Visual Abstract
TO THE EDITOR:
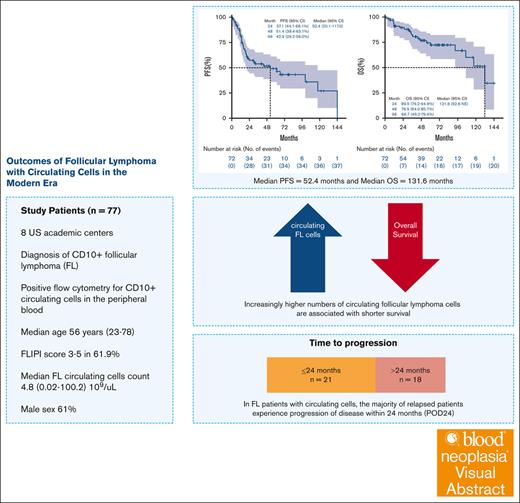
Overall survival (OS) rates are continuously improving for patients with follicular lymphoma (FL) treated in the modern era, with a 15-year OS reported at 65%.1 Circulating lymphoma cells in the peripheral blood can occur in FL and are detected by flow cytometry (FC). The Groupe d’Etude des Lymphomes Folliculaires (GELF) defines leukemic phase FL when the circulating lymphoma cell count exceeds 5 × 109/L.2-4 The Follicular Lymphoma International Prognostic Index (FLIPI) does not include circulating lymphoma cells, whereas FLIPI-2 and FLIPI-24 address this indirectly by including bone marrow involvement and leukocytosis, respectively.5,6 Various smaller studies (n = 7-39) reported an incidence of leukemic phase in 2% to 5% of patients with FL, which has been associated with shorter survival.7-9 To better understand the significance of the increasing number of circulating lymphoma cells, we conducted a multicenter, retrospective study in patients with leukemic phase FL treated in the modern era. We identified patients across 8 US academic institutions with the following inclusion criteria: (1) histopathologic diagnosis of FL, (2) accompanying peripheral blood circulating lymphoma cells by FC at diagnosis (decision to order FC was according to treating physician’s discretion), and (3) initial diagnosis of FL between 2008 and 2022. Criteria for positive FC study was in accordance with World Health Organization classification which included a surface immunoglobulin light-chain restricted population with expression of CD10.10 We excluded patients with FL grade 3b, transformed FL, or other active malignancies at diagnosis. This study was approved by the institutional review boards of participating institutions and was conducted following the Declaration of Helsinki.
Seventy-seven patients with FL and CD10+ circulating lymphoma cells in peripheral blood were identified. Patients’ demographics and disease characteristics are presented in Table 1. The median circulating lymphoma cell count was 4.8 × 109/L (interquartile range, 0.3 × 109/L to 13.4 × 109/L).
Baseline characteristics and response
| Characteristic . | Total patients, N (%) . | Patients treated with frontline therapy, n (%) . |
|---|---|---|
| Total patients | 77 (100) | 72 (100) |
| Age, y | ||
| <60 | 43 (55.8) | 42 (58.3) |
| ≥60 | 34 (44.2) | 30 (41.7) |
| Median (25th percentile-75th percentile) | 56 (46-67) | 55.5 (46-66) |
| Sex | ||
| Female | 30 (39) | 27 (37.5) |
| Male | 47 (61) | 45 (62.5) |
| Race, n (%) | 73 (100) | 68 (100) |
| White | 62 (84.9) | 57 (83.8) |
| Black | 7 (9.6) | 7 (10.3) |
| Asian | 4 (5.5) | 4 (5.9) |
| Ethnicity | ||
| Hispanic | 17 (22.1) | 16 (22.2) |
| Non-Hispanic | 60 (77.9) | 56 (77.8) |
| ECOG performance status, n (%) | 73 (100) | 69 (100) |
| 0-1 | 68 (93.2) | 65 (94.3) |
| 2-3 | 5 (6.8) | 4 (5.7) |
| Bulky disease, n (%) | 75 (100) | 70 (100) |
| No | 57 (76) | 52 (74.3) |
| Yes | 18 (24) | 18 (25.7) |
| FLIPI score, n (%) | 76 (100) | 71 (100) |
| 0-1 | 10 (13.1) | 8 (11.3) |
| 2 | 19 (25) | 17 (23.9) |
| 3-5 | 47 (61.9) | 46 (64.8) |
| B symptoms, n (%) | 76 (100) | 71 (100) |
| No | 44 (57.9) | 41 (57.7) |
| Yes | 32 (42.1) | 30 (42.3) |
| Bone marrow involvement, n (%) | 69 (100) | 66 (100) |
| No | 2 (2.9)∗ | 2 (3) |
| Yes | 67 (97.1) | 64 (97) |
| Elevated LDH, n (%) | 68 (100) | 65 (100) |
| No | 38 (55.9) | 36 (55.4) |
| Yes | 30 (44.1) | 29 (44.6) |
| WBC at diagnosis (×109/L), n (%) | 73 (100) | 69 (100) |
| Median (25th percentile-75th percentile) | 14.2 (8-25.1) | 16.3 (8-25.8) |
| ALC at diagnosis (×109/L), n (%) | 72 (100) | 68 (100) |
| Median (25th percentile-75th percentile) | 7.38 (1.75-16.51) | 8.09 (1.94-18.51) |
| Primary symptom at diagnosis, n (%) | 76 (100) | 71 (100) |
| B symptoms | 21 (27.6) | 21 (29.6) |
| Asymptomatic leukocytosis | 16 (21.1) | 12 (16.9) |
| Asymptomatic lymphadenopathy | 28 (36.8) | 27 (38) |
| Organ compromise | 4 (5.3) | 4 (5.6) |
| Other | 7 (9.2) | 6 (8.5) |
| Circulating lymphoma cells (×109/L) | ||
| <5 | 40 (52) | 36 (50) |
| ≥5 | 37 (48) | 36 (50) |
| Median (25th percentile-75th percentile) | 4.8 (0.3-13.4) | 5.07 (0.48-14.2) |
| Ki67, n (%) | 55 (100) | 51 (100) |
| 0-25 | 31 (56.4) | 29 (56.9) |
| 26-50 | 15 (27.3) | 15 (29.4) |
| 50-85 | 9 (16.4) | 7 (13.7) |
| Median (25th percentile-75th percentile) | 20 (10-40) | 20 (10-40) |
| CD10 positive in lymph node, n (%) | 75 (100) | 70 (100) |
| BCL-2 FISH positive, n (%) | 42 (100) | 41 (100) |
| No | 1 (2.4) | 0 (0) |
| Yes | 41 (97.6) | 41 (100) |
| MYC FISH positive, n (%) | 30 (100) | 30 (100) |
| No | 27 (90) | 27 (90) |
| Yes | 3 (10) | 3 (10) |
| Frontline therapy, n (%) | ||
| Anti-CD20 | 10 (13.0) | 10 (13.9) |
| Anti-CD20 + bendamustine | 36 (46.8) | 36 (50.0) |
| Anti-CD20 + CHOP | 22 (28.6) | 22 (30.6) |
| Surveillance | 5 (6.5) | N/A |
| Other | 4 (5.2) | 4 (5.6) |
| Best response from frontline therapy, n (%) | 73 (100) | 70 (100) |
| CR | 47 (64.4) | 47 (67.1) |
| PR | 16 (21.9) | 16 (22.9) |
| SD | 5 (6.8) | 2 (2.9) |
| PD | 5 (6.8) | 5 (7.1) |
| Death | ||
| No (alive) | 56 (72.7) | 52 (72.2) |
| Yes (dead) | 21 (27.3) | 20 (27.8) |
| POD24†, n (%) | 62 (100) | 50 (100)‡ |
| No | 37 (59.7) | 29 (58.0) |
| Yes | 25 (40.3) | 21 (42.0) |
| Characteristic . | Total patients, N (%) . | Patients treated with frontline therapy, n (%) . |
|---|---|---|
| Total patients | 77 (100) | 72 (100) |
| Age, y | ||
| <60 | 43 (55.8) | 42 (58.3) |
| ≥60 | 34 (44.2) | 30 (41.7) |
| Median (25th percentile-75th percentile) | 56 (46-67) | 55.5 (46-66) |
| Sex | ||
| Female | 30 (39) | 27 (37.5) |
| Male | 47 (61) | 45 (62.5) |
| Race, n (%) | 73 (100) | 68 (100) |
| White | 62 (84.9) | 57 (83.8) |
| Black | 7 (9.6) | 7 (10.3) |
| Asian | 4 (5.5) | 4 (5.9) |
| Ethnicity | ||
| Hispanic | 17 (22.1) | 16 (22.2) |
| Non-Hispanic | 60 (77.9) | 56 (77.8) |
| ECOG performance status, n (%) | 73 (100) | 69 (100) |
| 0-1 | 68 (93.2) | 65 (94.3) |
| 2-3 | 5 (6.8) | 4 (5.7) |
| Bulky disease, n (%) | 75 (100) | 70 (100) |
| No | 57 (76) | 52 (74.3) |
| Yes | 18 (24) | 18 (25.7) |
| FLIPI score, n (%) | 76 (100) | 71 (100) |
| 0-1 | 10 (13.1) | 8 (11.3) |
| 2 | 19 (25) | 17 (23.9) |
| 3-5 | 47 (61.9) | 46 (64.8) |
| B symptoms, n (%) | 76 (100) | 71 (100) |
| No | 44 (57.9) | 41 (57.7) |
| Yes | 32 (42.1) | 30 (42.3) |
| Bone marrow involvement, n (%) | 69 (100) | 66 (100) |
| No | 2 (2.9)∗ | 2 (3) |
| Yes | 67 (97.1) | 64 (97) |
| Elevated LDH, n (%) | 68 (100) | 65 (100) |
| No | 38 (55.9) | 36 (55.4) |
| Yes | 30 (44.1) | 29 (44.6) |
| WBC at diagnosis (×109/L), n (%) | 73 (100) | 69 (100) |
| Median (25th percentile-75th percentile) | 14.2 (8-25.1) | 16.3 (8-25.8) |
| ALC at diagnosis (×109/L), n (%) | 72 (100) | 68 (100) |
| Median (25th percentile-75th percentile) | 7.38 (1.75-16.51) | 8.09 (1.94-18.51) |
| Primary symptom at diagnosis, n (%) | 76 (100) | 71 (100) |
| B symptoms | 21 (27.6) | 21 (29.6) |
| Asymptomatic leukocytosis | 16 (21.1) | 12 (16.9) |
| Asymptomatic lymphadenopathy | 28 (36.8) | 27 (38) |
| Organ compromise | 4 (5.3) | 4 (5.6) |
| Other | 7 (9.2) | 6 (8.5) |
| Circulating lymphoma cells (×109/L) | ||
| <5 | 40 (52) | 36 (50) |
| ≥5 | 37 (48) | 36 (50) |
| Median (25th percentile-75th percentile) | 4.8 (0.3-13.4) | 5.07 (0.48-14.2) |
| Ki67, n (%) | 55 (100) | 51 (100) |
| 0-25 | 31 (56.4) | 29 (56.9) |
| 26-50 | 15 (27.3) | 15 (29.4) |
| 50-85 | 9 (16.4) | 7 (13.7) |
| Median (25th percentile-75th percentile) | 20 (10-40) | 20 (10-40) |
| CD10 positive in lymph node, n (%) | 75 (100) | 70 (100) |
| BCL-2 FISH positive, n (%) | 42 (100) | 41 (100) |
| No | 1 (2.4) | 0 (0) |
| Yes | 41 (97.6) | 41 (100) |
| MYC FISH positive, n (%) | 30 (100) | 30 (100) |
| No | 27 (90) | 27 (90) |
| Yes | 3 (10) | 3 (10) |
| Frontline therapy, n (%) | ||
| Anti-CD20 | 10 (13.0) | 10 (13.9) |
| Anti-CD20 + bendamustine | 36 (46.8) | 36 (50.0) |
| Anti-CD20 + CHOP | 22 (28.6) | 22 (30.6) |
| Surveillance | 5 (6.5) | N/A |
| Other | 4 (5.2) | 4 (5.6) |
| Best response from frontline therapy, n (%) | 73 (100) | 70 (100) |
| CR | 47 (64.4) | 47 (67.1) |
| PR | 16 (21.9) | 16 (22.9) |
| SD | 5 (6.8) | 2 (2.9) |
| PD | 5 (6.8) | 5 (7.1) |
| Death | ||
| No (alive) | 56 (72.7) | 52 (72.2) |
| Yes (dead) | 21 (27.3) | 20 (27.8) |
| POD24†, n (%) | 62 (100) | 50 (100)‡ |
| No | 37 (59.7) | 29 (58.0) |
| Yes | 25 (40.3) | 21 (42.0) |
ALC, absolute lymphocyte count; CHOP, cyclophosphamide, adriamycin, vincristine and prednisone; FISH, fluorescence in situ hybridization; LDH, lactate dehydrogenase; PB, peripheral blood; PD, progressive disease; SD, stable disease; WBC, white blood cell.
These 2 patients had circulating lymphoma cells of 0.1 × 109/L and 1.4 × 109/L. Negative bone marrow could be related to sampling error.
POD24: progression of disease at 24 months, which is not available for patients with follow-up <24 months.
Excluding patients who received single-agent anti-CD20 at first line.
Frontline treatment was heterogeneous among participating centers. An anti-CD20 antibody with bendamustine was most commonly used (n = 36 patients [46%]; Table 1). Maintenance therapy with an anti-CD20 agent was used in 10 of 18 patients with available data. Overall response rate in 70 evaluable patients was 90% (n = 63/70; complete response [CR] in 47 [67.1%] and partial response [PR] in 16 [22.9%] patients; Table 1). Thirty-seven of 70 patients had disease progression. Among them, 21 patients had progression of disease within 24 months of diagnosis (POD24; as defined by Casulo et al11) in the subset of 50 evaluable patients for POD24. Due to progression, 28 received second-line therapy (20 had repeat biopsy at progression; detailed subsequent therapy shown supplemental Table 1). Response was less favorable compared to the first-line therapy with an overall response rate of 64.2% (CR in 46.4% and PR in 17.8%), stable disease in 17.8% and progression of disease in 17.8%. In later lines, chimeric antigen receptor T cell was used in 6 patients (2 with transformation). Of the 4 patients with FL, CR was observed in 3 and PR was observed in 1 patient.
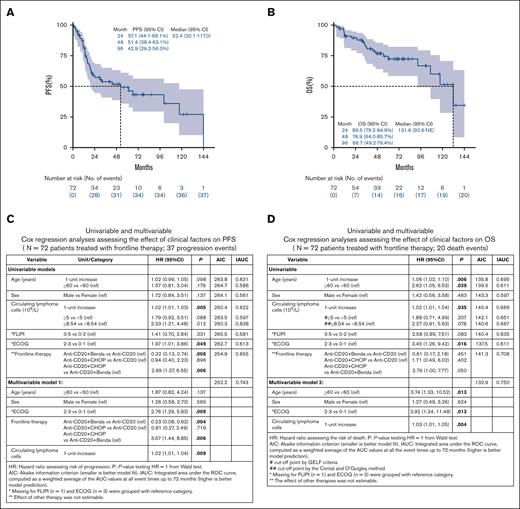
The median duration of follow-up was 49.9 months (range, 1.3-174.4). The median progression-free survival (PFS) was 52.4 months (95% confidence interval [CI], 20.1-117.0; Figure 1A), while the median OS was 131.6 months (95% CI, 92.6 to not estimable [NE]; Figure 1B). Kaplan-Meier curves for PFS and OS stratified by circulating lymphoma cells as dichotomized (<5 × 109/L vs ≥5 × 109/L) variables are shown in supplemental Figure 1. Univariable Cox analyses for PFS show that Eastern Cooperative Oncology Group performance status (ECOG PS) of ≥2 (vs 0-1 as reference hazard ratio [HR], 1.97; 95% CI, 1.01-3.86; P = .049) and higher circulating lymphoma cells count (1-unit increase HR, 1.02; 95% CI, 1.01-1.03; P = .005) were the factors significantly associated with shorter PFS. Circulating lymphoma cells as a dichotomous variable using a GELF cutoff of 5 × 109/L was not associated with PFS (P = .088). Using an outcome-oriented method to determine the cutoff point for risk stratification (low vs high) based on the PFS log-rank test, a cutoff of ≥8.54 × 109/L was significant (vs <8.54 × 109/L as reference HR, 2.33; 95% CI, 1.21-4.48; P = .012).12 Frontline treatment with anti-CD20 with doxorubicin, cyclophosphamide, vincristine and prednisone (anti-CD20 + CHOP) was associated with shorter PFS (vs single-agent anti-CD20 with bendamustine as reference HR, 2.99; 95% CI, 1.37-6.55; P = .006). In multivariable Cox analysis, circulating lymphoma cells as continuous variable (1-unit increase HR, 1.02; 95% CI, 1.01-1.04; P = .009), and treatment with anti-CD20 + CHOP vs anti-CD20 with bendamustine (HR, 3.57; 95% CI, 1.44-8.85; P = .006) were significantly associated with shorter PFS, adjusting for age, sex, and ECOG PS. In addition, treatment with anti-CD20 with bendamustine was associated with longer PFS in a multivariable analysis (vs single-agent anti-CD20 as reference HR, 0.23; 95% CI, 0.08-0.62; P = .004; Figure 1C).
Survival and prognosis factors in patients with leukemic phase follicular lymphoma. Kaplan-Meier curves for PFS (A) and OS (B) (n = 72 patients treated with frontline therapy). Cox regression analysis assessing effect of clinical factors on PFS (C) and OS (D).
Survival and prognosis factors in patients with leukemic phase follicular lymphoma. Kaplan-Meier curves for PFS (A) and OS (B) (n = 72 patients treated with frontline therapy). Cox regression analysis assessing effect of clinical factors on PFS (C) and OS (D).
Higher circulating lymphoma cells count (1-unit increase HR, 1.02; 95% CI, 1.01-1.04; P = .035) were significantly associated with shorter OS. However, circulating lymphoma cells as dichotomized variables (at cutoff points of 5 × 109/L and 8.54 × 109/L), were not associated with OS outcome (Figure 1D). The multivariable Cox model showed that circulating lymphoma cells as a continuous variable was significantly associated with shorter OS (1-unit increase HR, 1.03; 95% CI, 1.01-1.05; P = .004), adjusting for age, sex, and ECOG PS. When focusing on 50 evaluable patients for POD24, those experiencing POD24 after frontline therapy demonstrated shorter post-POD24 survival (HR, 20.77; 95% CI, 2.68-161.14; P = .004) in the univariable analysis. The significance of POD24 (HR, 17.34; 95% CI, 2.02-148.91; P = .009) was held in multivariable analysis adjusting for age, sex, and ECOG PS (supplemental Table 2). In addition, we performed logistic regression analysis to assess the association between circulating lymphoma cells and POD24 event (yes vs no). Consequently, circulating lymphoma cells as continuous and dichotomized using GELF cutoff of 5 × 109/L was not a significant predictor for POD24 event (P > .05); however, the cutoff of 8.54 × 109/L was significantly associated with POD24 event (≥8.54 × 109/L vs <8.54 × 109/L as reference odds ratio, 3.49; 95% CI, 1.01-12.06; P = .049).
Transformation occurred in 8 patients with a median time from diagnosis of 11.2 months (range, 3-40). At initial diagnosis, these patients had a median number of circulating lymphoma cells of 4.0 × 109/L (range, 0.1 × 109/L to 44.2 × 109/L). Median OS of 30.8 months (95% CI, 6.5 to NE) in 8 transformed patients, compared to 131.6 months (95% CI, 106.7 to NE) in 69 nontransformed patients. Five of 8 transformed patients died, and the median survival, from the date of transformation, was 17.3 months (95% CI, 3.5 to NE). Limitations of the current study include the retrospective design, lack of a control arm and detailed data on rituximab maintenance, and nonstandardized decision for FC introducing potential selection bias in our analysis.
In conclusion, to our knowledge, we present the largest cohort to understand the natural history of patients with FL and circulating lymphoma cells in the modern era. The prognostic value of circulating lymphoma cells in FL is debatable with some recent smaller studies suggesting lack of adverse prognostic implications.13 Our study provides evidence that increasingly higher numbers of circulating FL cells are associated with shorter survival. A new suggested cutoff point of 8.54 × 109/L, which was significantly associated with PFS (HR, 2.33; 95% CI, 1.21-4.48; P = .012) but not with OS, underscoring the development of effective therapies and might reflect that improved treatment modalities shift the cutoff point of aggressive biology to a higher threshold. More importantly, validation is needed to confirm the predictive value of this suggested cutoff point for POD24, a novel finding that can aid in tailoring newer agents in the frontline therapy to this high-risk population.
Acknowledgments: I.S.L., J.P.A., and A.T. are supported by the Sylvester Comprehensive Cancer Center National Cancer Institute core grant (grant P30CA240139). J.P.A. is additionally supported by the Peykoff Initiative from the Lymphoma Research Foundation (grant #905127), the Dwoskin Family Foundation, and the US Department of Defense (grant CA220385). I.S.L is additionally supported by grants R01CA233945 and U01 CA195568 from the National Cancer Institute, the Dwoskin and Anthony Rizzo Families Foundations, and the Jaime Erin Follicular Lymphoma Research Consortium.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the US Department of Defense.
The graphical abstract was created with BioRender.com.
Contribution: A.T., I.S.L., and J.P.A. designed research, performed research, analyzed data, and wrote the manuscript; S.H. and I.M.R. analyzed data and wrote the manuscript; and N.E., K.Annunzio., D.M.K., P.D., F.S.-P., B.M., L.F., J.R.C.-F., S.-H.K., N.B.O., K.Alhamad., T.O., G.S., R.K. F.B., M.A., J.S., S.D., and C.A. performed research and wrote the manuscript.
Conflict-of-interest disclosure: J.P.A. received research support from ADC Therapeutics, Genmab, AbbVie, and BeiGene; and provided consultancy services to ADC Therapeutics, Genentech, AbbVie, and Regeneron. I.S.L. received compensation for teaching from Kyowa Kirin Pharmaceutical Development INC; provided consultancy services to Adaptive Biotechnology; and is an Investigational New Drug (IND) holder at ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Juan Pablo Alderuccio, Division of Hematology, Department of Medicine, Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, 1475 NW 12th Ave, Miami, FL 33136; email: jalderuccio@med.miami.edu.
References
Author notes
I.S.L. and J.P.A. contributed equally to this study.
Nonconfidential data will be made available to qualified researchers upon reasonable request from the corresponding author, Juan Pablo Alderuccio (jalderuccio@med.miami.edu).
The full-text version of this article contains a data supplement.