TO THE EDITOR:
Pharmaceutical industry–initiated clinical trial protocols for patients with chronic lymphocytic leukemia (CLL) require not only frequent doctor’s visits and blood analyses but usually also repeated computerized tomography (CT) examinations. The value of such intense radiologic monitoring for efficacy evaluation has not been systematically analyzed and contrasts with the recommendations by the 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines, which state that CT is not generally indicated in clinical practice and, for clinical trials, desirable at the start of therapy and for response evaluation if previously abnormal, and otherwise with a CR and PR.1 Furthermore, when submitting clinical study protocols to the radiation protection authority for approval we have often experienced the authority questioning on the cumulative radiation doses in trials on patients with good prognosis. A meta-analysis reported that in 77% of the patients, progressive disease (PD) was detected by physical examination or blood counts and only in 15% by imaging techniques.2
We report here the results of a retrospective observational analysis including patients with CLL who had participated in pharma-conducted phase 2 or 3 clinical trials at 1 single center (Karolinska University Hospital, Stockholm, Sweden) between 13 February 2013 and 31 March 2022.3-9 The project was approved by the Swedish Ethical Review Authority (www.epm.se). Patient consent was not required. The project was performed in accordance with the Declaration of Helsinki and national laws.
All medical records were reviewed by a physician, and descriptive analyses were performed on a number of doctor’s visits, blood tests, and CT examinations. Each patient served as his/her own control, that is, was analyzed twice: once as specified in the respective clinical trial protocol and once as if the patient would have been followed-up in clinical routine according to iwCLL guidelines.1 When guidelines did not provide detailed instruction for clinical routine, we applied clinical practice methods at Karolinska University Hospital as an estimate, mainly frequency of blood analyses and doctor’s visits. Time to response (TTR), progression-free survival (PFS), and overall response rate (iwCLL criteria1) were recorded according to both principles (clinical trial protocol vs iwCLL clinical practice) of follow-up. Data were gathered in case record forms and transferred to a database. Information was systematically cross-checked by an independent CLL physician and validated for accuracy.
Statistical analysis was performed, and differences in numerical variables between the 2 groups were analyzed using the Wilcoxon signed-rank test. Percentages of response/death and progression/death were computed at each time, respectively; Kaplan-Meier survival plots were generated; and TTR and PFS were compared with the log-rank (Mantel-Cox) test.
Clinical characteristics are summarized in Table 1. In total, 42 patients were included. Their median age at start of therapy was 73 years (range, 52-87) and 43% received their CLL medication first-line therapy. The most frequently used agent was zanubrutinib monotherapy (38%), followed by ibrutinib monotherapy (24%) and rituximab-bendamustine (12%). Median follow-up time was 38 months (range, 2-108).
Monitoring according to the respective clinical trial protocol vs iwCLL clinical practice recommendations is provided in Table 2. Median number of doctor’s visits per protocol and according to iwCLL clinical guidelines1 were 18 (range, 3-34) and 10 (range, 1-18), respectively (P < .0001). The corresponding median number of blood tests were 30 (range, 3-73) and 18 (range, 1-45), respectively (P < .0001). The median total number of CT examinations according to clinical trial protocols during treatment and follow-up was 10 (range, 2-17), with an estimated median total radiation dose of 80-110 mSv (range, 16-22 mSv to 136-187 mSv), compared with 0 CT examinations in clinical practice as recommended by iwCLL1 (P < .0001).
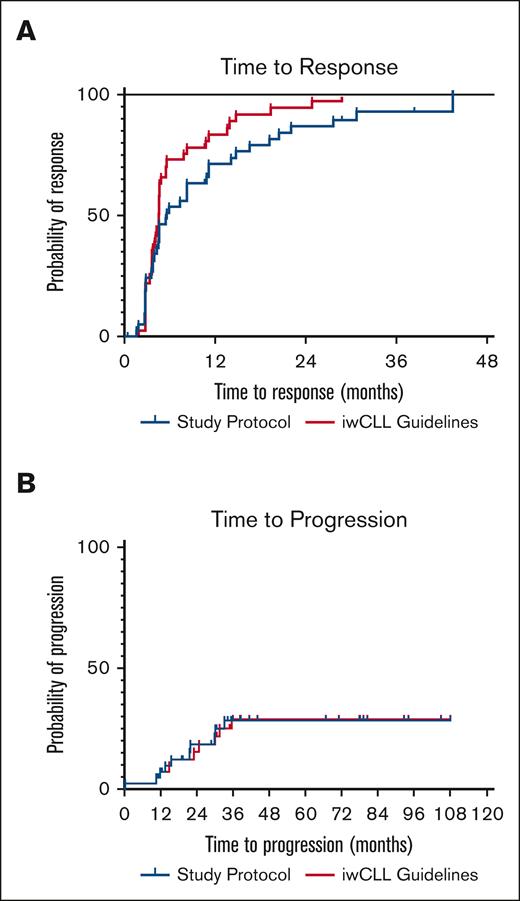
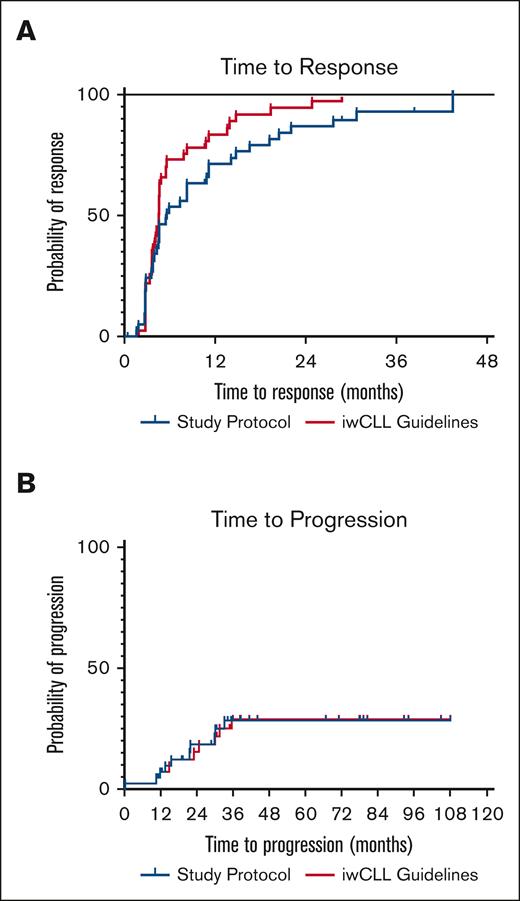
Next, we analyzed the impact of frequent monitoring on outcome measures. Overall response rate was 90% as analyzed per clinical trial protocol and 93% when analyzed clinically according to iwCLL1 (not significant). The median TTR was 5.6 months per protocol and 4.6 months when analyzed clinically1; there was a nonsignificant trend toward shorter TTR when analyzed according to iwCLL clinical recommendations (Figure 1A). PFS is shown in Figure 1B, and there was no difference between per protocol and clinical practice iwCLL1 analyses, respectively. Ten patients (24%) had fulfilled criteria for PD at last follow-up; the median time to progression had not been reached. Reasons for PD were increasing lymphocytosis (n = 6), palpable new lymph nodes or increasing lymphadenopathy (n = 3) and increasing lymphadenopathy by CT only (n = 1).
Time to Response and Time to Progression. (A) TTR according to clinical trial protocol or iwCLL Guidelines. (B) Time to progression according to clinical trial protocol or iwCLL guidelines.
Time to Response and Time to Progression. (A) TTR according to clinical trial protocol or iwCLL Guidelines. (B) Time to progression according to clinical trial protocol or iwCLL guidelines.
Seven patients (17 %) had died during the study period. A longer follow-up is required to estimate overall survival.
In conclusion, our analysis indicates that intense monitoring conducted in many pharma-designed clinical trial protocols for CLL may not advance the discovery of response or progression vs that of iwCLL clinical routine recommendations, while adding burden to both patients and health care. CT at baseline may, however, be recommended before the start of venetoclax therapy to address the risk of tumor-lysis syndrome. Frequent monitoring of blood chemistry and symptoms, albeit not necessarily by physical doctor’s visits, is still recommended for safety reasons in clinical trials, for which iwCLL recommend 1 CT at baseline and a second at final response assessment.1 Thus, CT examinations in pharma-initiated trials (here: median, 10) exceed not only clinical guidelines but also the iwCLL clinical trial recommendations, something we consider is better assessed in investigator-initiated trials. In our analyses, CT examinations were frequent both in the early and late treatment phases, yet PFS was not significantly affected. The impact on health following radiation doses at these levels remains uncertain, although nuclear power industry workers were reported to have an increased mortality, mainly due to cancer, if exposed to >100 mSv.10 A linear relationship between radiation exposure and cancer incidence was described among Japanese survivors after the nuclear bombs in 1945.11 The major limitation of our analysis is the relatively small number of individuals, including the lack of an external validation cohort or a matched set of controls managed off trial. Extended analyses on a larger number of patients on a multicenter basis are warranted.
Acknowledgments: This work was supported by grants from the Swedish Blood Cancer Foundation, The Swedish Cancer Society, the Cancer Society in Stockholm, Cancer and Allergy Foundation and Region Stockholm.
Contribution: A.M., S.E.S., A.Ö., and L.H. designed the study, analyzed the results, and wrote the draft manuscript; T.A.M. performed the statistical analyses; A.M. and J.L. audited the medical files and completed the case record forms; and all authors reviewed and edited revisions of the manuscript and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: A.Ö. and M.P. have received unrestricted scientific grants from BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Agnes Mattsson, Department of Oncology-Pathology, Karolinska Institutet, SE-17176, Stockholm, Sweden; email: agnes.mattsson@regionstockholm.se.
References
Author notes
L.H. and A.O. contributed equally to this study.
All data are available on request from the corresponding author, Agnes Mattsson (agnes.mattsson@regionstockholm.se).