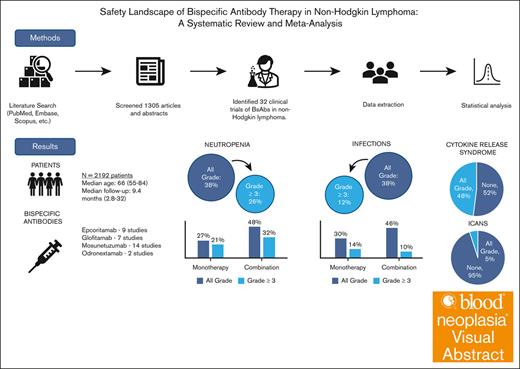
Visual Abstract
Bispecific antibodies (BsAbs) have emerged as a novel immunotherapy option for the treatment of non-Hodgkin lymphoma; however; their safety profiles remain underexplored. We conducted a systematic review and meta-analysis to better delineate the safety profiles of BsAbs, focusing on the prevalence and rates of infection, neutropenia, cytokine release syndrome (CRS), and immune effector cell–associated neurotoxicity syndrome (ICANS). A comprehensive literature search led to the inclusion of 32 trials, with a total of 2192 patients. Median age of participants was 66 years (range, 55-84) with a median of 2 prior lines of therapy (range, 0-5). At a median follow-up of 9.4 months (range, 2.8-32 , the pooled prevalence of all-grade and grade ≥3 neutropenia was 38% and 26%, respectively, with all-grade and grade ≥3 infections occurring at a rate of 38% and 12%, respectively. The prevalence of all grade CRS was 48% but only 2% was grade ≥3, whereas ICANS was infrequent (5% all grade). Stratification revealed both increased all-grade neutropenia and infection rates with combination therapy in comparison to BsAbs monotherapy. Despite these variations, BsAbs demonstrated an overall manageable safety profile, suggesting their viability as a treatment option in the relapsed/refractory setting. Standardized safety reporting and vigilant monitoring are essential to optimize their clinical use and improve patient outcomes.
Introduction
Most lymphomas are adequately treated with frontline conventional chemotherapy and immunotherapy, but a substantial proportion still relapse or develop refractory disease, of which only a fraction are candidates for salvage therapy.1,2 Bispecific antibodies (BsAbs) are the latest advancement in the immunotherapy field for the treatment relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL).3-6 Building upon the success of prior immunotherapeutic approaches, including immune checkpoint inhibitors, monoclonal antibodies, and chimeric antigen receptor (CAR) T-cell therapies, BsAbs have demonstrated significant efficacy. BsAbs are T-cell engagers that bridge CD3 on the surface of T cells to a target such as CD19 and/or CD20 on the surface of B cells, thereby resulting in T-cell activation, proliferation, and enhanced tumor killing. Multiple BsAbs have been evaluated clinically and recent clinical trials have led to the approval of 3 BsAbs in NHL: mosunetuzumab for R/R follicular lymphoma (FL) after ≥2 lines of therapy, and both epcoritamab and glofitamab for R/R diffuse large B-cell lymphoma (DLBCL) after ≥2 lines of therapy.7-9 Trials showcased notable overall and complete response rates and the potential of BsAbs to induce meaningful clinical outcomes, including for patients previously treated with CAR T-cell therapy.
As these agents become increasingly used in clinical practice and move to earlier lines of therapy, it becomes increasingly important to identify and understand their safety profile. Despite their therapeutic potential, BsAbs are associated with significant treatment-related adverse events (AEs). The dual targeting capability of BsAbs not only enhances tumor-killing capabilities of T cells but also modulate the immune system leading to unique side effects such as cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), and other hematological toxicities, including cytopenias. Infection remains a major concern, attributed not only to the immunomodulatory properties of BsAbs and their effects on CD20-expressing B cells but also to the fact that patients receiving BsAbs are usually heavily pretreated and already immunocompromised.
Although the safety for BsAbs is typically reported per protocol in individual clinal trials, we aim to provide a broader analysis by examining the safety profiles of BsAbs as a class across multiple studies in NHL. This comprehensive overview of common AEs, including infection, neutropenia, CRS, and ICANS, seeks to equip clinicians with a clearer understanding of safety considerations as the use of BsAbs continues to expand in clinical practice.
Methods
Eligibility criteria
Our primary objective was to assess the prevalence of AEs reported in prospective clinical trials in NHL. Primary outcomes of interest included rates of infection, neutropenia, CRS, and ICANS. Secondary outcomes of interest included rates of anemia and thrombocytopenia. All phase 1 to 3 trials evaluating CD20-targeting BsAbs in any type of lymphoma met inclusion criteria, including abstracts. CD19-targeting antibodies were excluded to reduce heterogeneity, because they are no longer used in lymphoma treatment. Retrospective studies were excluded because of inconsistent reporting and attribution of AEs.
Severity of AEs were assessed independently in each study, with severity most commonly defined based on the National Cancer Institute Common Terminology Criteria for Adverse Events. Neutropenia was defined as a reduction in the circulating absolute number of neutrophils, commonly defined as <1.5 x 109 cells/L, with grade ≥3 defined as <1.0 to 0.5 x 109 cells/L. Immune effector cell toxicities, including CRS and ICANS, were graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus criteria.10 In phase 1/2 studies that reported rates separately for dose escalation and fixed dose-expansion cohort, only patients from the dose-expansion cohort were included to reduce dose associated heterogeneity.
Data sources and search strategy
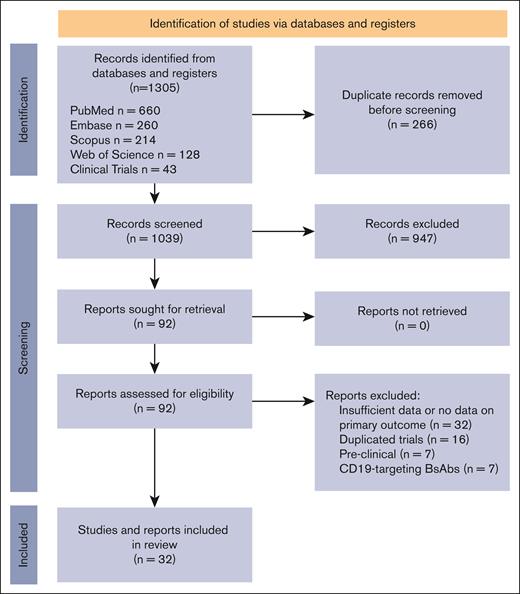
We conducted a systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 We performed a comprehensive literature search on PubMed, Embase, Scopus, Web of Science, and the Cochrane Central Register of Controlled Trials using a combination of keywords related to “bispecific antibodies” and “lymphoma” (supplemental Text 1). Our search strategy aimed to identify all clinical trials evaluating BsAbs in the treatment of any type of lymphoma. We initially identified 1305 articles. After duplicate removal, remaining articles were screened based on predefined inclusion criteria focusing on phase 1 to 3 trials, assessing safety and efficacy of BsAbs in any type of NHL, either as monotherapy or in combination with other therapies, irrespective of prior lines of therapy.
Data extraction and quality assessment
Prespecified data elements were extracted independently by 2 authors (R.F. and A.J.L.). Data elements extracted included study characteristics, patient demographics, treatment details, and AEs. Discrepancies were resolved through consensus or third-party adjudication (T.H.). Quality assessment of included trials was done using Cochrane risk of bias tool for randomized trials and the Risk of Bias 2 (ROB2) tool for nonrandomized studies. Risk of bias assessment was independently evaluated by 2 authors (R.F. and A.J.L.).
Statistical analyses
Prevalence of AEs grade >0, ≥3, and 5 were estimated using generalized linear mixed modeling meta-analysis of the proportions of patients with AEs within trials (equally weighted). Subgroup analyses compared infection rates between monotherapy and combination therapy regimens. Common and random effects model estimates of prevalence were reported with 95% confidence intervals (95% CI). Heterogeneity was assessed using I2 and τ2 statistics and tested using the Cochran Q test. P values ≤ .05 were considered statistically significant. Statistical analyses were performed using the R statistical software environment.
Results
In this systematic review conducted up to April 2024, we first identified 1305 articles. After duplicate removal, the remaining articles were screened based on predefined inclusion criteria focusing on phase 1 to 3 trials, assessing safety and efficacy of BsAbs in any type of NHL, either as monotherapy or in combination with other therapies, irrespective of prior lines of therapy. This led to the identification of 39 clinical trials evaluating BsAbs in NHL (Figure 1). Seven studies evaluated CD19-targeting BsAbs and were excluded. All 32 remaining studies evaluated CD20-targeting antibodies, encompassing a total of 2192 patients, as detailed in supplemental Table 1. Most of these studies, which included both abstracts and full-text papers, were phase 1 or 2 trials. The NHL subtypes under investigation included DLBCL, FL, and mantle cell lymphoma. The BsAbs evaluated were epcoritamab (9 studies), glofitamab (7 studies), mosunetuzumab (14 studies), and odronextamab (2 studies).
PRISMA flow diagram. Selection process of studies included in the systematic review following the PRISMA 2020 guidelines. Adapted from Page et al11 with permission.
PRISMA flow diagram. Selection process of studies included in the systematic review following the PRISMA 2020 guidelines. Adapted from Page et al11 with permission.
The median age of participants across the studies was 66 years (range, 55-84), and patients had received a median of 2 prior lines of therapy (range, 0-5). The overall median follow-up duration was 9.4 months (range, 2.8-32). Nineteen of the included studies used BsAbs in combination with other therapeutics, as shown in supplemental Table 1.
Pooled estimates
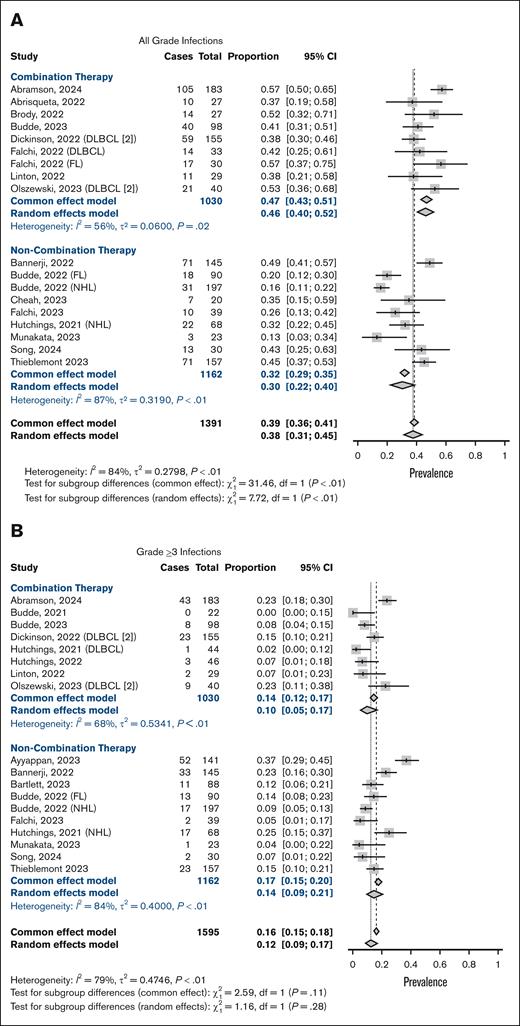
We used a random effect model to account for heterogeneity among the studies. The prevalence of all grade infections was 38% (95% CI, 31-45; I2 = 84%; Figure 2A), and 12% for grade ≥3 infections (95% CI, 9-17; I2 = 79%; Figure 2B). Grade 5 infections were seen in 2% (95% CI, 1-4, I2 = 59%; supplemental Figure 1). The overall prevalence of all-grade and grade ≥3 neutropenia was 38% (95% CI, 31-46; I2 = 83%; Figure 2C) and 26% (95% CI, 21-31; I2 = 75%; Figure 2D), respectively. A proportion of publications included in the analysis did not report results on infections (44%; 14/32 studies). Infections by affected organ system were reported in 8 studies and are summarized in supplemental Table 2. Pearson correlation between all grade infections and neutropenia showed a negligible linear relationship (Pearson r, 1653; P = .52), as well as for grade ≥3 infections and grade ≥3 neutropenia (Pearson r, −0.07; P = .77). Other reported hematological toxicities included anemia, seen in 31% (95% CI, 24-40; I2 = 86%; grade ≥3, 13%) and thrombocytopenia in 23% (95% CI, 12-39; I2 = 91%; grade ≥3, 8%; supplemental Figure 2).
Meta-analysis of neutropenia and infection rates. Forest plots depicting infection and neutropenia prevalence stratified by patients receiving BsAb therapy as monotherapy and those receiving in combination with chemoimmunotherapy. Panels A-B depict prevalence of all-grade infections, and panels C-D show neutropenia rates.
Meta-analysis of neutropenia and infection rates. Forest plots depicting infection and neutropenia prevalence stratified by patients receiving BsAb therapy as monotherapy and those receiving in combination with chemoimmunotherapy. Panels A-B depict prevalence of all-grade infections, and panels C-D show neutropenia rates.
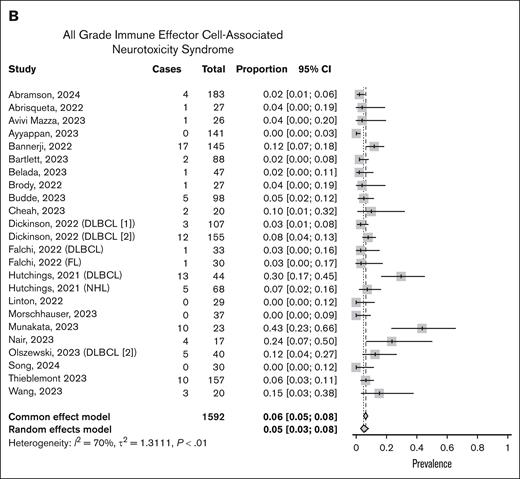
CRS was more consistently reported across the studies compared with infection rates, with all 32 studies reporting data. The overall prevalence of all-grade CRS was 48% (95% CI, 0.42-54; I2 = 83%; Figure 3A). However, grade ≥3 CRS was rare, occurring in only 2% of patients (95% CI, 2-3; I2 = 0%; supplemental Figure 3). Four studies used empiric steroids as part of study treatment. Neurotoxicity was addressed in 23 of the evaluated studies, but criteria to define ICANS was not always specified or consistent across studies. When reported, the prevalence of all-grade ICANS was 5% (95% CI, 3-8; I2 = 70%), and grade ≥3 was 1% (95% CI, 1-2; I2 = 0%; Figure 3B; supplemental Figure 4).
Meta-analysis of CRS and ICANS. Forest plots showing detailed rates of overall immune-related AEs including CRS (A) and ICANS (B).
Meta-analysis of CRS and ICANS. Forest plots showing detailed rates of overall immune-related AEs including CRS (A) and ICANS (B).
Data regarding treatment discontinuation was not consistently reported across studies and was not included in the analysis. Similarly, insufficient information regarding rates of hypogammaglobulinemia were available for analysis.
Monotherapy vs combination therapy
As previously mentioned, 19 (59%) studies evaluated the use of BsAbs in combination with chemotherapy and/or immunomodulating agents. In the attempt to discern the rates of AEs specific to BsAbs without the impact of combinations, the analysis was stratified by monotherapy (ie, BsAb alone) vs combination therapy. Specific regimens used in combination therapy are shown in supplemental Table 1.
Stratification showed significantly higher rates of all grade infections with combination therapy (46%, 95% CI, 40-52; I2 = 56%) than BsAbs monotherapy (30%, 95% CI, 22-40; I2 = 87%; X2 = 7.72; P = .05; Figure 2A). However, the difference in prevalence of grade ≥3 infection between monotherapy and combination therapy was not statistically significant (14%, 95% CI: 9-21 and 10%, 95% CI, 5-17, respectively; P = .28; Figure 2B). No significant difference in grade 5 infections was seen between monotherapy and combination therapy, with a prevalence of 2% (95% CI, 1-3; I2 = 0%) and 3% (95% CI, 1-7; I2 = 71%), respectively (X2 = 1.67; P = .20; supplemental Figure 4). Similarly, all-grade (48%, 95% CI, 39-57; I2 = 77%; vs 27%, 95% CI, 21-33; I2 = 68%; X2 = 14.8; P < .01) and grade ≥3 neutropenia (32%, 95% CI, 24-41; I2 = 77%; vs 21%, 95% CI, 17-25; I2 = 52%; X2 = 5.92; P = .01) were more common in combination therapy (Figure 2C-D). As for CRS and ICANS, no significant differences were seen in either all grade or grade ≥3 rates (supplemental Figure 5).
Lymphoma subtype and treatment setting
The analysis was stratified by lymphoma aggressiveness into 2 categories: indolent (FL) and aggressive (including DLBCL, LBCL, mantle cell lymphoma, and Richter transformation [RT]). Studies that included both subtypes were excluded. Grouping subtypes by aggressiveness rather than analyzing each individually allowed for more robust comparison, given the limited data available for some subtypes. Testing for subgroup differences revealed no statistically significant difference in all grade neutropenia rates but did reveal a significant difference in grade ≥3 neutropenia rates (X2 = 4.48; P = .03) with the highest rates seen in patients with DLBCL (35%, 95 CI, 26-44; supplemental Figure 6). No significant differences were seen in all-grade and grade ≥3 infections between subtypes (supplemental Figure 7). Grade 5 infections as well as ICANS and CRS were not evaluable given the small subgroup and limited instances of qualifying AEs.
No significant differences in AEs were seen when comparing use of BsAbs in the frontline vs R/R setting except for all-grade (X2 = 8.39; P < .01) and grade ≥3 anemia (X2 = 7.80; P < .01; supplemental Figure 8), which was more common in newly diagnosed, albeit only 13 studies reported data on anemia. Similarly, when comparing patients previously treated with CAR T-cell therapy vs not, no statistically significant differences in AE rates were seen, including CRS and ICANS (data not shown).
BsAb products
AEs per BsAbs product are shown in supplemental Figure 9. Statistically significant differences were seen in all grade infections (X2 = 9.16; P = .03), neutropenia (X2 = 9.80; P = .02), CRS (X2 = 16.61; P < .01), and ICANS (X2 = 9.37; P = .05); along with grade ≥3 infections (X2 = 20.75; P < .01) and anemia (X2 = 14.75; P < .01). Of particular interest were all grade infections, which were less common with mosunetuzumab (29%, 95% CI, 20-39) in comparison with epcoritamab (43%, 95% CI, 38-48) and glofitamab (48%, 95% CI, 35-61). Similarly, mosunetuzumab appeared to have the lowest rates of all grade neutropenia compared with epcoritamab and glofitamab.
Odronextamab was associated with the highest grade ≥3 infection rates (29%, 95% CI, 21-40), although only 2 studies were included. Grade ≥3 infection rates with other products included 11% with glofitamab (95% CI, 5-23), 16% with epcoritamab (95% CI, 11-24), and 10% with mosunetuzumab (95% CI, 7-13). The rates of all-grade CRS was lower with mosunetuzumab (37%, 95% CI, 29-46) than with epcoritamab (54%, 95% CI, 47-61) and glofitamab (57%, 95% CI, 49-64).
Discussion
To our knowledge, this analysis provides 1 of the first systematic evaluations of the safety profile of BsAbs in the treatment of NHL. An initial challenge encountered in the review process was the large number of data sets having been reported as abstracts rather than full manuscripts, which limited the extent to which AEs could be detailed. Published abstracts focused predominantly on clinical efficacy data and did not consistently report on all safety results collected. Nevertheless, toxicity associated with BsAbs is known to be primarily associated with immune-mediated phenomena, which led to our focus on hematological toxicity, infection, CRS, and ICANS rates.4
Unlike cytotoxic chemotherapy, hematological toxicity and infections seen with BsAbs are most likely tied to immune engagement rather than direct cytotoxicity. The pooled prevalence of all-grade infections at 35% and grade ≥3 infections at 12% underscore the immunocompromised state of this patient population and is consistent with the known risk associated with T-cell–engager therapies. Specific pathogens and infection types were not always delineated in the aggregated data. However, extrapolating from individual reports, some grade ≥3 infections were viral in nature.12-14 Of note, multiple trials coincided with the peak of the COVID-19 pandemic, during which a considerable proportion of grade ≥3 infections were attributed to severe acute respiratory syndrome coronavirus 2. It is challenging to isolate the pandemic’s impact on infection rates given the paucity of data separating COVID-19–related infections from other etiologies, which is important to consider when interpreting infection rates. Moreover, recent long-term follow-up data suggest that the rate of overall AEs, particularly infections, may rise over time. For instance, Thieblemont et al reported an initial infection rate of 14.6% with epcoritamab, which rose to 25% with 2-year follow-up, highlighting the potential for persistent immune dysregulation.15 The immunosuppressive effects of BsAbs may not be fully captured during the initial stages of therapy, because prolonged immune engagement and gradual depletion of reserves could result in increased susceptibility to opportunistic infections over time, all of which could be further complicated by the cumulative effects of prior therapies. Long-term follow-up will be essential to further elucidate the evolving safety profile of BsAbs.
The observed increased rate of infections with combination therapy compared with monotherapy highlights the compounded immunosuppressive risk when BsAbs are used in conjunction with traditional chemotherapy or other immunomodulators, such as lenalidomide. However, despite being more prevalent, this did not seem to translate to increased severity, because there were no significant differences in grade ≥3 infections between groups when stratified by monotherapy vs combination therapy. Neutropenia is another significant AE seen with BsAbs therapy, noted in 34% of patients for all-grade neutropenia and 21% for grade ≥3. Although these rates align with the anticipated myelosuppressive nature of immunotherapy, they also seem to be less severe than those associated with CAR T-cell therapy, probably because of the effects of lymphodepleting chemotherapy administered before CAR T-cell infusion.
Putting these findings in the context of a treatment landscape that includes CAR T-cell therapy, these rates appear to be more favorable. A recent meta-analysis showed higher rates of grade ≥3 infections in patients treated with CAR T-cell therapy than in those treated with BsAbs in DLBCL.16 Similarly, patients with lymphoma receiving CAR T-cell therapy found a grade ≥3 neutropenia incidence of 60%, with other retrospective analyses showing slightly higher rates.17,18 This difference may be attributable to the lack of need of lymphodepleting therapy with BsAbs, a known risk factor for exacerbated immunosuppression. Nevertheless, a significant proportion of patients included in the analysis had previously received CAR T cells and theoretically represent a group at increased risk for long-standing hematological and immune-related toxicities. However, our analysis did not reveal any significant difference in overall AEs between those previously exposed to CAR T cells vs not, although the analysis was limited to a small number of studies (data not shown).
Although there are no definitive guidelines for antimicrobial prophylaxis specific to patients treated with BsAbs, recent studies have also explored the use of immunoglobulin G replacement in multiple myeloma and chronic lymphocytic leukemia/NHL to address secondary immunodeficiency, particularly hypogammaglobulinemia, after treatment and have shown improved infection outcomes.19,20 Although the pathophysiology of NHL is distinct and theoretically associated with a lower risk of hypogammaglobulinemia compared with multiple myeloma, we cannot draw concrete conclusions because of limited published data on hypogammaglobulinemia associated with BsAbs in our cohort.
CRS, although commonly encountered, was predominantly low grade. All-grade CRS occurred in 43%, with only 2% experiencing grade ≥3 severity, in line with previous reports with BsAbs.21,22 CRS was most commonly encountered during the first cycle of treatment, and the implementation of step-up dosing has demonstrated to be an effective strategy for mitigating the onset of severe CRS.7,8,23,24 Additionally, the use of prophylactic and therapeutic corticosteroids, with or without tocilizumab, has also been instrumental in reducing rates and severity of CRS. This reflects the cumulative expertise derived from the management of CRS associated with CAR T-cell therapies. CRS prevalence rates seen with BsAbs seem to be lower than those associated with CAR T-cell therapy, however the lack of uniform reporting standards and differences in patient characteristics limits a formal comparative analysis.25-27
Although, prevalence of ICANS seems to be low across studies, drawing definitive conclusions is difficult given the lack of standardized criteria to define and classify ICANS. Mild neurological side effects including headaches, insomnia, and dizziness were commonly reported. However, other trials, particularly those evaluating blinatumomab, did document more severe, dose-limiting neurological events, including aphasia and encephalopathy.24,28 Our data suggest a spectrum of neurotoxicity associated with BsAbs with a potential for serious neurological events, which may vary according to individual products, which warrant closer monitoring.
Overall, the safety profile of BsAbs was consistent across trials, including different lymphoma subtypes. One noticeable difference was the increased risk of grade ≥3 neutropenia in DLBCL. Tolerability was emphasized by many trials highlighting high percentages of dose intensity and low proportion of treatment discontinuation from AEs.12,29,30 Notably, fatal events linked directly to treatment were also rare, underscoring the relative safety of BsAbs in this context.12,31 As mentioned above, compared with other treatment modalities, the incidence of fatal events seems to be lower than those reported in historical data. Similarly, in comparison with those receiving therapy in the R/R setting, BsAbs as frontline therapy may also be better tolerated.32
Limitations of our study include the heterogeneity observed across studies, which may affect the generalizability of our findings. Observed heterogeneity is secondary to differences in study design, treatment regimens, limited patient demographic information, and varying AE reporting standards. Disease subtypes may have also influenced heterogeneity, especially when considering differences in BsAbs regimens and lymphoma subtypes. To reduce dose-associated heterogeneity, we included only dose-expansion cohort data when available. However, in some phase 1 trials, in which this distinction was not provided, dose-escalation cohorts were included, potentially leading to an underestimation of AEs. Additionally, the underreporting of treatment discontinuation data limits a comprehensive analysis of the risk-benefit profile of BsAbs. Furthermore, most of the included trials are in early phases and will require additional follow-up to better assess the wide range of associated AEs. As the immunotherapy field continues to evolve, long-term safety data are crucial to understand the chronic effects, especially among those patients who achieve durable responses.
As BsAbs move to earlier lines of therapy either as monotherapy or in combination with cytotoxic chemotherapy or targeted agents, additional research should focus on AE profiles in comparison with standard therapies. This information could help clinicians balance the benefit of increased efficacy in a combination therapy against the increased risk for complications. Sequencing of BsAbs in relation to other treatment options would also likely affect their safety profile. For example, altered immune dynamics and immunosuppressive effects of prior CAR T-cell therapy or multiple prior lines of chemotherapy may synergistically elevate risk of certain AEs. All these factors could partially explain the variance seen across studies.
In conclusion, BsAbs are a promising addition to the treatment of NHL, and our analysis reveals a tolerable safety profile and low treatment-associated mortality rates, particularly in comparison with alternative therapies in the R/R setting. Efforts across the field have helped optimize dosing strategies and incorporate prophylactic measures that reduce the overall incidence of AEs. As BsAbs continue to be integrated into clinical practice, standardized reporting of safety and AEs data will aid in optimizing treatment by balancing tolerability and efficacy to maximize patient outcomes.
Authorship
Contribution: R.F., T.H., and A.J.L. were responsible for designing and writing the protocol; D.A.-D., R.F., and A.J.L. completed data collection; R.F., A.J.L., and T.H. wrote the first draft of the manuscript; B.T.L. performed data analysis; and T.H. and H.R.V. contributed to the writing and reviewing of the manuscript.
Conflict-of-interest disclosure: T.H. reports consulting for BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Talal Hilal, Division of Hematology and Oncology, Mayo Clinic in Arizona, 5881 E Mayo Blvd, Phoenix, AZ 85054; email: hilal.talal@mayo.edu.
References
Author notes
The data supporting the findings of this meta-analysis are derived from publicly available sources and previously conducted studies. All studies included in the analysis are cited in the references.
The full-text version of this article contains a data supplement.