Key Points
HRDE are associated with inferior survival in FL; early TFL and FL24 are different entities with distinct natural courses.
Baseline clinical and positron emission tomography parameters are associated with early TFL but not FL24.
Visual Abstract
Progression of follicular lymphoma (FL) or transformation (TFL) within 24 months of immunochemotherapy (ICT) represent high-risk defining events (HRDE) with poor overall survival (OS). We examined baseline clinical characteristics, imaging, and outcomes for patients experiencing HRDE with newly diagnosed FL requiring ICT. HRDE groups were: relapse or progression of FL within 24 months (FL24), early TFL (transformation <24 months of ICT), late TFL (transformation >24 months of ICT).433 patients were categorized as reference FL (Ref FL), n = 352 (no HRDE); FL24, n = 43; early TFL, n = 29; late TFL, n = 9. Chemotherapy included bendamustine (63%), CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone) (27%), or CVP (cyclophosphamide, vincristine, prednisone) (10%); 85% received rituximab/15% obinutuzumab and 48% received maintenance therapy. Compared with Ref FL group, OS from HRDE was inferior for FL24 (hazard ratio [HR], 3.93; 95% confidence interval [CI], 2.14-7.23), early TFL (HR, 8.16; 95% CI, 4.38-15.2), and late TFL (HR, 8.23; 95% CI, 3.18-21.25). OS from HRDE was inferior for early TFL compared with FL24 (HR, 2.08; 95% CI, 1.02-4.21). In multivariable analysis, performance status, lactate dehydrogenase, beta-2-microglobulin and grade 3A were associated with early TFL. Clinical characteristics did not differentiate early TFL from FL24. Maximum standardized uptake value was higher in early TFL but not FL24 compared to Ref FL. Early TFL and FL24 represent different HRDEs and are associated with inferior OS. Distinguishing early TFL from FL24 is important for biomarker development, management and to develop and interpret trials in this area of unmet need.
Introduction
Follicular lymphoma (FL) is the most common form of indolent non-Hodgkin lymphoma (NHL). It is considered to have a favorable prognosis with a median overall survival (OS) of >18 years owing to its indolent nature and the availability of effective therapies for patients requiring treatment.1-3 Despite this favorable long-term survival, lymphoma-related mortality (LRM) remains the leading cause of death in patients with FL.4,5 These LRM events are often preceded by a clinical inflection point, namely high-risk defining events (HRDEs), whereby patients show either evidence of early FL progression after therapy or transformation to a more aggressive NHL.
Transformation of FL (TFL) is a well-described event in the natural history of disease. It can occur at any time but most cases occur in the first 24 months after immunochemotherapy (ICT).6 TFL is a major contributor to treatment failure. Multiple studies have examined outcomes with TFL after ICT with reported 2 year OS ∼30% to 55%.7-13 Upfront treatment appears to affect the outcomes after transformation, with previously untreated or anthracycline-naïve patients experiencing more favorable outcomes after transformation.10,12 Late transformation events are less common, and although they remain associated with increased rates of LRM, they appear to have an improved prognosis compared with TFL earlier in the disease course.8,9,12
Patients who experience progression or relapse with FL early after frontline ICT also experience high rates of LRM.5,8,14,15 Progression of disease within 24 months of therapy (POD24) is the most widely adopted definition for these HRDE and occurs in ∼20% of patients with FL treated with ICT at diagnosis.16 POD24 confers a subsequent 5-year OS of 50% compared with a reference group of 90%. POD24 has subsequently been validated in >5000 patients across 13 clinical trials and is now accepted as an early surrogate end point for LRM in clinical trials.17 Risk factors for POD24 have been identified, including male gender, poor Eastern Cooperative Oncology Group performance status (ECOG PS), high-risk FL international prognostic index (FLIPI) score, and elevated beta-2-microglobulin (B2M).17 Baseline 18F-fluorodeoxyglucose positron emission tomography (PET)-computed tomography metrics are associated with inferior progression-free survival (PFS)18,19; however, their relationship with each HRDE remains uncertain.
A limitation of the POD24 definition is that it is histology agnostic, not distinguishing between progression and relapse with preserved indolent FL histology compared with TFL. The clinical and biological heterogeneity of these HRDE has confounded the development of pretreatment prognosticators and biologically directed clinical studies to alter disease course in these patients. Despite histological differences, POD24 patients with TFL or FL have been equally considered as HRDE and presumed to have a similar outcome.
There are limited data examining baseline characteristics and outcomes of distinct HRDE. In this study, we assessed the outcomes of HRDE in a large, international cohort of patients with newly diagnosed FL requiring ICT at diagnosis in the rituximab (R) era. We categorized HRDE as early FL (FL24), early TFL, or late TFL. We aimed to identify baseline characteristics and imaging parameters that could predict the nature of a subsequent HRDE.
Methods
Patient selection and HRDE definitions
We conducted an international, multicenter retrospective analysis of patients with newly diagnosed FL requiring treatment across 15 academic centers between 2002 and 2021. Patients with grade 1 to 3A FL requiring treatment at diagnosis with ICT using bendamustine, CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), or CVP (cyclophosphamide, vincristine, prednisone) were included. We excluded patients who remained untreated and were initially managed with a “watchful waiting” (WW) approach (defined as >6 months from diagnosis), patients managed with R monotherapy or radiotherapy alone, and patients with suspected or biopsy-proven TFL at diagnosis. Patients followed up for <24 months were also excluded unless experiencing progression, relapse, or TFL in this time. Baseline clinical, imaging and laboratory values, treatment details, and outcomes were collected, and FLIPI scores were calculated in patients with available data. Cases were contributed from academic centers with expert lymphoma multidisciplinary meeting histopathology review. Central pathology review of all cases from a large number of participating sites was not feasible. The institutional review boards of each participating facility approved the research, which was conducted in accordance with the Declaration of Helsinki.
Patients were classified into subsets based on the nature of HRDE: “FL24” defined as relapse or progression of low-grade FL occurring within 24 months of diagnosis, “Early TFL” defined as transformation to aggressive NHL occurring within 24 months of diagnosis, or “Late TFL” defined as transformation to aggressive NHL occurring >24 months from diagnosis. Patients not experiencing an HRDE formed the reference FL (Ref FL) group. Transformation events were defined as either “biopsy-proven” (if histologically confirmed) or “clinically-defined” as determined by the treating clinician where histological confirmation was not available.
Baseline PET scans were conducted according to institutional guidelines. Maximum standardized uptake values (SUVmax) were recorded from diagnostic imaging reports where available. In addition, 127 cases from 4 contributing centers were independently evaluated to calculate total metabolic tumor volume (TMTV) and total lesional glycolysis (TLG) using a semiautomated Syngo.via software package (vB60_HF08). Using Syngo.via “Lesion Scout” tool, a minimum threshold for identifying SUV was set at 2.5. Semiautomated segmentation of images was performed using a criteria threshold of 41% SUVmax and a minimal lesion volume of 1 cm3. The “Auto ID” option was used to exclude normal physiological [18F]Fluorodeoxyglucose(FDG) uptake. The results from the Lesion Scout tool were reviewed and adjusted as necessary to ensure accurate representation of disease by an independent radiologist and nuclear medicine specialist blinded to treatment outcomes.
Statistical analysis
Comparisons of baseline characteristics were performed using Pearson χ2 test or Fisher exact test for categorical variables and analysis of variance or Kruskal-Wallis analysis of variance for normally distributed and non-normally distributed continuous variables, respectively. Landmark analysis for OS from risk-defining event (OS-RDE) was defined as survival from the time of an HRDE or from 2 years after diagnosis for patients not experiencing an event (ie, Ref FL). The Kaplan-Meier method was used to estimate OS-RDE. Survival curves were compared and hazard ratios (HRs) and associated 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression. Univariable and multivariable multinomial logistic regression was used to estimate the associations between HRDE and potential predictor variables. For multivariable models, clinical variables were considered if they were associated with the outcome at P ≤ .3 on univariable analysis; PET metrics were not considered owing to the number of missing values. Initially all variables were included; they were then removed sequentially according to P value until only variables with P ≤ .05 were included in the final model. For multivariable models, missing data were imputed using multiple imputation by chained equations and regression estimates were combined according to Rubin’s rules. Statistical analysis was performed in Stata v17.0 (StataCorp LLC, College Station, TX). P value ≤.05 was considered significant.
Results
Patients and disease characteristics
Initially 501 patients with FL were identified across 15 institutions. We excluded patients initially managed with a WW approach (n = 34) or without ICT (n = 15). In addition, 19 patients died or were lost to follow-up before 24 months without documented HRDE preceding the cause of death and were also excluded. A total of 433 patients were included for further analysis (supplemental Figure 1). Eighty-one patients (19%) experienced an HRDE: FL24, n = 43; early TFL, n = 29; and late TFL, n = 9. Baseline characteristics are presented in Table 1. The mean age was 60 years (range, 30-88), 92.1% had an ECOG PS of 0 to 1, and 45.8% had a high-risk FLIPI score (≥3). Median follow-up from diagnosis was 5.2 years (range, 1.3-21.5 years). Frontline chemotherapy used was bendamustine (62.8%), CHOP (27.5%), or CVP (9.7%). All patients received an anti-CD20 monoclonal antibody with first-line chemotherapy: R was used in 85.2% of patients and obinutuzumab in 14.8%. Maintenance anti-CD20 monoclonal antibody therapy was used in 48.3% of patients.
Baseline patient characteristics for Ref FL and each HRDE subset
| . | All patients, N = 433 . | Ref FL, n = 352 . | HRDE combined, n = 81 . | HRDE subsets . | HRDE vs Ref FL, P value . | ||
|---|---|---|---|---|---|---|---|
| FL24, n = 43 . | TFL early, n = 29 . | TFL late, n = 9 . | |||||
| Age at diagnosis, y | 60.5 | 60.4 | 61.1 | 59.6 | 63.2 | 61.4 | .65 |
| Male | 248/433 (57.3%) | 198/352 (56.3%) | 50/81 (61.7%) | 25/43 (58.1%) | 19/29 (65.5%) | 6/9 (66.7%) | .73 |
| ECOG PS ≥2 | 34/432 (7.9%) | 21/352 (6.0%) | 13/81 (16%) | 5/43 (11.6%) | 8/29 (27.6%) | 0/9 (0.0%) | <.001 |
| SUVmax median (IQR) | 11.4 (8.6-15.1) | 11.1 (8.6-14.6) | 12.1 (8.1-19.0) | 11.4 (7.4-16.5) | 16.3 (9.9-21.9) | 12.1 (10.5-15.6) | .070 |
| TMTV (cm3) (IQR) | 251.9 (79.3-738.5) | 187.4 (81.3-536.1) | 333 (138-1197) | 259.7 (103.5-872.1) | 1197 (740.8-1618) | _ | .12 |
| TLG (IQR) | 1402 (423.8-4752) | 1163 (311.5-3111) | 2209 (535-6702) | 1427 (380.4-6257) | 8606 (5515-11914) | _ | .085 |
| Stage III-IV | 358/432 (82.9%) | 286/352 (81.5%) | 72/81 (88.9%) | 39/43 (90.7%) | 26/29 (89.7%) | 7/9 (77.8%) | .31 |
| LDH elevated | 128/433 (29.6%) | 88/352 (25.0%) | 40/81 (49.4%) | 21/43 (48.8%) | 16/29 (55.2%) | 3/9 (33.3%) | <.001 |
| Age >60 y | 230/433 (53.1%) | 184/352 (52.3%) | 46/81 (56.8%) | 24/43 (55.8%) | 18/29 (62.1%) | 4/9 (44.4%) | .71 |
| >4 nodal sites | 241/432 (55.8%) | 197/352 (56.1%) | 44/81 (54.3%) | 26/43 (60.5%) | 14/29 (48.3%) | 4/9 (44.4%) | .68 |
| Hb <120 g/L | 58/432 (13.4%) | 45/352 (12.8%) | 13/81 (16.0%) | 6/43 (14.0%) | 7/29 (24.1%) | 0/9 (0.0%) | .25 |
| Histological grade 3A | 32/278 (11.5%) | 25/232 (10.8%) | 7/46 (15.2%) | 1/43 (4.5%) | 6/20 (30.0%) | 0/5 (0.0%) | .069 |
| BM involvement | 144/335 (43.0%) | 116 (41.4%) | 28/55 (50.9%) | 14/30 (46.7%) | 12/20 (60.0%) | 2/5 (40.0%) | .42 |
| B2M elevated | 67/242 (27.7%) | 48/202 (23.8%) | 19/40 (47.5%) | 7/19 (36.8%) | 11/16 (68.8%) | 1/5 (20.0%) | .001 |
| FLIPI group | |||||||
| Low (0-1) | 96/432 (22.2%) | 83/351 (23.6%) | 13/81 (16.0%) | 6/43 (14.0%) | 3/29 (10.3%) | 4/9 (44.4%) | .013 |
| Int (2) | 138/432 (31.9%) | 120/351 (34.2%) | 18/81 (22.2%) | 12/43 (27.9%) | 5/29 (17.2%) | 1/9 (11.1%) | |
| High (3-5) | 198/432 (45.8%) | 148/351 (42.2%) | 50/81 (61.7%) | 25/43 (58.1%) | 21/29 (72.4%) | 4/9 (44.4%) | |
| 1L therapy | |||||||
| Bendamustine | 272/433 (62.8%) | 227/352 (64.5%) | 45/81 (55.6%) | 21/43 (48.8%) | 19/29 (65.5%) | 5/9 (55.6%) | .29 |
| CHOP | 119/433 (27.5%) | 93/352 (26.4%) | 26/81 (32.1%) | 14/43 (32.6%) | 8/29 (27.6%) | 4/0 (44.4%) | |
| CVP | 42/433 (9.7%) | 32/352 (9.1%) | 10/81 (12.3%) | 8/43 (18.6%) | 2/29 (6.9%) | 0/9 (0.0%) | |
| CD20 MAb | |||||||
| Rituximab | 369 /433 (85.2%) | 293/352 (83.2%) | 76/81 (93.8%) | 41/43 (95.3%) | 26/29 (89.7%) | 9/9 (100.0%) | .095 |
| Obinutuzumab | 64/433 (14.8%) | 59/352 (16.8%) | 5/81 (6.2%) | 2/43 (4.7%) | 3/29 (10.3%) | 0/9 (0.0%) | |
| Maintenance therapy | 209/433 (48.3%) | 177/352 (50.3%) | 32/81 (39.5%) | 16/43 (37.2%) | 11/29 (37.9%) | 5/9 (55.6%) | .25 |
| Median follow-up from diagnosis y (range) | 5.2 y (0.6-20.5) | 5.09 y (2.1-19.1) | _ | 7.2 y (0.6-20.5) | 5.66 y (0.7-16.5) | 10.29 y (2.5-17.9) | _ |
| . | All patients, N = 433 . | Ref FL, n = 352 . | HRDE combined, n = 81 . | HRDE subsets . | HRDE vs Ref FL, P value . | ||
|---|---|---|---|---|---|---|---|
| FL24, n = 43 . | TFL early, n = 29 . | TFL late, n = 9 . | |||||
| Age at diagnosis, y | 60.5 | 60.4 | 61.1 | 59.6 | 63.2 | 61.4 | .65 |
| Male | 248/433 (57.3%) | 198/352 (56.3%) | 50/81 (61.7%) | 25/43 (58.1%) | 19/29 (65.5%) | 6/9 (66.7%) | .73 |
| ECOG PS ≥2 | 34/432 (7.9%) | 21/352 (6.0%) | 13/81 (16%) | 5/43 (11.6%) | 8/29 (27.6%) | 0/9 (0.0%) | <.001 |
| SUVmax median (IQR) | 11.4 (8.6-15.1) | 11.1 (8.6-14.6) | 12.1 (8.1-19.0) | 11.4 (7.4-16.5) | 16.3 (9.9-21.9) | 12.1 (10.5-15.6) | .070 |
| TMTV (cm3) (IQR) | 251.9 (79.3-738.5) | 187.4 (81.3-536.1) | 333 (138-1197) | 259.7 (103.5-872.1) | 1197 (740.8-1618) | _ | .12 |
| TLG (IQR) | 1402 (423.8-4752) | 1163 (311.5-3111) | 2209 (535-6702) | 1427 (380.4-6257) | 8606 (5515-11914) | _ | .085 |
| Stage III-IV | 358/432 (82.9%) | 286/352 (81.5%) | 72/81 (88.9%) | 39/43 (90.7%) | 26/29 (89.7%) | 7/9 (77.8%) | .31 |
| LDH elevated | 128/433 (29.6%) | 88/352 (25.0%) | 40/81 (49.4%) | 21/43 (48.8%) | 16/29 (55.2%) | 3/9 (33.3%) | <.001 |
| Age >60 y | 230/433 (53.1%) | 184/352 (52.3%) | 46/81 (56.8%) | 24/43 (55.8%) | 18/29 (62.1%) | 4/9 (44.4%) | .71 |
| >4 nodal sites | 241/432 (55.8%) | 197/352 (56.1%) | 44/81 (54.3%) | 26/43 (60.5%) | 14/29 (48.3%) | 4/9 (44.4%) | .68 |
| Hb <120 g/L | 58/432 (13.4%) | 45/352 (12.8%) | 13/81 (16.0%) | 6/43 (14.0%) | 7/29 (24.1%) | 0/9 (0.0%) | .25 |
| Histological grade 3A | 32/278 (11.5%) | 25/232 (10.8%) | 7/46 (15.2%) | 1/43 (4.5%) | 6/20 (30.0%) | 0/5 (0.0%) | .069 |
| BM involvement | 144/335 (43.0%) | 116 (41.4%) | 28/55 (50.9%) | 14/30 (46.7%) | 12/20 (60.0%) | 2/5 (40.0%) | .42 |
| B2M elevated | 67/242 (27.7%) | 48/202 (23.8%) | 19/40 (47.5%) | 7/19 (36.8%) | 11/16 (68.8%) | 1/5 (20.0%) | .001 |
| FLIPI group | |||||||
| Low (0-1) | 96/432 (22.2%) | 83/351 (23.6%) | 13/81 (16.0%) | 6/43 (14.0%) | 3/29 (10.3%) | 4/9 (44.4%) | .013 |
| Int (2) | 138/432 (31.9%) | 120/351 (34.2%) | 18/81 (22.2%) | 12/43 (27.9%) | 5/29 (17.2%) | 1/9 (11.1%) | |
| High (3-5) | 198/432 (45.8%) | 148/351 (42.2%) | 50/81 (61.7%) | 25/43 (58.1%) | 21/29 (72.4%) | 4/9 (44.4%) | |
| 1L therapy | |||||||
| Bendamustine | 272/433 (62.8%) | 227/352 (64.5%) | 45/81 (55.6%) | 21/43 (48.8%) | 19/29 (65.5%) | 5/9 (55.6%) | .29 |
| CHOP | 119/433 (27.5%) | 93/352 (26.4%) | 26/81 (32.1%) | 14/43 (32.6%) | 8/29 (27.6%) | 4/0 (44.4%) | |
| CVP | 42/433 (9.7%) | 32/352 (9.1%) | 10/81 (12.3%) | 8/43 (18.6%) | 2/29 (6.9%) | 0/9 (0.0%) | |
| CD20 MAb | |||||||
| Rituximab | 369 /433 (85.2%) | 293/352 (83.2%) | 76/81 (93.8%) | 41/43 (95.3%) | 26/29 (89.7%) | 9/9 (100.0%) | .095 |
| Obinutuzumab | 64/433 (14.8%) | 59/352 (16.8%) | 5/81 (6.2%) | 2/43 (4.7%) | 3/29 (10.3%) | 0/9 (0.0%) | |
| Maintenance therapy | 209/433 (48.3%) | 177/352 (50.3%) | 32/81 (39.5%) | 16/43 (37.2%) | 11/29 (37.9%) | 5/9 (55.6%) | .25 |
| Median follow-up from diagnosis y (range) | 5.2 y (0.6-20.5) | 5.09 y (2.1-19.1) | _ | 7.2 y (0.6-20.5) | 5.66 y (0.7-16.5) | 10.29 y (2.5-17.9) | _ |
P values obtained using Pearson χ2 test or Fisher exact test (if expected cell counts <1 in any cell or expected counts <5 in >20% of cells) for categorical variables and analysis of variance or Kruskal-Wallis analysis of variance for approximately normally distributed and nonnormally distributed continuous variables respectively. Continuous variables are reported as a median value.
BM, bone marrow; Hb, hemoglobin; Int, Intermediate; IQR, Interquartile range; MAb, monoclonal antibody; SUVmax, maximum standardized uptake value, 1L, frontline.
Survival after clinically defined HRDEs
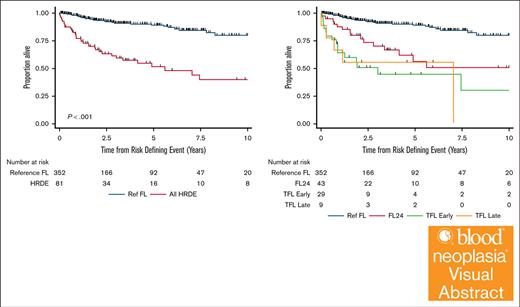
OS-RDE was significantly shorter in those experiencing any HRDE than the Ref FL group (HR, 5.54; 95% CI, 3.41-9.00; P < .001; 5-year OS-RDE 52% any HRDE [95% CI, 38-65] vs 5-year OS-RDE 89% for Ref FL [95% CI, 84-93]) (Figure 1A). There were differences in OS-RDE among HRDE subtypes (Table 2; Figure 1B). Compared with the Ref FL group, there was an increased risk of death for patients experiencing FL24 (HR, 3.93; 95% CI, 2.14-7.23; P < .001; 5-year OS-RDE 56% [95% CI, 36-73]), early TFL (HR, 8.16; 95% CI, 4.38-15.2; P < .001; 5-year OS-RDE 44% [95% CI, 24-63]), and late TFL (HR, 8.23; 95% CI, 3.18-21.25; P < .001; 5-year OS-RDE, 56% [95% CI, 20-80]). Considering only events in the first 24 months of follow-up, there was an increased risk of death for patients experiencing early TFL compared with FL24 (HR, 2.08; 95% CI, 1.02-4.21; P = .04). There was no difference in OS-RDE between early TFL and late TFL (HR, 1.01; 95% CI, 0.37-2.78; P = .99) (Table 2; Figure 1B). From diagnosis, OS at 5 years was significantly longer in the Ref FL group (92% [95% CI, 89-96]) than FL24 (5-year OS 70% (95% CI, 57-87), early TFL 5-year OS 41% (95% CI, 23-70), and late TFL 5-year OS 56% (95% CI, 31-99; P < .001) (supplemental Figure 2).
OS after HRDE. Kaplan-Meier curves of OS-DE in the Ref FL group compared with (A) any HRDE event or (B) clinically defined HRDE subsets.
OS after HRDE. Kaplan-Meier curves of OS-DE in the Ref FL group compared with (A) any HRDE event or (B) clinically defined HRDE subsets.
Results of univariable Cox regression for landmark survival analysis by HRDE (n = 433)
| . | n . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|
| Ref FL | 352 | 1.00 (reference) | |||||
| FL24 | 43 | 3.93 (2.14-7.23) | <.001 | 1.00 (reference) | |||
| TFL early | 29 | 8.16 (4.38-15.20) | <.001 | 2.08 (1.02-4.21) | .04 | 1.00 (reference) | |
| TFL late | 9 | 8.23 (3.18-21.25) | <.001 | 2.09 (0.76-5.75) | .15 | 1.01 (0.37-2.78) | .99 |
| . | n . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|
| Ref FL | 352 | 1.00 (reference) | |||||
| FL24 | 43 | 3.93 (2.14-7.23) | <.001 | 1.00 (reference) | |||
| TFL early | 29 | 8.16 (4.38-15.20) | <.001 | 2.08 (1.02-4.21) | .04 | 1.00 (reference) | |
| TFL late | 9 | 8.23 (3.18-21.25) | <.001 | 2.09 (0.76-5.75) | .15 | 1.01 (0.37-2.78) | .99 |
Association of baseline clinical characteristics with HRDE subtypes
The baseline characteristics of the Ref FL group and each HRDE subset are presented in Table 1. Elevated lactate dehydrogenase (LDH), elevated B2M, FLIPI scores, and ECOG PS >2 were enriched in patients who experienced any HRDE compared with the Ref FL group. To establish whether baseline characteristics were associated with specific HRDE subsets, we performed univariable multinomial logistic regression (Table 3). Compared with Ref FL, elevated LDH was associated with increased rates of both FL24 (relative risk ratio [RRR], 2.86; 95% CI, 1.5-5.46; P = .001) and early TFL (RRR, 3.69; 95% CI, 1.71-7.98; P < .001). Baseline clinical features associated with early TFL only included ECOG PS ≥2 (RRR, 5.98; 95% CI, 2.37-15.11; P < .001), high FLIPI score (RRR, 3.93; 95% CI, 1.14-13.55; P = .012), grade 3A histology (RRR, 3.55; 95% CI, 1.25-10.07; P = .02), and elevated B2M (RRR, 7.06; 95% CI, 2.34-21.32; P < .001). Of these, elevated LDH (RRR, 3.29; 95% CI, 1.41-7.66; P = .006), ECOG PS ≥2 (RRR, 4.36; 95% CI, 1.58-12.13; P = .005), elevated B2M (RRR, 3.09; 95% CI, 1.03-9.29; P = .044), and grade 3A histology (RRR, 3.08; 95% CI, 1.00-9.52; P = .05) remained independently associated with early TFL in multivariable analysis. No clinical features at diagnosis were significantly associated with increased rates of late TFL.
Univariable multinomial logistic regression for baseline clinical and PET metrics for HRDE subsets
| Variables . | FL24 . | Early TFL . | Late TFL . | FL24 vs early TFL, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RRR . | 95% CI . | FL24 vs Ref FL, P value . | RRR . | 95% CI . | Early TFL vs Ref FL, P value . | RRR . | 95% CI . | Late TFL vs Ref FL, P value . | ||
| Female | 0.93 | 0.49-1.76 | .81 | 0.68 | 0.31-1.50 | .34 | 0.64 | 0.16-2.61 | .54 | .53 |
| ECOG PS >2 | 2.07 | 0.74-5.80 | .17 | 5.98 | 2.37-15.11 | <.001 | NA | _ | _ | .092 |
| Stage III-IV | 2.22 | 0.76-6.42 | .14 | 1.97 | 0.58-6.71 | .28 | 0.80 | 0.16-3.92 | .78 | .88 |
| Elevated LDH | 2.86 | 1.50-5.46 | .001 | 3.69 | 1.71-7.98 | <.001 | 1.50 | 0.37-6.12 | .57 | .60 |
| Age >60 | 1.15 | 0.61-2.18 | .66 | 1.49 | 0.69-3.26 | .31 | 0.73 | 0.19-2.77 | .64 | .60 |
| >4 nodal sites | 1.20 | 0.63-2.28 | .59 | 0.73 | 0.34-1.56 | .42 | 0.63 | 0.17-2.37 | .49 | .31 |
| Hb < 120 g/L | 1.10 | 0.44-2.76 | .83 | 2.16 | 0.87-5.36 | .09 | NA | _ | _ | .28 |
| Grade 3A | 0.39 | 0.05-3.06 | .37 | 3.55 | 1.25-10.07 | .02 | NA | _ | _ | .053 |
| BM involvement | 1.24 | 0.58-2.63 | .58 | 2.12 | 0.84-5.35 | .11 | 0.94 | 0.16-5.73 | .95 | .36 |
| Elevated B2M | 1.87 | 0.70-5.02 | .21 | 7.06 | 2.34-21.32 | <.001 | 0.80 | 0.09-7.35 | .85 | .065 |
| FLIPI low (0-1) | 1.00 | Reference | .13 | 1.00 | Reference | .012 | 1.00 | Reference | .28 | .83 |
| FLIPI Int (2) | 1.38 | 0.50-3.83 | 1.15 | 0.27-4.96 | 0.17 | 0.02-1.57 | ||||
| FLIPI high (3-5) | 2.34 | 0.92-5.93 | 3.93 | 1.14-13.55 | 0.56 | 0.14-2.30 | ||||
| Variables . | FL24 . | Early TFL . | Late TFL . | FL24 vs early TFL, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RRR . | 95% CI . | FL24 vs Ref FL, P value . | RRR . | 95% CI . | Early TFL vs Ref FL, P value . | RRR . | 95% CI . | Late TFL vs Ref FL, P value . | ||
| Female | 0.93 | 0.49-1.76 | .81 | 0.68 | 0.31-1.50 | .34 | 0.64 | 0.16-2.61 | .54 | .53 |
| ECOG PS >2 | 2.07 | 0.74-5.80 | .17 | 5.98 | 2.37-15.11 | <.001 | NA | _ | _ | .092 |
| Stage III-IV | 2.22 | 0.76-6.42 | .14 | 1.97 | 0.58-6.71 | .28 | 0.80 | 0.16-3.92 | .78 | .88 |
| Elevated LDH | 2.86 | 1.50-5.46 | .001 | 3.69 | 1.71-7.98 | <.001 | 1.50 | 0.37-6.12 | .57 | .60 |
| Age >60 | 1.15 | 0.61-2.18 | .66 | 1.49 | 0.69-3.26 | .31 | 0.73 | 0.19-2.77 | .64 | .60 |
| >4 nodal sites | 1.20 | 0.63-2.28 | .59 | 0.73 | 0.34-1.56 | .42 | 0.63 | 0.17-2.37 | .49 | .31 |
| Hb < 120 g/L | 1.10 | 0.44-2.76 | .83 | 2.16 | 0.87-5.36 | .09 | NA | _ | _ | .28 |
| Grade 3A | 0.39 | 0.05-3.06 | .37 | 3.55 | 1.25-10.07 | .02 | NA | _ | _ | .053 |
| BM involvement | 1.24 | 0.58-2.63 | .58 | 2.12 | 0.84-5.35 | .11 | 0.94 | 0.16-5.73 | .95 | .36 |
| Elevated B2M | 1.87 | 0.70-5.02 | .21 | 7.06 | 2.34-21.32 | <.001 | 0.80 | 0.09-7.35 | .85 | .065 |
| FLIPI low (0-1) | 1.00 | Reference | .13 | 1.00 | Reference | .012 | 1.00 | Reference | .28 | .83 |
| FLIPI Int (2) | 1.38 | 0.50-3.83 | 1.15 | 0.27-4.96 | 0.17 | 0.02-1.57 | ||||
| FLIPI high (3-5) | 2.34 | 0.92-5.93 | 3.93 | 1.14-13.55 | 0.56 | 0.14-2.30 | ||||
The association between first-line treatment and each HRDE was examined (supplemental Table 1). HRDE subtype was not significantly associated with chemotherapy regimen (P = .29), monoclonal antibody (P = .095), or the use of maintenance therapy after induction ICT (P = .25). The incidence of FL24 events was higher in R- than in obinutuzumab-treated patients (11.1% vs 3.1%; P = .042). When adjusted for HRDE, chemotherapy (HR, 1.38; 95% CI, 0.79-2.32; P = .26), monoclonal antibody (HR, 1.82; 95% CI, 0.65-5.10; P = .25), and maintenance therapy (HR, 0.75; 95% CI, 0.44-1.27; P = .28) were not associated with OS-RDE.
Association between baseline PET metrics and HRDE subtypes
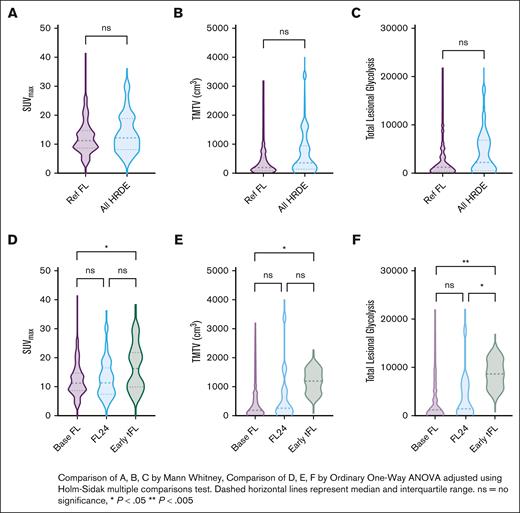
Baseline SUVmax data were available in 255 patients (Ref FL, n = 211; FL24, n = 30; early TFL, n = 14) and TMTV/TLG data were available in 125 patients (Ref FL, n = 104; FL24, n = 18; early TFL, n = 3). Overall, there was no difference in SUVmax, TMTV, or TLG between Ref FL and all HRDEs (Figure 2A-C; supplemental Table 2). However, when examining HRDE subsets, the SUVmax was higher in those with early TFL (median, 16.3; interquartile range [IQR], 9.9-21.9; Padj = .027) compared with Ref FL (median, 11.1; IQR, 8.6-14.6) but not FL24 (median, 11.4; IQR, 7.4-16.5; Padj = .904) (Figure 2D). TMTV and TLG were both higher in early TFL than Ref FL (TMTV Padj = .046; TLG Padj = .0044) but there was no difference between Ref FL and FL24 (TMTV Padj = .195; TLG Padj = .171) (Figure 2E-F).
Comparison of baseline PET metrics among HRDE subsets. Violin plots showing the distribution of baseline PET metrics between Ref FL and HRDE (FL24 and early TFL). (A,D) SUVmax, (B,E) TMTV, and (C,F) TLG.
Comparison of baseline PET metrics among HRDE subsets. Violin plots showing the distribution of baseline PET metrics between Ref FL and HRDE (FL24 and early TFL). (A,D) SUVmax, (B,E) TMTV, and (C,F) TLG.
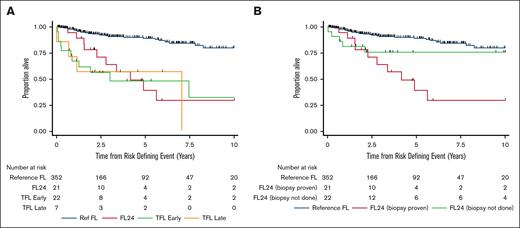
Survival after “biopsy-proven” HRDEs
Overall the HRDE was confirmed by biopsy in 50 of 81 (62%); however, this varied across the HRDE subtypes. In the FL24 group, a biopsy was only performed in 49% of cases. Conversely, a biopsy was performed in 84% of clinically suspected TFL cases. We examined the outcomes of patients where the nature of the HRDE was biopsy proven (n = 402 overall: FL24, n = 21; early TFL, n = 22; late TFL, n = 7). Baseline characteristics were similar to the original cohort (supplemental Tables 3 and 4). As seen in the clinically defined cohort, analysis of biopsy-proven HRDE subsets demonstrated significantly shorter OS-RDE (Figure 3A; supplemental Table 5). Compared with the Ref FL group (5-year OS-RDE, 89% [95% CI, 84-93]), there was an increased risk of death for patients experiencing FL24 (HR, 6.00; 95% CI, 3.00-12.00; P < .001; 5-year OS-RDE 39% [95% CI, 14-64]), early TFL (HR, 7.20; 95% CI, 3.61-14.39; P < .001; 5-year OS-RDE 48% [95% CI, 24-69]), and late TFL (HR, 7.42; 95% CI, 2.61-21.15; P < .001; 5-year OS-RDE 57% [95% CI, 17-84]). However, unlike in the clinically defined cohort, there was no significant difference in OS-RDE for patients experiencing FL24 compared with either early TFL (HR, 1.20; 95% CI, 0.52-2.78; P = .67) or late TFL (HR, 1.24; 95% CI, 0.39-3.91; P = .72). Patients with FL24 without histological confirmation (ie, clinically defined) had a similar OS to the Ref FL group (HR, 2.19; 95% CI, 0.85-5.68; P = .11; 5-year OS-RDE 76% [95% CI, 51-89]) (Figure 3B; supplemental Table 6).
OS after biopsy-proven HRDE. Kaplan-Meier curves of OS-RDE comparing Ref FL group with (A) biopsy-proven HRDE subsets and (B) biopsy-proven and clinically defined FL24 groups.
OS after biopsy-proven HRDE. Kaplan-Meier curves of OS-RDE comparing Ref FL group with (A) biopsy-proven HRDE subsets and (B) biopsy-proven and clinically defined FL24 groups.
Discussion
Early POD or transformation represent critical junctures in FL. This study demonstrated the clinical characteristics and outcomes of patients experiencing HRDE after ICT. The OS for all HRDEs was inferior to the Ref FL group (5-year OS-RDE 89%). Separating the outcomes of HRDE subsets revealed an inferior OS-RDE for early TFL (5-year OS-RDE 44%) compared with FL24 (5-year OS-RDE 56%). Multiple baseline conventional clinical (ECOG PS, elevated LDH, elevated B2M, grade 3A) and imaging prognosticators (SUVmax) were associated with early TFL, whereas only LDH was predictive of FL24 events. Taken together, FL24 and early TFL represent unique entities with distinct clinical trajectories in FL after ICT.
The development of POD24 as a validated outcome measure for FL marked a significant advancement, allowing for early trial assessment that would otherwise require long follow-up. One of the limitations of the original definition was the inability to distinguish among relapse, progression, or transformation given that this information was not available within the National LymphoCare Study.16 Consequently, POD24 encompasses a diverse cohort of high-risk patients characterized by variations in disease biology, clinical and radiological features, and treatment responses. This heterogeneity has impeded efforts to establish clinical prognostic indicators for identifying high-risk patients before treatment initiation, hindering the development of risk-stratified clinical trials. Although attempts to use established clinical scores (eg, FLIPI, FLIPI-2, PRIMA-PI) and molecular biomarkers for identifying POD24 events have been made, their specificity and sensitivity remain modest.20-23 Similarly, the FLIPI24 score, tailored to predict POD24 events using clinical and laboratory parameters, lacks the ability to differentiate among relapse, progression, transformation, or death.24 Furthermore, the recruitment of patients for trials aimed at improving outcomes after POD24 events remains a challenge, partly owing to the exclusion of TFL, which constitutes a substantial portion of POD24 cases in the contemporary setting.25
Our data for patients experiencing early TFL are consistent with previous reports with 75% of all cases within 24 months and a subsequent median OS of ∼2.5 years.7-10,12,13 These early transformations may arise from clonal expansion of occult transformed disease present at baseline under ICT selection pressures.26 Unlike previous studies,8,9 early and late TFL had a similarly poor median OS of 2.5 years from the time of landmark event. For the FL24 group, survival (5-year OS-RDE 56%) was comparable with the original POD24 definition.16 In contrast to early TFL, FL24 may represent treatment failure with resistant clones that emerge early.26 In this study, the chemotherapy backbone or the use of maintenance therapy did not significantly affect the incidence of HRDE (overall or for any specific HRDE) or outcomes after these events. As observed in the GALLIUM trial, although the total HRDE was similar across R- and obinutuzumab-treated patients, those treated with obinutuzumab demonstrated fewer early progressions with FL24 but not cases of transformation.3
Clinical features that predict for POD24 have been described.7,12,13 Established clinical prognosticators were examined to attempt to differentiate early TFL from FL24. Clinical biomarkers at diagnosis did not differentiate early TFL from FL24 in our data. Compared with the Ref FL group, multiple established prognostic features remained significant for early TFL in multivariable testing including elevated LDH, ECOG PS, elevated B2M, and grade 3A histology. Only elevated LDH was a significant biomarker for predicting FL24 and it was not specific given that it was also significant in early TFL. No features at were significantly associated with late TFL. This suggests that prognosticators (eg, FLIPI24) developed using end points of PFS or OS are largely identifying cases of transformation but are insensitive to the occurrence of FL24.
Baseline quantitative PET metrics may also identify patients at high risk of HRDE, although the optimal application of these indices is unclear. Higher SUVmax values have been reported in patients with de novo aggressive NHL histology compared with FL.27 Tumor bulk (assessed as TMTV) is prognostic for POD24 and OS in FL19,28; however, the contribution of each HRDE (early TFL and FL24) to these outcomes remains to be elucidated. The composite measure of bulk and SUVmean as TLG was predictive in identifying early transformation events compared with late.29 These PET parameters may drive clinical decisions with large real-world data showing patients with PET-staged FL more frequently treated with anthracycline-containing regimens.30 Our study demonstrated a considerable range in SUVmax across the entire cohort, including those experiencing HRDE, reflecting biological heterogeneity within FL. There was no difference in SUVmax, TMTV, or TLG between all HRDEs and Ref FL. However, examining HRDE individually demonstrated SUVmax, TMTV, and TLG were all higher in early TFL than Ref FL. Conversely, there were no differences in these same metrics in FL24 compared with Ref FL. Our small numbers limit firm conclusions. The lack of availability of PET data in all patients precluded the inclusion of PET metrics in multivariable analysis. These findings require validation in larger prospective data sets that separate HRDEs into early TFL and FL24 to clarify the role of baseline PET metrics in predicting HRDE.
In previous studies, the frequency of a biopsy at progression ranged from 14% to 41%.2,3,6 Consequently, these data are not based on systematic biopsies of all patients and the true frequency of early TFL vs FL24 is unknown. In clinical practice, the nature of an HRDE is often based on clinician judgment. When a biopsy is not feasible, patients are often treated for TFL based on a high index of clinical suspicion. In our data, HRDE without a biopsy were clinically defined by treating physicians; however, we did not specify the criteria for clinical TFL. Our biopsy-proven HRDE demonstrated similar baseline characteristics to the clinically defined cohort and similar patterns of inferior OS-RDE for FL24, early TFL, and late TFL groups compared with the Ref FL group. OS-RDE was similar between biopsy-proven FL24 (5-year OS-RDE 39%) and early TFL groups. Conversely, FL24 patients without a biopsy had a similar OS-RDE to the Ref FL group. Although numbers are small, this highlights the heterogeneity among FL24 events and the poor prognosis of patients with biopsy-proven FL24. HRDEs are heterogeneous and clinicians consider timing, tumor volume and kinetics, symptoms, and laboratory values in determining whether to perform a biopsy.
Our study has several limitations inherent to retrospective real-world data with nonrandom allocation of treatment. A universal limitation in the field is that histological confirmation of the HRDE was only available in a subset of patients. However, limiting the analysis to only patients undergoing a biopsy introduces a selection bias and is more difficult to apply to real-world clinical practice. Although clinical definitions of TFL are subjective, our clinically defined cases appeared to have similar clinical features and outcomes to the biopsy-proven group. Our patients experiencing early TFL after ICT represent a high-risk group given that they have experienced an event after optimal frontline treatment; however, these data are less applicable to patients experiencing transformation after initial management with a WW approach, R monotherapy, or radiotherapy alone. Given that there was no defined requirement for imaging after treatment, the use of PFS as an end point would not be directly comparable with a trial with imaging at predefined timepoints. Information regarding second-line treatment was incomplete and therefore precluded an analysis of its impact. Central pathology review was not performed. Conversely, the strengths of this study are the use of the original POD24 definition to separate HRDE, the use of OS-RDE as a robust end point, and the valuable insights into baseline PET metrics and the role of a biopsy in HRDE in the real world.
In summary, all HRDEs in the real world after ICT are associated with inferior OS. Separating HRDE within the first 24 months after ICT is a priority to understand differences between early TFL and FL24. Given that baseline clinical factors were not able to differentiate early TFL from FL24, future strategies should investigate the role of baseline PET metrics, molecular data, and circulating tumor DNA to develop robust biomarkers for early TFL and FL24. The availability of pretherapy prognostic tools to identify patients predicted to experience HRDE would facilitate risk-stratified frontline trials with novel strategies (eg, bispecific antibodies, chimeric antigen receptor T-cell therapy) to reduce the incidence of HRDE. Similarly, there is a need to separate early TFL from FL24 to provide biologically relevant treatment to these distinct subgroups for patients experiencing an event. Considering early FL and FL24 as distinct entities will be critical to improving the understanding of the biology of each and to design and interpret strategies to improve the survival of patients with HRDE.
Authorship
Contribution: J.W.D.T. and G.H. were involved in project design; J.W.D.T., R.C., R.S., A.G., H.B., T.H.T., C.B., S.J., E.G., S.S., T.T., E.S., Y.C., G.B., N.G., K.M., E.A.H., T.C., J.T., D.T., J.S., G.H., C.Y.C., A.B., K.M., A.J., J.R., P.M., S.M.A., and G.H. contributed data; J.W.D.T., A.G., G.H., V.A.C., and M.M. analyzed data; J.W.D.T., A.G., and G.H. were involved in manuscript writing; and all authors approved the final manuscript.
Conflict-of-interest disclosure: J.W.D.T. reports research funding from BeiGene, Cellectar, and Janssen. D.T. reports research funding from Janssen and Roche; and honoraria/speaker bureau fees from Roche, Janssen, Takeda, Amgen, CSL, EUSA, and Antengene. C.Y.C. reports honoraria/advisory/consulting fees from Roche, Janssen, Gilead, AstraZeneca, Lilly, BeiGene, Menarini, Dizal, AbbVie, Genmab, Sobi, and Bristol Myers Squibb; and research funding from Bristol Myers Squibb, Roche, AbbVie, MSD, and Lilly. E.H. reports research funding (paid to institution) from Roche, Bristol Myers Squibb, Merck KgA, AstraZeneca, TG Therapeutics, and Merck; consultancy or advisory role fees (paid to institution) from Roche, Merck Sharp & Dohme, AstraZeneca, Gilead, Antengene, Novartis, Regeneron, Janssen, Specialised Therapeutics, and Sobi; and travel expenses from AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Greg Hapgood, Princess Alexandra Hospital, Brisbane, QLD 4102, Australia; email: greg.hapgood@health.qld.gov.au.
References
Author notes
Data are available on request from the corresponding author, Greg Hapgood (greg.hapgood@health.qld.gov.au).
The full-text version of this article contains a data supplement.