytometry; PI, propidium iodide; qPCR, quantitative polymerase chain reaction. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To validate the essential role of RAD51 in MCL cell genomic integrity and survival, the biologic outcomes associated with silencing RAD51 itself were assessed. Using 2 RAD51-targeting shRNA in JeKo-1 and SP-53 cells, efficient RAD51 KD (Figure 5H-I) resulted in pronounced DNA damage (Figure 5I) and marked cytotoxicity (Figure 5J). Similarly, pharmacologic targeting of RAD51 with small molecule inhibitors, B02 and RI-1, resulted in significant cytotoxicity (Figure 5K; supplemental Figure 7) and DNA damage in the same MCL cell lines (Figure 5L; supplemental Figure 7). Together, these findings confirm that intact RAD51 function is essential for MCL cell survival and DNA integrity in this disease.
Combining tegavivint with a PARP inhibitor results in synergistic cell killing in preclinical models of MCL
Finally, we sought to identify a novel therapeutic combination strategy for MCL leveraging our observation that TBL1X targeting results in DNA damage in MCL cells via RAD51 depletion. We hypothesized that combining tegavivint with an agent that blocks other DDR mechanisms would result in synergistic MCL cell death. The orally available inhibitor of PARP-1/2 catalytic activity, talazoparib, works by blocking several DNA repair activities (single strand break excision repair, nonhomologous end-joining, HR, DNA mismatch repair, and replication fork stabilization) resulting in accumulation of unrepaired DNA damage and eventual cell death.42,43 In vitro, combining tegavivint with talazoparib resulted in additive or synergistic MCL cell killing in all cell lines (n = 3; Figure 6A). This combination was then tested in vivo using a patient-derived MCL xenograft (PDX AA) developed in our laboratory from a patient with MCL with acquired ibrutinib resistance, a common clinical therapeutic challenge for which novel treatment strategies are needed. After systemic engraftment (5 million PDX AA cells), mice were randomized to receive tegavivint (30 mg/kg, IV, twice weekly) and/or talazoparib (0.33 mg/kg, oral gavage, once daily for 5 days per week), or the relevant VC. All drug treatments conferred a survival advantage over their respective VC, and combination treatment of tegavivint plus talazoparib (OS = 108 days [range, 103-109]) prolonged median OS compared with single-agent tegavivint (OS = 79 days [range, 74-80]; P = .0007, hazard ratio, 3.698; 95% CI, 1.129-12.12) and single-agent talazoparib (OS = 96 days [range, 96-99]; P = .0003, hazard ratio, 3.725; 95% CI, 1.134-12.23; Figure 6B). In summary, these findings demonstrate the therapeutic efficacy of combining tegavivint with talazoparib in preclinical MCL models.
Tegavivint with PARP inhibitor talazoparib is an effective combination against MCL in vitro and in vivo. (A) MCL cell killing activity of the combination of tegavivint plus talazoparib in vitro in MCL cell lines, JeKo-1, UPN-1, and Maver. Matrices display calculated Loewe additivity scores ± standard deviation from dose concentration combinations of tegavivint and talazoparib viability studies. Matrices are color-coded; blue indicates synergistic cell killing; red, antagonism; and green represents dose combinations for which additive effect was found. Cells were treated with the indicated concentrations of talazoparib, tegavivint, and/or DMSO control, and cell viability was determined by acridine orange (AO) and propidium iodide (PI) staining and Nexcelom Cellaca cell counter. Combenefit was used to generate isobologram analyses and determine synergism (Loewe additivity model).34 (B) Kaplan-Meier plot showing OS in an MCL PDX mouse model treated with tegavivint (30 mg/kg, IV via tail vein, twice weekly) and/or talazoparib (0.33 mg/kg, oral gavage, once daily for 5 days per week), or the relevant VC. Note: because of tail edema, all treatments were discontinued on day 63 (indicated on plot with black dotted line). Mean OS was 73 days for the tegavivint VC cohort (n = 7), 68 days for talazoparib VC (n = 7), 70 days for combination VC (n = 7), 79 days for tegavivint (n = 8), 96 days talazoparib (n = 8), and 108 days for combination (n = 6). P values were calculated by the log-rank Mantel-Cox test. Tala, talazoparib; Teg, tegavivint; Veh, vehicle control. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Tegavivint with PARP inhibitor talazoparib is an effective combination against MCL in vitro and in vivo. (A) MCL cell killing activity of the combination of tegavivint plus talazoparib in vitro in MCL cell lines, JeKo-1, UPN-1, and Maver. Matrices display calculated Loewe additivity scores ± standard deviation from dose concentration combinations of tegavivint and talazoparib viability studies. Matrices are color-coded; blue indicates synergistic cell killing; red, antagonism; and green represents dose combinations for which additive effect was found. Cells were treated with the indicated concentrations of talazoparib, tegavivint, and/or DMSO control, and cell viability was determined by acridine orange (AO) and propidium iodide (PI) staining and Nexcelom Cellaca cell counter. Combenefit was used to generate isobologram analyses and determine synergism (Loewe additivity model).34 (B) Kaplan-Meier plot showing OS in an MCL PDX mouse model treated with tegavivint (30 mg/kg, IV via tail vein, twice weekly) and/or talazoparib (0.33 mg/kg, oral gavage, once daily for 5 days per week), or the relevant VC. Note: because of tail edema, all treatments were discontinued on day 63 (indicated on plot with black dotted line). Mean OS was 73 days for the tegavivint VC cohort (n = 7), 68 days for talazoparib VC (n = 7), 70 days for combination VC (n = 7), 79 days for tegavivint (n = 8), 96 days talazoparib (n = 8), and 108 days for combination (n = 6). P values were calculated by the log-rank Mantel-Cox test. Tala, talazoparib; Teg, tegavivint; Veh, vehicle control. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Discussion
Despite recent advances in the MCL treatment landscape, nearly all patients eventually progress and succumb to their disease,44,45 highlighting an urgent need for novel therapeutic strategies. Although complex and heterogeneous, MCL pathogenesis often involves deregulation of DDR mechanisms and significant genomic instability. However, defects in DDR pathways may represent an “Achilles heel” of MCL and an opportunity for therapeutic exploitation.46 In this study, we characterize TBL1X as a novel oncogenic target in MCL, showing that TBL1X is abundantly expressed in MCL; TBL1X expression is associated with poor clinical outcomes; and that targeting TBL1X genetically and pharmacologically results in cell cycle arrest, DNA damage, and ultimately in MCL cell death in vitro and in vivo. To our knowledge, the oncogenic role of TBL1X in MCL biology was previously unexplored. Here, we mechanistically demonstrated that TBL1X stabilizes key components of the cell cycle and DDR machinery and serves as a master regulator of the cyclin D1–RAD51-DDR axis. Furthermore, pharmacologic and genetic downregulation of RAD51 was found to phenotypically recapitulate the cytotoxicity and DNA damage observed with TBL1X targeting, indicating that targeting the DDR in MCL results in significant cell death because of the inability of MCL cells to repair high levels of endogenous/spontaneous DNA damage. At present, our work does not indicate a role for TBL1X in MCL pathogenesis, rather these studies provide mechanistic evidence of the key role this adapter protein plays in oncoprotein stability in maintaining the malignant phenotype. Our findings demonstrate that TBL1X represents a novel therapeutic target for this incurable disease and that the TBL1X-RAD51 axis is essential for genomic stability and cell viability in MCL.
Cyclin D1, which is overexpressed in most patients with MCL, is the primary driver of cell cycle dysregulation in MCL.8 However, cyclin D1 overexpression is independently not sufficient for MCL development.47,48 Our data, and work from others, have shown that silencing cyclin D1 in MCL cells induces G1 cell cycle arrest but not major cell death.41 Of clinical importance, cyclin D1 depletion after tegavivint treatment of MCL cells does not result in compensatory upregulation of cyclin D2 and D3, suggesting that TBL1X might be involved in controlling the stability of the cyclin D family of proteins, resulting in a deeper response to treatment. Published work describes the roles of cyclin D1 in the DDR in cancer, including a link between cyclin D1 and HR.24,49 In MCL specifically, CCND1 depletion has been shown to increase origin firing, leading to replication stress and DNA DSBs.50 Relevant to our own research, the Sicinski group has performed proteomic screening of cyclin D1 in several human cancer models including MCL and showed a direct cyclin D1–RAD51 interaction by IP that was enhanced upon radiation-induced DNA damage.24 They showed that shRNA-mediated cyclin D1 depletion attenuated recruitment of RAD51 to damaged DNA, resulting in impaired HR, suggesting that cyclin D1 serves as a RAD51 chaperone in this disease.24 RAD51 recombinase is crucial mediator of HR, an essential and tightly regulated DNA DSB repair mechanism involved in maintaining genomic integrity and stability.51 In cancer cells, blockade of RAD51 HR activity leads to accumulation of unrepaired DNA damage and tumor cell death.24,52 Furthermore, upregulation of RAD51 has been previously documented in solid tumors and hematologic malignancies, in association with cancer progression and poor clinical outcomes.52-56
Notably, although we found CCND1 depletion to induce robust G1 cell cycle arrest and reduced proliferation in MCL cells, we did not observe DNA damage. Moreover, we were unable to reproduce the cyclin D1/RAD51 protein–protein interaction in our MCL models using mass spectrometry, co-IP, and reverse co-IP; however, the work by Jirawatnotai et al24 prompted us to mechanistically investigate the role of TBL1X in controlling the stability of RAD51. Interestingly, our data show that genetic and pharmacologic targeting of TBL1X resulted in rapid depletion of RAD51 in a posttranscriptional and translation-independent manner. Because TBL1X regulates many prosurvival pathways, we do not claim tegavivint-induced MCL death to be mediated exclusively by RAD51 destabilization. However, pharmacologic and genetic targeting of RAD51 also results in significant DNA damage and MCL cell death, suggesting a prominent prosurvival role of this protein in MCL and a mechanism of tegavivint-induced MCL cell death.
Contributing to the dysfunctional DDR pathways that define MCL, ATM is among the most frequently mutated DDR genes.19 Although targeting of TBL1X did not significantly affect ATM levels (not shown), our data show that TBL1X plays an essential role in controlling the stability of other critical DDR proteins, such as CDT1, Chk1, and Chk2. Similar to what has been shown with Chk1 inhibitors in MCL,57 in assessing tegavivint activity against a panel of samples from patients with MCL with various genetic backgrounds and treatment histories (eg, treatment naïve vs relapsed/refractory disease), TP53 and ATM statuses do not appear to correlate with tegavivint sensitivity. This is particularly important because TP53 mutations have been detected in up to 75% of patients with MCL who progress on ibrutinib.58 In addition to low IC50 concentrations in MCL cell lines considered to be ibrutinib resistant (such as Granta-519 and Mino),59 we found tegavivint treatment to result in significant cytotoxicity in primary MCL patient samples, including samples from individuals who had relapsed after, or progressed on, ibrutinib. This activity translated to our in vivo study, in which we found single-agent tegavivint to significantly prolong the survival in a PDX generated from a patient who progressed on ibrutinib. This provides further support of the translational relevance of our work.
DDR-targeting strategies are effective against lymphoma preclinically and in clinical trials with inhibitors for PARP, ataxia-telangiectasia mutated and Rad3-related (ATR), CHK1, DNA-dependent protein kinase (DNA-PK), and WEE1.46 By targeting multiple aspects of the DDR pathway, combination therapies can circumvent or overcome resistance mechanisms that might develop with single-agent DDR inhibitors. We showed, herein, that the rationally developed, novel combination of tegavivint and PARP inhibitor talazoparib is therapeutically effective in preclinical MCL models, including cell lines and our ibrutinib-resistant MCL PDX. Leveraging our mechanistic work defining the role of TBL1X in supporting HR via stabilization of RAD51, our goal was to maximize the therapeutic potential of tegavivint in MCL by cotargeting other essential DDR mechanisms via PARP inhibition.
The SCF complex is an E3 ubiquitin ligase responsible for polyubiquitination of target proteins, promoting proteolytic degradation via the proteasome.60 Substrate selectivity is conferred via specific subunits of this multiprotein supercomplex. Interestingly, TBL1X has an F-box motif for substrate recognition, suggesting that TBL1X might interact directly with specific targets to modulate their turnover. Because our experiments failed to show a direct interaction between TBL1X, TBL1XR1 (a TBL1X homolog), cyclin D1, and RAD51, alternatively, this interaction could be indirect. Studies by Mansoor et al showed a significant association between TBL1XR1 expression and key DDR genes, such as RAD51 and RAC1, in B-cell lymphomas.61 TBL1X and TBLXR1 are core components of the nuclear receptor corepressor complex,62,63 and it is intriguing to speculate that this complex may have roles in protein stability and transcriptional regulation. We attribute the oncogenic TBL1X-mediated stabilization of cyclin D1 and RAD51 in MCL to activity of a SCFTBL1X complex, because we have previously shown that this complex regulates the turnover of critical oncoproteins in DLBCL.33 Specifically, we showed that tegavivint-induced dissociation of SCF inhibitor CAND1 drives proteasomal degradation of SCF targets.33 Further work is needed to fully characterize the exact mechanisms through which TBL1X regulates cyclin D1 and RAD51 stability in MCL.
In summary, through our studies, we have mechanistically characterized the key role of TBL1X in controlling DNA integrity in MCL. We provided evidence for translating these findings into a novel therapeutic approach and the rationale for combination strategies to maximize the therapeutic potential of tegavivint in patients with this incurable lymphoma.
Acknowledgments
The authors are grateful for the patients who donated tissue samples to The Ohio State University (OSU) Hematology Tissue Bank Shared Resource (HTB SR) Comprehensive Cancer Center (CCC). The authors acknowledge the resources provided by OSU CCC SR, including the HTB SR, Target Validation SR, Comparative Pathology and Digital Imaging SR, and the OSU Campus Microscopy and Imaging Facility (Columbus, OH; supported, in part, by P30-CA16058). The authors also thank the OSU University Laboratory Animal Resources for providing support for the animal studies. All mouse studies were approved by The OSU institutional animal care and use committee and the animals were maintained under compliance with institutional guidelines. The University of Arizona Genetic Cores provided cell line validation services. Brightfield microscopy was conducted at The OSU College of Veterinary Medicine (CVM) Department of Veterinary Biosciences (VBS).
This work was funded primarily by the National Institutes of Health (NIH) National Cancer Institute (NCI; Bethesda, MD) grants (K08-CA226352 [L.A.]; R01-CA266682 [L.A.]). B.P. was supported via the OSU CVM VBS Comparative Biomedical Sciences Graduate Program, an NIH NCI loan repayment program clinical research grant (L30CA284424), and an OSU Presidential Graduate Fellowship. N.J. is supported by an American Society of Hematology global research award (Washington, DC); a Ramalingaswami grant (BT/RLF/Re-entry/18/2017) from the Department of Biotechnology, Ministry of Science and Technology, Government of India; and a CORE research grant (CRG/2020/003377) from the Science and Engineering Research Board, Government of India.
Authorship
Contribution: B.P. and L.A. conceptualized and designed the study and wrote the manuscript; B.P., E.B., and S.L. performed the bulk of the experimentation and data acquisition, with the support of B.B., S.K., L.T., I.H., C.H., J.C.-M., S.N., N.J., and U.J.; B.P., L.T., L.A., J.C.-M., N.J., and U.J. analyzed the data; W.K.C., W.H., S.S., L.S., J.Y., and R.A.B. provided conceptual and technical advice and edited the manuscript; and L.A. served as study supervisor and provided funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lapo Alinari, The Ohio State University Wexner Medical Center, 400 W 12th Ave, 481D Wiseman Hall, Columbus, OH 43210; email: lapo.alinari@osumc.edu.
References
Author notes
The authors confirm that the data supporting the findings of this study are available within the article, figures, and its supplemental materials. Original data are available on request from the corresponding author, Lapo Alinari (lapo.alinari@osumc.edu).
The full-text version of this article contains a data supplement.


![TBL1X is highly expressed in MCL, and high TBL1X expression is associated with poorer prognosis in patients with MCL. Immunoblot analyses for TBL1X expression in (A) MCL cell lines (n = 9) and (B) primary MCL patient samples (n = 10) of variable genetic backgrounds, as compared with normal B cells. (C) Comparison of relative TBL1X transcript levels (ΔΔCT) in normal B cells (n = 10) compared with MCL samples from patients with MCL (n = 36). (D) OS for patients with MCL (n = 33) having the highest (top 33%) and lowest (bottom 33%) levels of TBL1X expression (TBL1XHigh [n = 19] and TBL1XLow [n = 14], respectively) depicted by Kaplan-Meier plot and analyzed with the log-rank Mantel-Cox test. Median OS for patients with MCL with high TBL1X expression was 21.37 vs 31.38 months for those with low expression (P = .0405; hazard ratio = 2.8). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/8/10.1182_bloodadvances.2024015769/1/m_blooda_adv-2024-015769-gr1.jpeg?Expires=1769092648&Signature=edILMi5jv7jzYqwIX8j77WYmf6c8FxhrPU41xQ4~vQW2ka8O9oLDk4VC6LzTGJIxtxcZ-Ln7YEFD2ZdRMoVl6wEPHkoYATxX-8IjsMtn6UT~yEzuIFgIqe3xtOU2dAnXteIE7~l9QRX64FmxwdUDwRoVIgKwzJEzZ7d9Mxpd0djfE8NdnX7-t0RiBrPL9FnqhAla4SSHN-g2PhXe2QYxMw3HgGcf93qwjpebkRSD9vUMYf0Z9fBQj9Q0YyW7ZSuXBbb8qH1-yl0M6fYs7wJHWyHz9WUpZTDZqKjMEX-mqeNWHO5LQ~SmnIT5u8ZP1mi-gbM~8iyRAeYRlUPGRxd11w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)