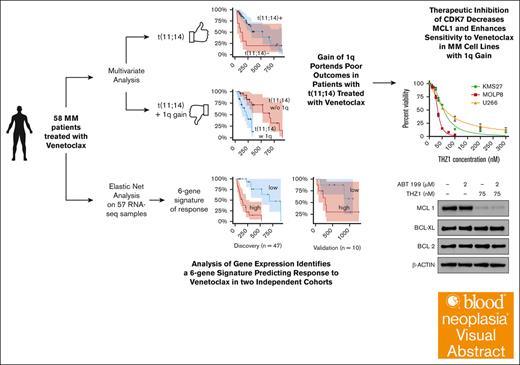
Key Points
RNA-seq analysis reveals a 6-gene signature that stratifies patients with MM by their predicted response to venetoclax.
This study proposes a novel approach to overcoming this resistance through CDK7 inhibition.
Visual Abstract
Venetoclax, a selective B-cell lymphoma 2 (BCL2) inhibitor, has emerged as a promising therapeutic agent for multiple myeloma (MM), particularly in patients harboring the t(11;14) translocation. In this study, we set out to identify markers of sensitivity and resistance to venetoclax in a real-world patient population, aiming to facilitate the development of personalized therapeutic strategies. Through the analysis of RNA sequencing (RNA-seq) data from relapsed/refractory patients treated with venetoclax, either as a single agent or in combination with other drugs, we unveiled a novel 6-gene signature that significantly stratified patients into risk groups for relapse and further validated its clinical relevance in 2 independent clinical and ex vivo data sets. Our analysis also highlighted the negative impact of chromosome 1q gain, which harbors the myeloid cell leukemia-1 (MCL1) gene, on progression-free survival, even in t(11;14)-positive patients. Encouraged by the well-documented role of MCL1 in resistance to venetoclax in various malignancies and the prognostic importance of the BCL2/MCL1 ratio in our cohort, we explored Cyclin-Dependent Kinase 7 (CDK7) inhibition as a potential strategy to overcome venetoclax resistance. In vitro experiments demonstrated that CRISPR-Cas9–mediated CDK7 depletion led to decreased MCL1 levels, enhancing the sensitivity of MM cells to venetoclax. Moreover, the combination of the CDK7 inhibitor THZ1 with venetoclax markedly induced cell death in venetoclax-resistant MM cells harboring 1q gain, thus offering a rational therapeutic approach, particularly for patients with this aberration. Overall, these findings provide important insights for optimizing venetoclax-based therapeutic strategies in MM.
Introduction
Multiple myeloma (MM) is a hematologic malignancy characterized by the clonal proliferation of malignant plasma cells in the bone marrow.1 This leads to the overproduction of monoclonal immunoglobulins, which can cause organ damage and impair the immune system. MM is the second most common blood cancer, with a global incidence of 6 to 7 cases per 100 000 individuals annually. Despite recent advances in treatment options, MM remains an incurable disease, with a high rate of relapse and resistance to therapy.
Venetoclax, a selective oral BCL2 inhibitor, has emerged as a promising therapeutic agent in the management of various hematologic malignancies and is currently approved for use in chronic lymphocytic leukemia and acute myeloid leukemia. In recent years, several clinical trials have investigated the efficacy and safety of venetoclax, both as a monotherapy and in combination with other agents, in the treatment of relapsed/refractory MM (RRMM).2-4 These studies have shown promising results, particularly in patients with the t(11;14) translocation involving cyclin D1 (CCND1) and those with high B-cell lymphoma 2 (BCL2) expression. However, not all patients with t(11;14) achieved a partial response (PR) or better with venetoclax, whereas some patients without t(11;14) did, underscoring the fact that the presence of t(11;14) alone may not be a definitive predictor of venetoclax response.2 In our previous work, RNA-seq analysis revealed the expression of B-cell–specific genes enriched in venetoclax-sensitive MM, suggesting a potential biomarker independent of t(11;14).5 Identifying factors contributing to sensitivity or resistance to venetoclax may aid in patient selection, thereby allowing for personalized treatment strategies and improved clinical outcomes.
The BCL2 protein family, with its proapoptotic and antiapoptotic members, plays a pivotal role in regulating cell survival and apoptosis. Aberrant expression of these proteins contributes to the development of chemoresistance and poor prognosis in MM and other malignancies.6,MCL1, in particular, has emerged as a critical gene for the survival of MM cells and conferring resistance to venetoclax.7-9 MCL1's importance in modulating venetoclax response has been demonstrated in preclinical studies, in which increased MCL1 expression was associated with reduced sensitivity to venetoclax treatment.6 The BCL2/MCL1 ratio has also been proposed as a potential biomarker for predicting venetoclax response. However, the association between MCL1 expression and venetoclax resistance in patients is inconsistent, and MCL1's precise role in the context of venetoclax treatment remains to be fully elucidated.5 Thus, further exploration of these roles is crucial to optimize patient selection and guide therapeutic strategies.
In this study, we present new data from a single-center cohort of patients with RRMM treated with venetoclax-based therapies. Our primary objective was to identify specific markers of sensitivity and resistance to venetoclax in a real-world patient population, which may help guide future therapeutic decision-making. We also explored potential correlations between outcomes and clinical and pathological features, including disease stage, cytogenetic risk profile, and prior treatment history.
Methods
Sample collection and processing
Patients with MM treated at the Icahn School of Medicine at Mount Sinai were included in the study, with sample collection and analysis approved by institutional review boards (Multiple Myeloma Biorepository: ID 18-00456; Precision Medicine for Multiple Myeloma: STUDY-18-00458). Bone marrow aspirates were used to generate RNA-seq libraries, which were then sequenced on Illumina platforms.
Data processing
RNA-seq data were processed using STAR and featureCounts for alignment and gene-level quantification. Normalization and batch effect correction were applied using edgeR and Limma for the Mount Sinai data set, with similar methods applied to the Gene Expression Omnibus data set GSE167968.
Statistical and bioinformatics analysis
Survival analyses were performed using the R “survival” package, using Cox proportional hazards models and Kaplan-Meier tests. A Wilcoxon-Mann-Whitney test was used for other statistical comparisons. An elastic net regression identified a 6-gene signature, which was then validated across independent data sets.
Cellular assays
Cell viability was assessed in myeloma cell lines treated with venetoclax, THZ1, and mevociclib using the CellTiter-Glo assay, whereas western blotting was used to confirm protein expression levels and genetic knockout efficiency. Drug synergy was evaluated using SynergyFinder software.
Additional method details are provided as the supplemental Material.
Results
Study population characteristics
We identified a total of 58 patients who received venetoclax-based therapy between 2017 and 2021 and whose response could be evaluated (Table 1). Among these patients, 5 (8.6%) received venetoclax as monotherapy. In contrast, the remaining 53 (91.4%) were treated with various combinations of drugs to enhance therapeutic efficacy and avoid potential drug resistance. Notably, proteasome inhibitors (PIs; n = 40 [75.4%]) were frequently used in combination with venetoclax, based on the premise that PIs can increase susceptibility to venetoclax-induced apoptosis.10-12 Other combinations included immunomodulatory drugs (n = 13 [24.5%]), the CD38-targeting monoclonal antibody daratumumab (n = 25 [47.1%]), and the histone deacetylase (HDAC) inhibitor panobinostat (n=1, 0.02%). It should be noted that these categories are not mutually exclusive, and some patients received multiple types of drugs (see supplemental Table 1 for detailed treatment regimens). The patients were predominantly male (n = 44 [75.8%]), with a median age of 67.5 years (range, 35-86) at the beginning of therapy. Patients self-identified as White/European (n = 34 [58.6%]), Black/African American (n = 4 [6.8%]), and Asian (n = 3 [5.2%]), whereas the ethnicity of the remaining patients (n = 15 [25.8%]) was unknown. In terms of the International Staging System classification, 22 patients (37.9%) were in stage I, 10 (17.2%) in stage II, 16 (27.6%) in stage III, and the stage was unknown for 10 patients (17.2%). The median number of prior lines of therapy was 4 (range, 1-18).
Fluorescence in situ hybridization and cytogenetic analyses revealed that 48 patients (82.7%) harbored the t(11;14) translocation involving CCND1, and 23 patients (39.6%) exhibited 1q gain, with 19 of them (32.7%) presenting with both abnormalities. Immunohistochemistry demonstrated high levels of BCL2 expression in 26 patients (44.8%), whereas 4 patients exhibited medium levels (6.9%), 3 had low levels (5%), and for 17 patients (29.3%), data were not available (Table 1). The observed predominance of patients with t(11;14) translocation and high BCL2 immunohistochemistry levels reflects the influence of these markers in guiding the selection of venetoclax-based therapy.
The median progression-free survival (PFS) was 7.2 months (range, 1.2-30.4 months). The overall response rate was 53%, comprising stringent complete response in 10 patients (17%), complete response in 7 patients (12%), very good PR in 3 patients (5%), and PR in 11 patients (19%). A minimal response was observed in 2 patients (3%), whereas stable disease was noted in 21 patients (36%). Progressive disease was seen in 2 patients (3%). For 2 patients, the depth of response could not be evaluated (see supplemental Table 1).
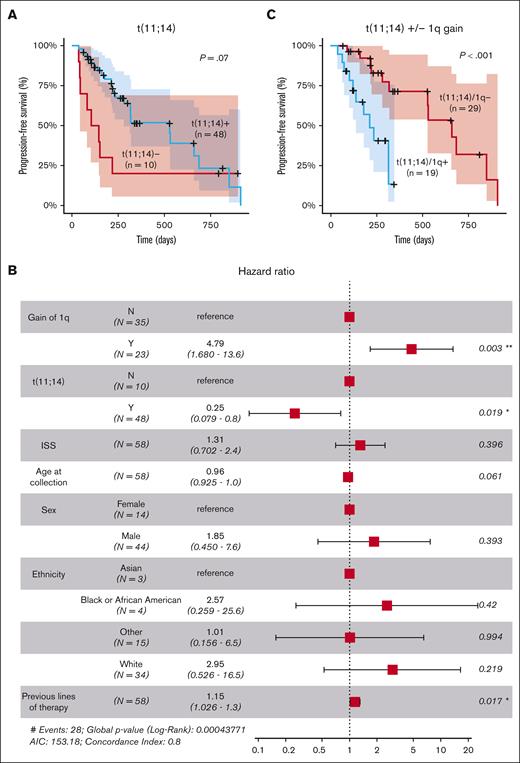
Gain of 1q portends poor outcomes in patients with t(11;14) treated with venetoclax
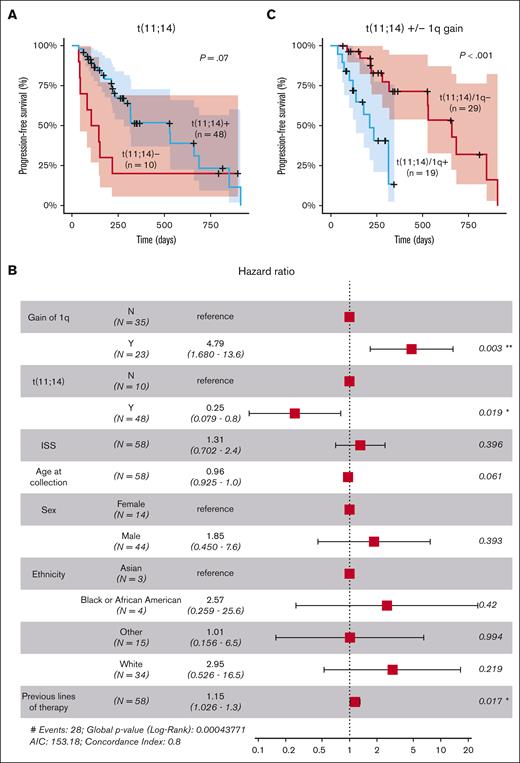
Univariate analysis using Kaplan-Meier and log-rank tests revealed a trend toward significance for the association of t(11;14) with longer PFS, although the difference did not reach statistical significance (P = .07; Figure 1A). However, when adjusting for other factors in a multivariate analysis, t(11;14) demonstrated a statistically significant effect on the outcome (hazard ratio [HR], 0.25; 95% confidence interval [CI], 0.079-0.8; P = .02), suggesting that the influence of t(11;14) on the outcome might have been confounded by other factors in the univariate analysis (Figure 1B). Multivariate analysis also identified a strong significant association between 1q gain and shorter PFS (HR, 4.79; 95% CI, 1.68-13.6; P = .003). The impact of 1q gain was also observed within the group of patients with t(11;14), in which the co-occurrence of 1q gain was associated with significantly shorter PFS (P < .01; Figure 1C). A modest negative effect of the number of previous lines of therapy was also observed (HR, 1.15; 95% CI, 1.026-1.3; P = .017; Figure 1B).
Clinical and cytogenetic markers associated with response to venetoclax. (A) Stratification of patients based on the presence of the t(11;14) translocation involving CCND1. (B) Multivariate analysis of clinical and cytogenetic markers for PFS. (C) Stratification of patients with t(11;14) based on the presence of 1q gain (n = 48). ∗, P<0.05; ∗∗, P<0.01; AIC, Akaike Information Criterion; ISS, International Staging System.
Clinical and cytogenetic markers associated with response to venetoclax. (A) Stratification of patients based on the presence of the t(11;14) translocation involving CCND1. (B) Multivariate analysis of clinical and cytogenetic markers for PFS. (C) Stratification of patients with t(11;14) based on the presence of 1q gain (n = 48). ∗, P<0.05; ∗∗, P<0.01; AIC, Akaike Information Criterion; ISS, International Staging System.
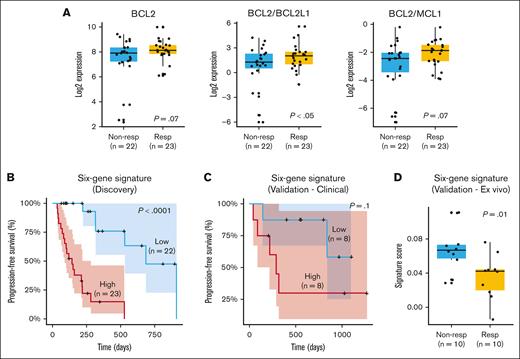
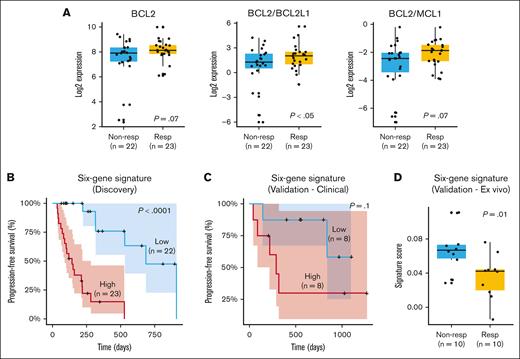
Analysis of gene expression identifies a 6-gene signature predicting response to venetoclax in 2 independent cohorts
We performed a gene expression analysis on 45 of the 58 patients for whom RNA-seq data were available. Initially, we assessed previously identified markers associated with response or resistance to venetoclax, such as BCL2, MCL1, and the ratios BCL2/MCL1 and BCL2/BCL2L1 (Figure 2A). Our results demonstrated a trend toward a better outcome in terms of depth of response (PR or better; P = .07) and a significant association between BCL2 expression and longer PFS (P = .01; supplemental Figure 1A). MCL1 alone did not correlate with PFS or depth of response (data not shown). However, the BCL2/MCL1 ratio exhibited a trend toward higher values in patients achieving PR or better (P = .07). Patients with a higher BCL2/MCL1 ratio also exhibited a trend toward prolonged PFS (supplemental Figure 1B). This observation suggests that the BCL2/MCL1 ratio may be relevant for predicting outcomes, but its statistical significance might be affected by the limited sample size or other factors. In contrast, the BCL2/BCL2L1 ratio was consistently and significantly associated with both depth of response (P < .05) and PFS (P < .05; supplemental Figure 1C).
Gene expression markers and response to venetoclax. (A) Gene expression differences for BCL2, BCL2/BCL2L1, and BCL2/MCL1 in responders (PR or better) and nonresponders (less than PR). (B) Six-gene signature (ATP1B3, MYL2, CNR1, FKBPL, LRRK2-DT, and LINC02541) significantly stratifies patients according to PFS and depth of response. (C) Validation of 6-gene signature in an independent cohort of venetoclax-treated patients with clinical response data. (D) Validation of 6-gene signature in a data set of primary MM cells treated ex vivo with venetoclax. Nonresp, nonresponders; Resp, responders.
Gene expression markers and response to venetoclax. (A) Gene expression differences for BCL2, BCL2/BCL2L1, and BCL2/MCL1 in responders (PR or better) and nonresponders (less than PR). (B) Six-gene signature (ATP1B3, MYL2, CNR1, FKBPL, LRRK2-DT, and LINC02541) significantly stratifies patients according to PFS and depth of response. (C) Validation of 6-gene signature in an independent cohort of venetoclax-treated patients with clinical response data. (D) Validation of 6-gene signature in a data set of primary MM cells treated ex vivo with venetoclax. Nonresp, nonresponders; Resp, responders.
Despite the observed trends, it is acknowledged that messenger RNA levels of BCL2 and MCL1 do not consistently correlate with their protein expression or functional impact on venetoclax response due to the potential for posttranscriptional modifications and complex cellular interactions.13 This discrepancy, especially prominent in MCL1, underscores the necessity for more comprehensive genomic indicators to predict drug response.
In this context, we extended our analysis to include a comprehensive gene expression profile, using elastic net regression to identify a prognostic gene signature for PFS. This method combines the strengths of both ridge and lasso regression, effectively managing multicollinearity with a regularization term. It is also efficient at variable selection, making it especially suitable for data sets such as ours in which there are more predictors (gene expression levels) than observations (patient samples). Through this analysis, we identified a novel 6-gene signature composed of ATP1B3, MYL2, CNR1, FKBPL, LRRK2-DT, and LINC02541. ATP1B3 is involved in maintaining cellular ion balance, whereas MYL2 plays a role in muscle contraction. CNR1 mediates the effects of endocannabinoid system neurotransmitters, and FKBPL is implicated in cellular stress responses and cancer progression19–22. LRRK2-DT and LINC02541 are long noncoding RNAs with potential roles in gene regulation, although their specific functions have yet to be elucidated.
The signature is a composite value calculated from the expression of 6 genes, each weighted by positive or negative coefficients as determined by the elastic net regression (see the supplemental Material). An analysis of the Multiple Myeloma Research Foundation CoMMpass data set, encompassing >600 patients with MM, confirmed that all 6 genes are consistently expressed across a broad spectrum of cases, underscoring the potential universality of this signature in MM.
The signature significantly stratified patients into low- and high-risk groups for relapse, as evidenced by Kaplan-Meier curves (Figure 2B). Patients with high signature expression had a median PFS of ∼4 months, whereas those with low expression experienced a more extended median PFS of ∼10 months (P < .0001). More specifically, higher expression levels of ATP1B3 and FKBPL were associated with poorer prognosis, whereas higher expression levels of MYL2, CNR1, LRRK2-DT, and LINC02541 were linked to better outcomes. Subanalysis of the signature's predictive value in subsets receiving venetoclax with PIs (n = 34) and daratumumab (n = 21) confirmed its significant association with PFS across different treatment regimens. Higher signature scores were also linked to less favorable responses (less than PR; P < .05; supplemental Figure 1D).
Among the identified genes, we selected ATP1B3 for further analysis due to its involvement in maintaining cellular ion balance, a critical function in cellular homeostasis and potential impact on drug response. Our analysis revealed that ATP1B3 alone is a significant prognostic indicator, with higher expression correlating with significantly lower PFS (supplemental Figure 2A). Additionally, ATP1B3 was highly expressed in cell lines with t(11;14) and 1q gain, specifically U266 and MOLP8, as shown by western blot analysis (supplemental Figure 2B). Knockdown of ATP1B3 by small interfering RNA in the resistant cell line MOLP8 increased sensitivity to venetoclax, as evidenced by reduced 50% inhibitory concentration (IC50) values (supplemental Figure 2C-D).
We evaluated the signature using 2 independent data sets from our prior studies.5,14,15 The first comprised 16 patients with clinical response data to venetoclax treatment. The second involved 20 primary MM samples subjected to ex vivo venetoclax titration, in which plasma cell apoptosis was quantified after 24-hour drug exposure to establish sensitivity profiles based on calculated IC50 values. Our results indicated that patients with elevated signature scores experienced a notably shorter median PFS of ∼8 months than those with lower scores who had a median PFS of ∼22 months. This distinction is visually evident in the Kaplan-Meier curve (Figure 2C). However, due to the limited size of the data set, statistical significance was not achieved (P = .1). When assessing ex vivo data, we observed that patients with higher signature scores had inferior responses, providing further support for the predictive potential of the signature (Figure 2D). To confirm the specificity of the signature to venetoclax response, we conducted a Cox proportional hazards analysis for PFS in the CoMMpass data set, which includes samples from patients with MM at diagnosis, none of whom were treated with venetoclax as a first-line therapy. The signature did not show prognostic value in this broader MM patient cohort for PFS (P = 1), even among patients with t(11;14) and/or gain of 1q (supplemental Figure 3). This finding suggests that the prognostic utility of the signature is specific to the context of venetoclax treatment.
Therapeutic inhibition of CDK7 decreases MCL1 and enhances sensitivity to venetoclax in MM cell lines with 1q gain
Considering the existing evidence highlighting the role of MCL1 in the development of resistance to venetoclax in MM and other malignancies,16,17 along with our findings on the prognostic value of the BCL2/MCL1 ratio in venetoclax response within our patient cohort, we aimed to explore the potential of targeting MCL1 to increase the sensitivity of MM cells to venetoclax. Importantly, MCL1 is located on chromosome 1q, and our analysis demonstrated that patients with 1q gain exhibited increased resistance to venetoclax. Therefore, we hypothesized that MCL1 depletion could overcome resistance to venetoclax and potentially improve treatment strategies for patients with 1q gain.
Although direct inhibition of MCL1 could be an intuitive strategy, this approach has been associated with potential cardiotoxicity due to the vital role of MCL1 in cardiac muscle cells.13,18 Hence, we sought to explore an alternative route of MCL1 suppression by targeting Cyclin-Dependent Kinase 7 (CDK7). It has been reported that inhibition of CDK7 indirectly leads to MCL1 downregulation, presenting a promising strategy to overcome venetoclax resistance.19,20 Furthermore, recent findings have demonstrated that targeting CDK7 with THZ1 can suppress STAT transcriptional activity and sensitize T-cell lymphomas to BCL2 inhibitors, which supports our approach in MM.21 Additionally, another study demonstrated the role of CDK7 as a master regulator of oncogenic cellular programs supporting MM growth and survival.22 This study showed that CDK7 inhibition via the covalent small-molecule inhibitor YKL-5-124 elicits a strong therapeutic response with minimal effects on normal cells and causes in vivo tumor regression, increasing survival in several mouse models of MM. This approach could help evade the cardiotoxic effects typically associated with direct MCL1 inhibition, offering a promising balance between efficacy and safety in treatment strategies.
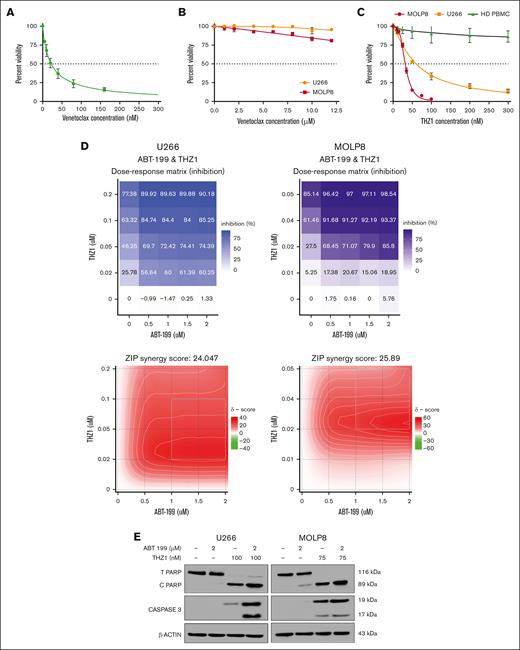
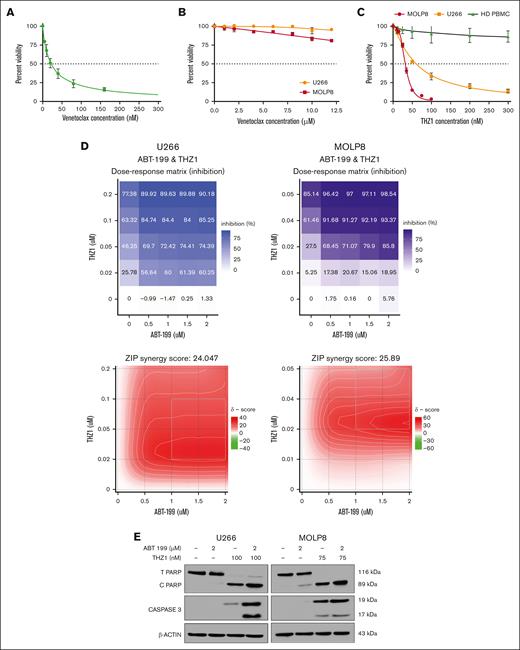
First, we chose appropriate cell lines for these experiments, based on previous literature and guided by our multiomics network model MM-PSN: KMS27 cells, harboring t(11;14), were selected as venetoclax-sensitive controls, whereas U266 and MOLP8 cells carrying 1q gain and t(11;14) were selected as representative resistant cells.23 Venetoclax sensitivity profiling validated these cells' characteristics, with KMS27 exhibiting high sensitivity (IC50, 22.26 ± 4.28 nM) and both U266 and MOLP8 demonstrating resistance (IC50, ≥12 μM; Figure 3A-B). Moreover, western blot analysis of the cell lines confirmed a high BCL2/MCL1 ratio in KMS27 and low values in U266 and MOLP8 (supplemental Figure 4).
Cytotoxic effect of venetoclax (ABT 199) and THZ1 alone and in combination on MM cell lines. (A-B) MM cell lines KMS 27 (A) and U266 and MOLP8 cells (B) were treated with varying concentrations of venetoclax (0-15 μM) for 24 hours. Cell viability, expressed as a percentage of untreated control, was assessed using the CellTiter-Glo (CTG) assay on triplicate cultures. (C) U266 and MOLP8 cells and healthy PBMCs (n = 5) were treated with THZ1 (0-300 nM) for 24 hours, with cell viability assessed using the CTG assay. (D) THZ1 and venetoclax synergistically induce cell death in venetoclax-resistant MM cells. The dose-response matrix and synergy map show the percentage inhibition of U266 and MOLP8 cells after 24 hours of treatment with venetoclax alone and in combination with THZ1 at different concentrations. Cell viability was determined using the CTG assay followed by ZIP score analysis. A ZIP score >10 denotes synergistic interaction. (E) U266 and MOLP8 cells were incubated with the indicated concentrations of ABT-199 ± THZ1 for 24 hours, after which PARP and CASPASE3 cleavage was monitored by immunoblotting analysis. β-actin was assayed to ensure equivalent loading and transfer. ABT, Abbott Laboratories; HD PBMC, healthy peripheral blood mononuclear cell.
Cytotoxic effect of venetoclax (ABT 199) and THZ1 alone and in combination on MM cell lines. (A-B) MM cell lines KMS 27 (A) and U266 and MOLP8 cells (B) were treated with varying concentrations of venetoclax (0-15 μM) for 24 hours. Cell viability, expressed as a percentage of untreated control, was assessed using the CellTiter-Glo (CTG) assay on triplicate cultures. (C) U266 and MOLP8 cells and healthy PBMCs (n = 5) were treated with THZ1 (0-300 nM) for 24 hours, with cell viability assessed using the CTG assay. (D) THZ1 and venetoclax synergistically induce cell death in venetoclax-resistant MM cells. The dose-response matrix and synergy map show the percentage inhibition of U266 and MOLP8 cells after 24 hours of treatment with venetoclax alone and in combination with THZ1 at different concentrations. Cell viability was determined using the CTG assay followed by ZIP score analysis. A ZIP score >10 denotes synergistic interaction. (E) U266 and MOLP8 cells were incubated with the indicated concentrations of ABT-199 ± THZ1 for 24 hours, after which PARP and CASPASE3 cleavage was monitored by immunoblotting analysis. β-actin was assayed to ensure equivalent loading and transfer. ABT, Abbott Laboratories; HD PBMC, healthy peripheral blood mononuclear cell.
Next, we treated the resistant cell lines with THZ1, a covalent inhibitor of CDK7 as well as CDK12/13 that has demonstrated activity against several tumor types, including MM.22,24,25 CDK7 has been reported to effectively modulate MCL1 expression levels, thus sensitizing cancer cells to apoptosis.26 Interestingly, our results showed that both cell lines were responsive to THZ1 treatment, as indicated by reduced cell viability (IC50U266, 32.21 ± 4.59 nM; IC50MOLP8, 60.35 ± 3.87 nM; Figure 3C). Importantly, we found that THZ1 treatment did not significantly reduce viability in peripheral blood mononuclear cells, suggesting a potential hematologic safety profile for this therapeutic approach.
We then investigated the combination of venetoclax and THZ1. Strikingly, cotreatment synergistically induced cell death in the venetoclax-resistant U266 and MOLP8 cell lines. This synergistic effect was quantified using the zero interaction potency (ZIP) synergy model,27 yielding scores of 24.047 and 25.89 for U266 and MOLP8, respectively. In the ZIP model, values >10 indicate synergy; thus these scores suggest a highly synergistic interaction between the 2 drugs in these cell lines (Figure 3D).
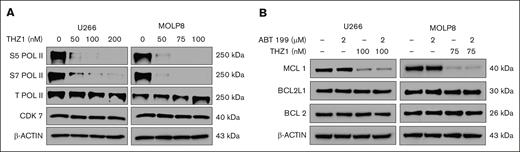
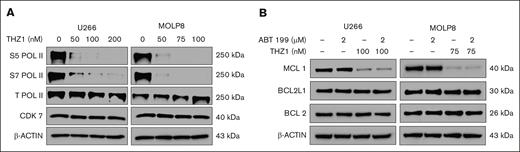
To investigate the underlying mechanism, we evaluated the effects of THZ1 on phosphorylation of RNA polymerase II (RNA Pol II). U266 and MOLP8 cells were treated with increasing concentrations of THZ1 for 24 hours, and we assessed the levels of phosphorylated RNA Pol II at serine 5 and serine 7, as well as the total levels of RNA Pol II and CDK7 by immunoblotting. Our findings showed that THZ1 effectively reduced RNA Pol II phosphorylation at both serine 5 and serine 7, without significantly affecting total Pol II or CDK7 levels, thus suggesting that THZ1 indeed halts transcription by impairing RNA Pol II function (Figure 4A).
Effects of THZ1 on RNA Pol II phosphorylation and MCL1 expression. (A) U266 and MOLP8 cells were treated with various concentrations of THZ1 for 24 hours. Lysates were prepared and subjected to immunoblot analysis to assess S5 Pol II, S7 Pol II, Total Pol II, and CDK7 levels. β-actin was assayed to ensure equivalent loading and transfer. (B) U266 and MOLP8 cells were exposed to venetoclax (ABT199) ± THZ1 for 24 hours. Lysates were prepared for western blotting to assess the expression of apoptosis-related proteins, with β-actin used as a loading control. ABT, Abbott Laboratories; S5 Pol II, RNA Pol II at serine 5; S7 Pol II, RNA Pol II at serine 7.
Effects of THZ1 on RNA Pol II phosphorylation and MCL1 expression. (A) U266 and MOLP8 cells were treated with various concentrations of THZ1 for 24 hours. Lysates were prepared and subjected to immunoblot analysis to assess S5 Pol II, S7 Pol II, Total Pol II, and CDK7 levels. β-actin was assayed to ensure equivalent loading and transfer. (B) U266 and MOLP8 cells were exposed to venetoclax (ABT199) ± THZ1 for 24 hours. Lysates were prepared for western blotting to assess the expression of apoptosis-related proteins, with β-actin used as a loading control. ABT, Abbott Laboratories; S5 Pol II, RNA Pol II at serine 5; S7 Pol II, RNA Pol II at serine 7.
We then further explored the synergistic interaction of venetoclax and THZ1 on apoptotic signaling in these resistant cell lines. U266 and MOLP8 were treated with venetoclax and THZ1 for 24 hours, after which we monitored apoptosis induction through the cleavage of poly (adenosine 5′-diphosphate-ribose) polymerase (PARP) and caspase-3 (CASP3). In U266 cells, THZ1 alone induced significant PARP cleavage, as evidenced by the reduction of total PARP and the increase of cleaved PARP. This cleavage was notably enhanced when cells were cotreated with venetoclax and THZ1 but was absent with venetoclax alone or no treatment. CASP3 cleavage was substantially pronounced when both drugs were administered but was absent with venetoclax alone or no drugs (Figure 3E).
In MOLP8 cells, we observed a similar pattern, with notable differences. Although the cleavage of CASP3 was already visible with THZ1 alone, it was substantially enhanced when cells were treated with the combination of both drugs. These results suggest that THZ1, either alone or in combination with venetoclax, significantly induces apoptosis in venetoclax-resistant MM cells. Importantly, the combination of both drugs leads to a more pronounced apoptotic response, thus supporting their potential as a combination treatment strategy for overcoming venetoclax resistance in MM cells with 1q gain.
Moreover, we demonstrated that THZ1, either alone or in conjunction with venetoclax, significantly downregulated the expression of MCL1 in both cell lines. Interestingly, no substantial changes were observed in the expression levels of BCL2 or BCL2L1 (Figure 4B).
These findings further suggest that CDK7 inhibition via THZ1 directly affects MCL1 expression, thus sensitizing venetoclax-resistant MM cells to apoptosis. The absence of changes in BCL2 and BCL2L1 expression suggests that the effect of THZ1 is selective toward MCL1 in these settings. This further highlights the potential benefit of this combination strategy in overcoming venetoclax resistance associated with 1q gain in MM.
Finally, to confirm that the observed effects of THZ1 were specifically due to CDK7 inhibition, given the broad activity of THZ1 against CDK12 and CDK13, we evaluated mevociclib, a selective CDK7 inhibitor. Mevociclib effectively reduced cell viability in MOLP8 and U266 cells, comparable with THZ1 (supplemental Figure 5A). Moreover, mevociclib synergized with venetoclax, yielding ZIP synergy scores of 13.359 in MOLP8 and 22.754 in U266, further confirming the role of CDK7 in sensitizing MM cells to venetoclax (supplemental Figure 5B).
MCL1 targeting increases venetoclax sensitivity in MM cell lines with 1q gain
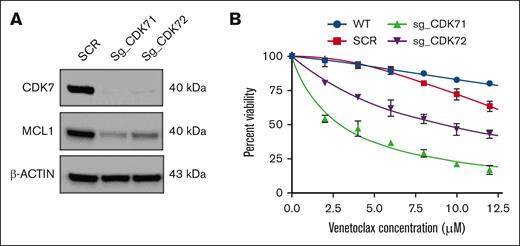
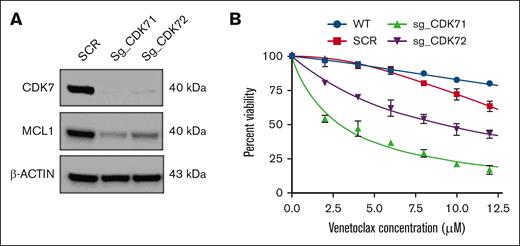
To further corroborate these findings and validate the specificity of our approach targeting the CDK7-MCL1 axis, we performed genetic knockout of CDK7 using CRISPR-Cas9 in the MOLP8 cell line. Depletion of CDK7 was confirmed through western blot analysis, which showed a substantial decrease in the expression of both CDK7 and MCL1 (Figure 5A). Moreover, CDK7 knockout resulted in enhanced sensitivity of these cells to venetoclax, as assessed by cell viability measurements. These findings support the hypothesis that THZ1's effectiveness is mediated through its inhibition of CDK7, despite its known broader activity against CDK12 and CDK13, and highlight the potential therapeutic benefit of selective CDK7 targeting in overcoming venetoclax resistance (Figure 5B).
MCL1 targeting increases venetoclax sensitivity in MM cell lines with 1q gain. (A) CDK7 knockout cells show diminished expression levels of the antiapoptotic protein MCL1. (B) CDK7 knockout cells were treated with varying concentrations of venetoclax (0-12 μM) for 24 hours. Cell viability was assessed by CTG assay. SCR, scramble; sg_CDK71, single-guide RNA 1 (CRISPR); sg_CDK72, single-guide RNA 2 (CRISPR); WT, wild-type.
MCL1 targeting increases venetoclax sensitivity in MM cell lines with 1q gain. (A) CDK7 knockout cells show diminished expression levels of the antiapoptotic protein MCL1. (B) CDK7 knockout cells were treated with varying concentrations of venetoclax (0-12 μM) for 24 hours. Cell viability was assessed by CTG assay. SCR, scramble; sg_CDK71, single-guide RNA 1 (CRISPR); sg_CDK72, single-guide RNA 2 (CRISPR); WT, wild-type.
Discussion
Our study aimed to elucidate potential markers of sensitivity and resistance to venetoclax in patients with MM and explore potential therapeutic strategies to overcome such resistance. The data we presented highlight multiple potential predictive factors for venetoclax response and also suggest potential routes for therapeutic intervention in the case of resistance.
The first finding of our study is the validation of the prognostic value of t(11;14) translocation in a real-world cohort and a strong significant association between gain of chromosome 1q and shorter PFS.2-4 This aberration has previously been associated with poor prognosis in MM, potentially through increasing the expression of genes crucial for MM progression and chemoresistance, including MCL1.23,28-30 MCL1 is an antiapoptotic protein that has been implicated in the development of resistance to venetoclax, a BCL2 inhibitor, in MM and other malignancies.7-9 This underscores the critical balance between proapoptotic and antiapoptotic members of the BCL2 protein family in shaping sensitivity to venetoclax.
Considering the potential role of MCL1 in venetoclax resistance, we explored therapeutic interventions that could target MCL1. We opted against direct MCL1 inhibition due to associated cardiotoxicity, choosing instead to target CDK7, which indirectly modulates MCL1 levels through transcriptional regulation.13,18,31 CDK inhibitors have been shown to induce cell cycle arrest and promote apoptosis in various cancer cells, including MM cells, and their inhibition has been reported to downregulate MCL1 expression, potentially sensitizing cancer cells to venetoclax-induced apoptosis.25,32
Our in vitro experiments demonstrated that a combination of the CDK7 inhibitor THZ1 and venetoclax effectively induced cell death in venetoclax-resistant MM cells. This is consistent with recent studies demonstrating the potential of combining CDK inhibitors, such as dinaciclib, with venetoclax to achieve synergistic effects in killing cancer cells.33 Thus, this combination therapy may not only enhance the effectiveness of venetoclax but also tackle the issue of MCL1-mediated resistance. Our findings provide a promising avenue and rationale for further investigating this therapeutic strategy in MM and other malignancies in which MCL1 plays a critical role in drug resistance.34
Lastly, we identified a 6-gene signature associated with PFS in patients with t(11;14), with higher scores being consistently associated with less favorable responses. This signature is characterized by genes with diverse biological functions, such as ATP1B3, which is implicated in maintaining cellular ion balance, and FKBPL, known for its role in managing cellular stress responses and cancer progression.35,36 Intriguingly, although the direct relationship between these genes and BCL2 or venetoclax will be investigated in future studies, their collective influence as a signature demonstrates substantial prognostic value. The preliminary data from our experiments revealed that knockdown of ATP1B3 significantly sensitizes MM cell lines with 1q gain to venetoclax, underscoring not only its prognostic impact but also its potential as a key mediator of drug response. This novel gene signature presents a valuable tool for enhancing our understanding of patient-specific venetoclax responses, paving the way toward more tailored treatment strategies. Future studies will be needed to further elucidate the mechanistic relationship between these genes and venetoclax resistance. Nevertheless, it will be critical to integrate this molecular signature with other clinical and biological factors to improve risk stratification and guide treatment decisions for patients with t(11;14) MM.
In conclusion, our study identified multiple factors associated with venetoclax response in patients with RRMM, including BCL2 and MCL1 expression, gain of 1q, and a novel 6-gene signature. We also demonstrated a potential therapeutic strategy to overcome venetoclax resistance by combining venetoclax with a CDK7 inhibitor. Although our findings offer promising insights, they should be viewed in the context of some limitations. Our sample size was relatively modest, and future studies with larger cohorts will be needed to validate our findings. Moreover, the retrospective nature of our study may have introduced bias in the selection of patients. Lastly, although our in vitro findings provide a compelling rationale, this combination should be further evaluated in clinical trials to confirm its potential application in clinical practice. Despite these caveats, these findings highlight the potential for personalized therapeutic strategies in MM, offering valuable insights to enhance venetoclax treatment response and overcome resistance, and laying the groundwork for future investigations in this field.
Acknowledgments
The authors thank the Genomics Core Facility at the Icahn Institute and Department of Genetics and Genomic Sciences at Mount Sinai, the Mount Sinai Hematological Malignancies Tissue Bank, and the Human Immune Monitoring Core for their support. This work was also supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
A.L. was supported by the American Society of Hematology Bridge Grant award. S.P. is supported by National Cancer Institute R01 CA244899, CA252222 and Tisch Cancer Institute P30 CA196521 grants. L.H.B. was supported by the Paula and Rodger Riney Foundation. This study was also supported by the Multiple Myeloma Center of Excellence Philanthropic fund.
Authorship
Contribution: A.L. and S.P. provided conceptualization and designed the study; R.P.D. performed laboratory experiments and provided interpretation; A.L. performed data analysis and provided data interpretation; V.L. performed sample processing; M.R., D.T.M., S.B., P.R., V.A.G., B.G.B., and S.N. performed sequencing data processing; S.T. and J.M. performed medical record data curation; F.H. provided sequencing services; H.J.C., S.R., J.R., C.R., A.R., L.S., A.C., S.J., A.K.N., L.H.B., and S.P. provided samples and patients; R.P.D., A.L., and S.P. wrote the original draft of the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: A.L. reports research support from Celgene/Bristol Myers Squibb. A.C. reports research support from Amgen, Array BioPharma, Celgene, GSK, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Oncoceutics, Pharmacyclics, and Seattle Genetics; consultancy fees from Amgen, Bristol Myers Squibb, Celgene, Millennium/Takeda, Janssen, and Karyopharm; and membership on the scientific advisory boards for Amgen, Celgene, Millennium/Takeda, Janssen, Karyopharm, Sanofi, and Seattle Genetics. A.R. is a consultant for Sanofi, Bristol Myers Squibb, Janssen, and Adaptive. C.R. is a consultant for Janssen, Bristol Myers Squibb, Takeda, Sanofi, and Artiva. H.J.C. is employed by the Multiple Myeloma Research Foundation; and has received research support from Bristol Myers Squibb, Takeda, and Genentech, outside the submitted work. J.R. is a consultant/advisor for Janssen, Bristol Myers Squibb, Pfizer, Karyopharm, Sanofi, Takeda, and AbbVie; and a part of speakers bureau for Janssen, Bristol Myers Squibb, Sanofi, and Adaptive Biotechnologies. L.H.B. reports consultancy fees and honoraria from AstraZeneca; and consultancy fees from AbbVie. S.J. is a consultant for Janssen, Bristol Myers Squibb, Legend Biotech, Regeneron, Caribou, Sanofi, Takeda, and Karyopharm; is the Data Monitoring Committee chairman for Genmab and Sanofi; and has membership on an entity's board of directors or advisory committees for International Myeloma Society, American Society of Hematology, and Society of Hematologic Oncology. S.P. receives research support from Amgen Inc, Celgene/Bristol Myers Squibb, the Multiple Myeloma Research Foundation, GRAIL, and Caribou; and is also on the advisory board for GRAIL and Genentech. S.R. has received honoraria from Janssen and research support from Janssen, Bristol Myers Squibb, C4 Therapeutics, Heidelberg Pharma, and Gracell Biotechnologies; and is also a part of Bristol Myers Squibb advisory board/Gracell Biotechnologies steering committee. The remaining authors declare no competing financial interests.
Correspondence: Alessandro Laganà, Icahn School of Medicine at Mount Sinai, 1470 Madison Ave, Room 5-107, New York, NY 10029; email: alessandro.lagana@mssm.edu.
References
Author notes
The RNA-Seq data are available from the NCBI Sequence Read Archive (SRA) under Project ID PRJNA1140228. The RNA-seq data sets used for validation have been deposited in the NCBI Gene Expression Omnibus database (accession number GSE167968).
The complete deidentified clinical data for the Mount Sinai patients in the study are provided as the supplemental Material.
The full-text version of this article contains a data supplement.