Key Points
HASPIN is a novel kinase dependency in AML regulating growth, transcription, and RNA-binding protein splicing functionality.
HASPIN inhibition suppresses various AML subtypes, synergizes with BCL-2 inhibitor venetoclax, and resensitizes venetoclax-resistant cells.
Visual Abstract
Acute myeloid leukemia (AML) is a blood cancer complicated by acquired drug resistance, disease relapse, and low overall survival rates. Combination therapies using multiple targeted inhibitors have been effective in treating patients with AML. However, combination treatments are limited by the number of usable targets and our ability to create rational pairings using complimentary molecular mechanisms. Here, we used a human kinase domain–targeted CRISPR screen to identify histone H3-associated protein (HASPIN) kinase as a significant, understudied dependency in AML. HASPIN depletion significantly reduced growth rate, induced a cell cycle arrest, and dysregulated transcription in AML. A proteomics data mining study characterized serine and arginine repeat enriched splicing factors (SR proteins) as a major category of HASPIN kinase substrates and highlighted the role of HASPIN as a splicing regulatory kinase. Accordingly, HASPIN depletion strongly dysregulated splicing in AML cells. HASPIN inhibitor CHR-6494 effectively reduced cell viability across AML subtypes while sparing healthy cells. Furthermore, a novel combination therapy consisting of CHR-6494 and B-cell lymphoma 2 (BCL-2) inhibitor venetoclax synergistically reduced AML cell viability and resensitized venetoclax-resistant AMLs to treatment. Our study presents HASPIN kinase as a novel therapeutic target for AML, underscores an underappreciated role of HASPIN in splicing regulation, and proposes a viable combination treatment for clinical testing.
Introduction
Acute myeloid leukemia (AML) is a cancer defined by an expansion of abnormal, undifferentiated hematopoietic cells in the body. AML constitutes one-third of all leukemia cases diagnosed per year.1 With the current available treatments, 5-year overall survival of patients with AML is just 24%. Furthermore, survival rates decrease but incidence rates increase with advancing patient age. Although recommended treatment strategies are revised annually,2,3 AML remains a significant clinical burden that demands additional research into underlying molecular mechanisms so that improved therapeutics can be devised.
Hematopoietic stem cell maintenance, proliferation, and differentiation are regulated by families of protein kinases. Receptor and nonreceptor tyrosine kinases initiate and propagate signaling cascades to strongly affect cellular behavior. Similarly, serine/threonine protein kinases directly phosphorylate substrates to regulate function. Signaling modulator and kinase mutations are present in at least two-thirds of AML cases and are the most common genetic lesion in patients with AML.4 In addition, concurrent kinase mutations confer worse prognoses, higher risk of disease relapse, and, consequently, lower overall survival rates.2 Pathogenic mutations grant proliferative advantages by constitutively activating receptor kinases and associated downstream pathways, such as with mutant fms related receptor tyrosine kinase 3 (FLT3) or KIT proto-oncogene, receptor tyrosine kinase (c-KIT) activating phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathways.4,5 Importantly, leukemia can also develop “addictions” to nonmutated protein kinases.6,7 Normally, dispensable kinases can become critical vulnerabilities in leukemia after adopting key roles in pathways mediating unchecked proliferation,8 metabolic alterations,9 and blocked differentiation.10 Notably, because these dependencies are specific to cancerous genetic environments, they are uniquely amenable to use as therapeutic targets.
AML treatment has not changed significantly for several decades.2 Intensive chemotherapy is broadly prescribed to newly diagnosed patients with AML aimed at achieving prolonged remission. However, patients still experience high relapse rates and poor survival after relapse,11 indicating a need for better treatment strategies. Protein kinases are actively being researched as candidates for targeted inhibition therapy in AML.12,13 Several multireceptor tyrosine kinase inhibitors have been approved for AML, such as FLT3 mutant-specific inhibitors, and have demonstrated clinical success.2 Furthermore, selective inhibitors for nonmutated serine/threonine kinases critical to proleukemic processes are actively being applied in clinical trial.14,15 However, despite improved outcomes, many patients treated with these agents relapse and develop drug resistance.11 Combination therapies are proven to bypass drug resistance and increase treatment efficacy in patients with cancer.16-18 Effective combinations target multiple dysregulated pathways to produce simultaneous toxic effects that cannot be easily adapted to by cancer cells.19 Consequently, this strategy demands a growing repertoire of effective molecular inhibitors and a deep understanding of associated disease mechanisms from which new and rational combinations are designed. Currently, only ∼30% of the human kinome is being researched for potential use in targeted treatment strategies,12,13 indicating that significant potential remains for the discovery of new drug targets. CRISPR screening has been highly effective at identifying novel genetic dependencies in AML.10,20,21 Domain-targeted CRISPR screens extend the approach by targeting genes at functional domains to enhance screen sensitivity.22
Here, we use a human kinase domain–targeted CRISPR screen to discover novel kinase dependencies in human AML. We identify histone H3-associated protein (HASPIN) kinase as a novel kinase dependency. HASPIN has been reported as a mitotic kinase responsible for coordinating spindle fiber attachments, but it has not been investigated in the context of hematopoiesis and leukemia. Using a series of functional and bioinformatic analyses, we highlight the underappreciated role of HASPIN as a messenger RNA (mRNA) splicing regulator. In addition, in vitro testing revealed robust and leukemia-specific therapeutic effects from HASPIN inhibitor modulation both alone and in combination with the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax, highlighting the potential clinical value of HASPIN as a novel target for AML.
Methods
See the supplemental Methods for complete description of methods.
Domain-targeted CRISPR screening
CRISPR screen was performed using the human kinase domain–focused CRISPR knockout (KO) library10 (Addgene 117725; a gift from Christopher Vakoc) and 2 t(8;21) AML cell lines. Kasumi-1 and SKNO-1 were transduced with LentiV_Cas9_Puro to generate Cas9-expressing cell lines. Cells equal to 300 to 500 times in library size (3051 single-guide RNAs [sgRNAs]) were transduced with the pooled, green fluorescent protein (GFP)-tagged library sgRNAs at low multiplicity of infection (MOI) (∼0.3) to ensure singular transductions and sufficient sgRNA coverage. GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS). Genomic DNA was harvested at initial (3 days after transduction) and final (13 doublings) time points using Qiagen DNeasy Blood and Tissue Kit. Cell lines were screened in duplicate. Sequencing libraries were constructed by 2-step polymerase chain reaction amplification, barcoded with Illumina Nextera XT V2 primers, pooled, and sequenced by Illumina HiSeq 4000. Raw sequencing reads were trimmed and processed using Model-based Analysis of Genome-wide CRISPR/Cas9 Knockout (MAGeCK) analysis pipelines.
Results
A kinase domain–targeted CRISPR screen identifies HASPIN as a novel dependency in AML cells
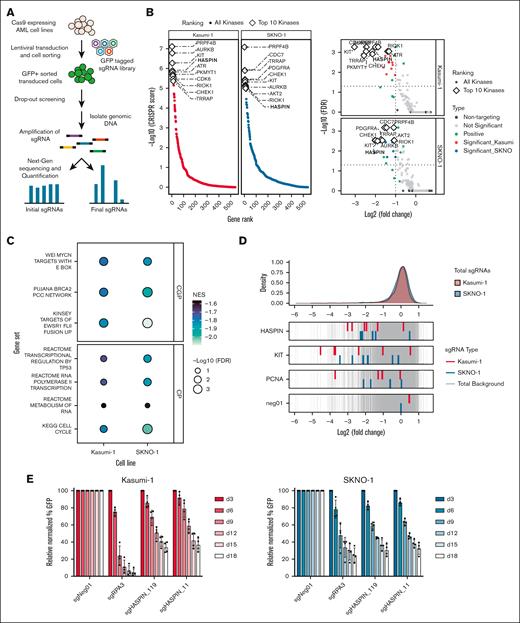
To identify new and therapeutically viable kinase targets for AML, we used a GFP-tagged, domain-targeted sgRNA library consisting of 3051 sgRNAs targeting 482 kinases (Figure 1A). We prioritized kinases with a minimum average log2(fold change) of −1.0 and top 10 ranking by CRISPR score in each of 2 AML cell lines (Figure 1B; supplemental Table 1). Seven kinases were shared between the most critical dependencies (supplemental Figure 1A). Encouragingly, several established AML kinase dependencies were contained within this list.5,23 To explore biological pathways enriched with AML kinase dependencies, we performed preranked gene set enrichment analysis (Figure 1C). Cell cycle, TP53 regulation, RNA metabolism, and progenitor status gene sets were most enriched with kinase dependencies, highlighting recurrent vulnerabilities in AML. We decided to focus our subsequent experiments on HASPIN, which scored highly in both cell lines. Five unique, HASPIN-targeting sgRNAs had significant log2-fold depletions in both cell lines relative to known dependencies and positive controls (Figure 1D). Finally, to validate our CRISPR screen findings, we performed a competitive growth assay10,22 (Figure 1E). HASPIN targeting sgRNAs had reduced growth rates relative to negative controls confirming the HASPIN dependency observed initially.
A kinase domain–targeted CRISPR screen identifies HASPIN as a novel dependency in AML cells. (A) Schematic of human kinase domain–targeted CRISPR screen in 2 t(8;21) AML cell lines, Kasumi-1 and SKNO-1. (B) Gene rank plots (left) and volcano plots (right) depicting significant kinase hits in the kinase domain–targeted CRISPR screens. Top candidates determined by CRISPR score as calculated by MAGeCK robust ranking aggregation (RRA) (left) and a log2(fold change) ≤−1.0 and false discovery rate (FDR) ≤0.05 significance cutoff (right). White diamonds indicate top 10 kinase hits in each plot. (C) Bubble plot of preranked gene set enrichment analysis results performed on top kinases identified by CRISPR screen in Kasumi-1 and SKNO-1 AML cell lines. Fill color indicates normalized enrichment score (NES). Size indicates significance by –log10(FDR). Facets indicate Molecular Signatures Database (MSigDB) gene set collection. (D) Density plot of all individual sgRNA log2(fold change) values in the kinase domain–targeted library. For selected genes, log2(fold change) values of corresponding sgRNAs depicted for Kasumi-1 and SKNO-1 cell lines relative to all other library sgRNAs (red, blue, and gray, respectively). (E) Competitive proliferation assay of Kasumi-1 or SKNO-1 cells expressing nontargeting negative control, RPA3-targeting positive control, or 1 of 2 HASPIN-targeting sgRNAs derived from CRISPR screen. Relative changes in cell proliferation rate measured by percentage of GFP-positive cells relative to nontargeting control on each day. Data are mean ± standard deviation (SD) of 4 independent experiments. CGP, chemical, genetic perturbation; CP, canonical pathway.
A kinase domain–targeted CRISPR screen identifies HASPIN as a novel dependency in AML cells. (A) Schematic of human kinase domain–targeted CRISPR screen in 2 t(8;21) AML cell lines, Kasumi-1 and SKNO-1. (B) Gene rank plots (left) and volcano plots (right) depicting significant kinase hits in the kinase domain–targeted CRISPR screens. Top candidates determined by CRISPR score as calculated by MAGeCK robust ranking aggregation (RRA) (left) and a log2(fold change) ≤−1.0 and false discovery rate (FDR) ≤0.05 significance cutoff (right). White diamonds indicate top 10 kinase hits in each plot. (C) Bubble plot of preranked gene set enrichment analysis results performed on top kinases identified by CRISPR screen in Kasumi-1 and SKNO-1 AML cell lines. Fill color indicates normalized enrichment score (NES). Size indicates significance by –log10(FDR). Facets indicate Molecular Signatures Database (MSigDB) gene set collection. (D) Density plot of all individual sgRNA log2(fold change) values in the kinase domain–targeted library. For selected genes, log2(fold change) values of corresponding sgRNAs depicted for Kasumi-1 and SKNO-1 cell lines relative to all other library sgRNAs (red, blue, and gray, respectively). (E) Competitive proliferation assay of Kasumi-1 or SKNO-1 cells expressing nontargeting negative control, RPA3-targeting positive control, or 1 of 2 HASPIN-targeting sgRNAs derived from CRISPR screen. Relative changes in cell proliferation rate measured by percentage of GFP-positive cells relative to nontargeting control on each day. Data are mean ± standard deviation (SD) of 4 independent experiments. CGP, chemical, genetic perturbation; CP, canonical pathway.
HASPIN is known as a mitotic protein kinase responsible for ensuring correct spindle fiber attachment to the centromere during metaphase.24,25 HASPIN is a dependency in several solid tumors,26-28 but it has not been evaluated in leukemia. Nevertheless, HASPIN has the highest median expression in hematopoietic cell lines (supplemental Figure 1B), supporting its potential functional relevance in blood cancers. Moreover, Haspin KO mice are both fertile and viable,29,30 suggesting the status of HASPIN as a genetic dependency is cancer specific.
HASPIN depletion reduces AML proliferation, induces cell cycle arrest, and dysregulates oncogenic transcription
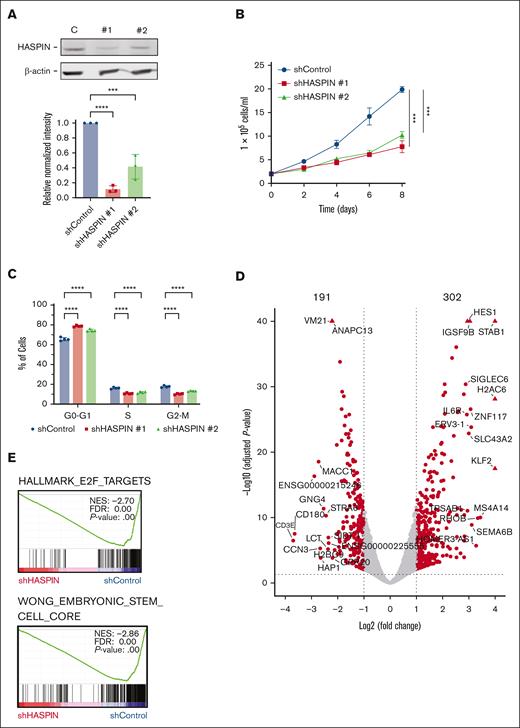
To assess the functional role of HASPIN in AML, we transduced Kasumi-1 cells with 2 unique, HASPIN-targeted short hairpin RNAs (shRNAs) and confirmed protein depletion by western blot (Figure 2A). HASPIN loss resulted in a consistent 50% reduction in Kasumi-1 proliferation rate (Figure 2B). To determine the cause of the reduction in growth, we performed cell cycle analysis. HASPIN depletion caused a notable cell cycle arrest into the G0 to G1 phase with corresponding reductions in the S and G2 to M phases (Figure 2C). HASPIN inhibition has also been associated with accumulation of S and G2/M phase cells24,25 and cell death.31 To determine whether apoptosis contributed to proliferation reductions, we performed Annexin-V/7-AAD staining. HASPIN depletion produced a moderate increase in apoptosis, suggesting most observed growth reductions were due to cell cycle arrest or checkpoint delays (supplemental Figure 2A).
HASPIN depletion reduces AML proliferation, induces cell cycle arrest, and dysregulates oncogenic transcription. (A) Western blot analysis of HASPIN and β-actin (loading control) in Kasumi-1 cells expressing either nontargeting control (C), or 1 of 2 unique HASPIN-targeting shRNAs (1 and 2). Data are mean ± SD of 3 independent experiments. Representative blot revealed. Significance determined by 1-way analysis of variance (ANOVA) with Holm-Sidak method for multiple comparison correction. ∗∗∗∗P < .0001; ∗∗∗P < .001. (B) Growth curve of Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. Data on curve are mean ± SD of technical triplicates. Representative curve of 3 independent experiments. Significance of day 8 determined by 2-way ANOVA with Holm-Sidak method for multiple comparison correction. ∗∗∗P < .001. (C) Cell cycle analysis of Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. Data are mean ± SD of 4 independent experiments. Significance determined by 2-way ANOVA with Holm-Sidak method for multiple comparison correction. ∗∗∗∗P < .0001. (D) Volcano plot of genes differentially expressed on HASPIN depletion in Kasumi-1 cells. Differentially expressed genes were determined using DESeq2. Points represent genes. Genes meeting a |log2(fold change)| ≥1.0 and Benjamini-Hochberg adjusted P value ≤.05 cutoff (dotted lines) are considered significant and colored red. Nonsignificant genes are colored gray. (E) Selected gene set enrichment analysis plots of genes differentially expressed on HASPIN depletion. NES, FDR q value, and P value are indicated within each plot. shControl, control shRNA; shHASPIN, HASPIN-targeting shRNA.
HASPIN depletion reduces AML proliferation, induces cell cycle arrest, and dysregulates oncogenic transcription. (A) Western blot analysis of HASPIN and β-actin (loading control) in Kasumi-1 cells expressing either nontargeting control (C), or 1 of 2 unique HASPIN-targeting shRNAs (1 and 2). Data are mean ± SD of 3 independent experiments. Representative blot revealed. Significance determined by 1-way analysis of variance (ANOVA) with Holm-Sidak method for multiple comparison correction. ∗∗∗∗P < .0001; ∗∗∗P < .001. (B) Growth curve of Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. Data on curve are mean ± SD of technical triplicates. Representative curve of 3 independent experiments. Significance of day 8 determined by 2-way ANOVA with Holm-Sidak method for multiple comparison correction. ∗∗∗P < .001. (C) Cell cycle analysis of Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. Data are mean ± SD of 4 independent experiments. Significance determined by 2-way ANOVA with Holm-Sidak method for multiple comparison correction. ∗∗∗∗P < .0001. (D) Volcano plot of genes differentially expressed on HASPIN depletion in Kasumi-1 cells. Differentially expressed genes were determined using DESeq2. Points represent genes. Genes meeting a |log2(fold change)| ≥1.0 and Benjamini-Hochberg adjusted P value ≤.05 cutoff (dotted lines) are considered significant and colored red. Nonsignificant genes are colored gray. (E) Selected gene set enrichment analysis plots of genes differentially expressed on HASPIN depletion. NES, FDR q value, and P value are indicated within each plot. shControl, control shRNA; shHASPIN, HASPIN-targeting shRNA.
To identify molecular changes associated with HASPIN depletion, we performed RNA sequencing on Kasumi-1 cells expressing either HASPIN targeting or control shRNAs and differential gene expression analysis (supplemental Figure 2B). We identified 493 significantly differentially expressed genes (302 upregulated, 191 downregulated; Figure 2D; supplemental Table 2). Gene set enrichment analysis revealed significant negative enrichments of gene sets associated with cell cycle regulation, stemness, and cellular proliferation after HASPIN knockdown (Figure 2E; supplemental Figure 2C). Conversely, we found significant positive enrichment of gene sets associated with TP53, differentiation, and other tumor-suppressive transcription factors (supplemental Figure 2C). Accordingly, in a hematopoietic cell-type enrichment analysis, significantly downregulated genes were strongly associated with progenitor cell types (hematopoietic progenitor cell (HPC), common myeloid progenitor (CMP), megakaryocyte-erythroid progenitor (MEP), granulocyte-monocyte progenitor (GMP)), whereas upregulated genes were associated with more differentiated myeloid cell types (monocytic, myelocytic; supplemental Figure 2D). Therefore, HASPIN seems to regulate transcriptional programs critical for leukemia survival either directly or as a byproduct of other mechanisms.
Integrated data mining characterizes HASPIN as a potential splicing regulatory kinase
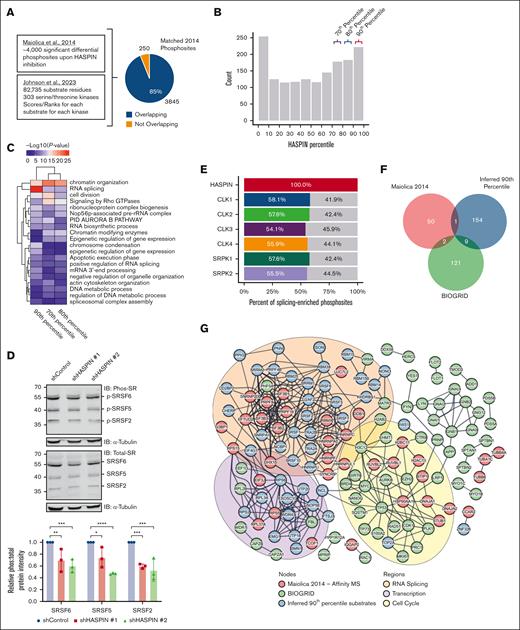
HASPIN has been reported to phosphorylate histone H3 at threonine 3 (H3T3) to regulate mitosis in nonhematopoietic cells.24,32-34 We analyzed H3T3 phosphorylation in AML cells and observed moderate reductions on HASPIN depletion (supplemental Figure 2E). This result supports the role of HASPIN in mitotic regulation in AML. Interestingly, HASPIN is also active during interphase,25 indicating its possible nonmitotic roles. We, therefore, explored whether HASPIN phosphorylates other key cellular regulators by integrating mass spectrometry analyses of peptides differentially phosphorylated on semispecific HASPIN inhibition reported by Maiolica et al35 in 2014 with an atlas of serine/threonine kinase substrate specificities published by Johnson et al36 in 2023. Of the 4095 differential phosphosites affected by HASPIN inhibition, 3845 residues mapped onto the substrate atlas (Figure 3A). Matched residues were assigned kinase percentile scores from the atlas, which represent their specificity as a given kinase’s substrate relative to all other residues in the atlas. A total of 583 matched residues scored as 70th to 90th percentile HASPIN substrates (Figure 3B). Next, we performed gene ontology analysis on proteins in the top 3 deciles of HASPIN substrates to ascertain pathways potentially regulated by HASPIN kinase activity (Figure 3C). RNA splicing was uniquely enriched with 90th percentile substrates. Several serine/Arginine-rich splicing factor (SRSF) family SR proteins, spliceosome components, and other notable RNA-binding proteins (RBPs) were among the top splicing-related substrate proteins, supporting the possible involvement of HASPIN in splicing.
Integrated data mining characterizes HASPIN as a potential splicing regulatory kinase. (A) Schematic depicting integration of 2 proteomics data sets (Maiolica et al35 and Johnson et al36) to assign HASPIN specificity scores to residues differentially phosphorylated on semispecific HASPIN inhibition. (B) Histogram of HASPIN percentile scores for 3845 differential phosphosites reported by Maiolica et al35 that mapped to the serine/threonine kinase substrate atlas by Johnson et al36. Y-axis is the number of phosphosites with HASPIN percentile scores within indicated histogram bucket range. X-axis is histogram bucket ranges corresponding to substrate atlas HASPIN percentile scores. Colored brackets indicate top deciles of HASPIN substrates. (C) Heat map depicting gene ontology (GO) enrichment for proteins belonging to 70th, 80th, and 90th HASPIN substrate percentiles correspond to bracketed bins in panel B. GO terms and percentiles were grouped by hierarchical clustering as indicated by column and row dendrograms. Cell color represents –log10(P value) for corresponding GO term enrichment. (D) Western blot analysis of phospho-SR, total SR, and α-tubulin (loading control) in Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. SRSF6, SRSF5, and SRSF2 are labeled according to size. Bar plot quantifies the ratio of phosphorylated SR protein signal to total SR protein signal where both values are normalized to respective loading controls and presented as values relative to the control condition. Representative blot of 3 independent experiments revealed. Data are mean ± SD of 3 independent experiments. Significance determined by 2-way ANOVA with Holm-Sidak correction for multiple comparison testing. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (E) Bar plot depicting the fraction of RNA splicing-related HASPIN substrates that are also predicted substrates for indicated splicing regulatory kinases. A total of 229 phosphosites corresponding to RNA splicing proteins enriched in panel C were analyzed. Substrates with >90% kinase percentile score for both HASPIN and indicated kinase were considered mutual or shared targets. Colored and gray areas represent percent of shared or exclusive substrates, respectively. (F) Venn diagram depicting overlap of proteins belonging to experimentally validated HASPIN-interactor data (Maiolica et al35 and BIOGRID) and inferred 90th percentile HASPIN kinase substrates. (G) STRING-DB protein-protein interaction network of experimentally validated HASPIN interacting proteins and inferred 90th percentile HASPIN kinase substrates. Nodes are proteins. Edges are STRING relationships at the highest confidence level (STRING confidence score >0.900). Node color indicates data origin. Inferred 90th percentile HASPIN substrate proteins are colored blue. HASPIN interacting proteins from affinity mass spectrometry data as reported by Maiolica et al35 are colored red. Curated HASPIN interacting proteins from the BIOGRID database are colored green. Colored ellipses represent prominent GO term enriched in nodes contained within. RNA splicing enrichments are colored in orange. Transcription enrichments are colored in purple. Cell cycle enrichments are colored in yellow. A total of 84 singleton nodes are hidden.
Integrated data mining characterizes HASPIN as a potential splicing regulatory kinase. (A) Schematic depicting integration of 2 proteomics data sets (Maiolica et al35 and Johnson et al36) to assign HASPIN specificity scores to residues differentially phosphorylated on semispecific HASPIN inhibition. (B) Histogram of HASPIN percentile scores for 3845 differential phosphosites reported by Maiolica et al35 that mapped to the serine/threonine kinase substrate atlas by Johnson et al36. Y-axis is the number of phosphosites with HASPIN percentile scores within indicated histogram bucket range. X-axis is histogram bucket ranges corresponding to substrate atlas HASPIN percentile scores. Colored brackets indicate top deciles of HASPIN substrates. (C) Heat map depicting gene ontology (GO) enrichment for proteins belonging to 70th, 80th, and 90th HASPIN substrate percentiles correspond to bracketed bins in panel B. GO terms and percentiles were grouped by hierarchical clustering as indicated by column and row dendrograms. Cell color represents –log10(P value) for corresponding GO term enrichment. (D) Western blot analysis of phospho-SR, total SR, and α-tubulin (loading control) in Kasumi-1 cells expressing either nontargeting control or 1 of 2 unique HASPIN-targeting shRNAs. SRSF6, SRSF5, and SRSF2 are labeled according to size. Bar plot quantifies the ratio of phosphorylated SR protein signal to total SR protein signal where both values are normalized to respective loading controls and presented as values relative to the control condition. Representative blot of 3 independent experiments revealed. Data are mean ± SD of 3 independent experiments. Significance determined by 2-way ANOVA with Holm-Sidak correction for multiple comparison testing. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (E) Bar plot depicting the fraction of RNA splicing-related HASPIN substrates that are also predicted substrates for indicated splicing regulatory kinases. A total of 229 phosphosites corresponding to RNA splicing proteins enriched in panel C were analyzed. Substrates with >90% kinase percentile score for both HASPIN and indicated kinase were considered mutual or shared targets. Colored and gray areas represent percent of shared or exclusive substrates, respectively. (F) Venn diagram depicting overlap of proteins belonging to experimentally validated HASPIN-interactor data (Maiolica et al35 and BIOGRID) and inferred 90th percentile HASPIN kinase substrates. (G) STRING-DB protein-protein interaction network of experimentally validated HASPIN interacting proteins and inferred 90th percentile HASPIN kinase substrates. Nodes are proteins. Edges are STRING relationships at the highest confidence level (STRING confidence score >0.900). Node color indicates data origin. Inferred 90th percentile HASPIN substrate proteins are colored blue. HASPIN interacting proteins from affinity mass spectrometry data as reported by Maiolica et al35 are colored red. Curated HASPIN interacting proteins from the BIOGRID database are colored green. Colored ellipses represent prominent GO term enriched in nodes contained within. RNA splicing enrichments are colored in orange. Transcription enrichments are colored in purple. Cell cycle enrichments are colored in yellow. A total of 84 singleton nodes are hidden.
To validate our findings, we probed for changes in phosphorylated and total SR protein levels on HASPIN depletion (Figure 3D). We observed significant reductions in phospho–SR protein levels in predicted HASPIN kinase substrates such as SRSF2, SRSF5, and SRSF6 without significant change in corresponding total protein levels. HASPIN knockdown did not cause complete loss of phospho–SR protein signal because other splicing regulatory kinases are likely able to cross-regulate splicing factor substrates.37,38 To investigate this possibility, we calculated what fraction of predicted, RNA splicing–enriched HASPIN substrate phosphosites (Figure 3C) were also high confidence substrates for known splicing regulatory kinases (Figure 3E). Half of HASPIN-specific substrates were shared with other splicing regulatory kinases, suggesting that HASPIN potentially cross-regulates target RBPs with other kinases and functions similarly. Next, we investigated how mechanistically HASPIN phosphorylates splicing factors. We compared our inferred 90th percentile HASPIN substrates with HASPIN protein-protein interaction data from published studies35 and the BIOGRID database. We observed minor overlaps between each set (Figure 3F), suggesting that HASPIN may not interact with these substrates directly and may require adaptor or scaffold proteins to coordinate modifications. Accordingly, STRING protein-protein interaction analysis returned a robust network between HASPIN interactors and inferred substrates (Figure 3G), supporting the adaptor-dependent model for the HASPIN kinase functionality.35 Cluster analysis identified 3 major clusters of proteins enriched in cell cycle, RNA splicing, and transcription with HASPIN substrates present in each. Altogether, our results describe HASPIN as a novel splicing regulatory kinase that may phosphorylate substrates while associated with protein complexes.
HASPIN depletion dysregulates RNA splicing in AML
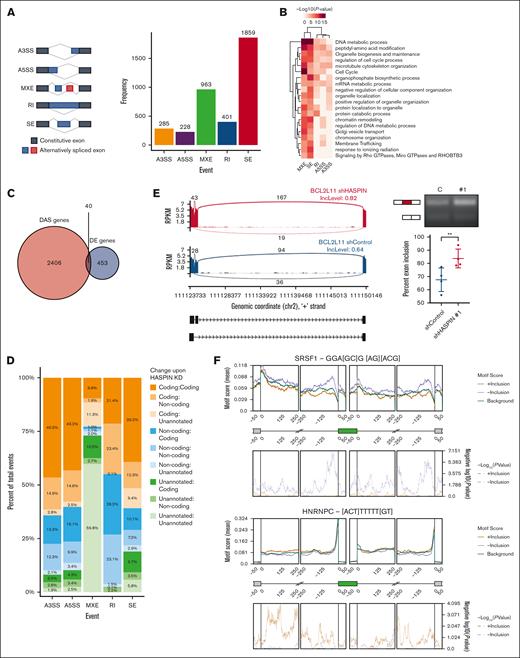
The reported HASPIN phosphorylation sites are mainly localized to the regulatory serine/arginine-rich (RS) domains of these SR protein splicing factors.35,36 Phosphorylation at these RS domains is critical for regulating SR protein splicing activity.39-46 We, therefore, investigated how HASPIN depletion affects RNA splicing in AML. To begin, we analyzed global mRNA splicing changes using replicate multivariate analysis of transcript sequences after shRNA-mediated HASPIN depletion in Kasumi-1 cells. The following are the 5 types of splicing events reported by the replicate multivariate analysis of transcript sequences: alternative 3′ and 5′ splice site, mutually exclusive exons, retained intron, and skipped exon. We detected the following significant splicing events: 285 alternative 3′ splice sites, 228 alternative 5′ splice sites, 963 mutually exclusive exons, 401 retained intron, and 1859 skipped exons (Figure 4A; supplemental Figure 3A). Differentially spliced genes were enriched in DNA repair, mRNA metabolism, and cell cycle pathways (Figure 4B). Furthermore, differentially spliced genes had low overlap with differentially expressed genes after HASPIN knockdown, suggesting 2 distinct functional effects resulting from protein depletion (Figure 4C). To evaluate the functional consequences of HASPIN-related splicing dysregulation, transcript isoforms were matched to control or knockdown conditions and assigned functional biotypes consisting of protein coding, noncoding, or unannotated (Figure 4D). HASPIN depletion caused reciprocal biotype changes, where protein coding becomes noncoding or unannotated and vice versa, and preserved biotypes, suggesting broad alterations in coding potential and functional transcript diversity. Transcripts also switched into nonsense-mediated decay and vice versa, but these constituted fewer events overall (supplemental Figure 3B). We next identified specific examples of transcripts undergoing functional alterations in leukemia-dependent pathways (Figure 4E; supplemental Figure 3C). BCL2-like 11 (BCL2L11, BIM) is a BH3-only proapoptotic protein. On HASPIN depletion, we observed a switch from a nonsense-mediated decay BCL2L11 transcript to BIML, an isoform more active than the canonical BIMEL isoform,47,48 through greater exon 2C inclusion indicating potentially increased apoptotic responses. Reverse transcription polymerase chain reaction analysis validated this increased exon inclusion in BCL2L11 transcripts after HASPIN depletion (Figure 4E).
HASPIN depletion dysregulates RNA splicing in AML. (A) Bar plot revealing replicate multivariate analysis of transcript sequences quantification of significant splicing events induced on shRNA-mediated HASPIN depletion in Kasumi-1 cells. Major splicing patterns (left) are as follows: A3SS, alternative 3′ splice site; A5SS, alternative 5′ splice site; MXE, mutually exclusive exon; RI, retained intron; SE, skipped exon. Events that had at least 50 junction-spanning reads, FDR ≤0.05, and absolute delta percent spliced in (|dPSI|) ≥0.10 were considered significant. (B) Heat map depicting GO enrichments of significantly spliced events for splicing pattern. Terms and event type were grouped by hierarchical clustering as indicated by column and row dendrograms. Cell color represents –log10(P value) for corresponding GO term enrichment. (C) Venn diagram of genes with differential alternative splicing events (DAS genes) and genes with differential expression (DE genes) on HASPIN depletion in Kasumi-1 cells. Intersection represents genes that are both DAS and DE on HASPIN depletion. Significant splicing events or differentially expressed genes were overlapped based on respective cutoffs. (D) Proportional bar plots reporting changes between transcript functional biotypes in significant splicing events of each pattern on HASPIN depletion in Kasumi-1 cells. Notation is “Initial:Final.” Each color corresponds with a transition between biotypes. Transcript biotypes assigned according to ENSEMBL transcriptome annotation. “Unannotated” indicates that the transcript was not located in the annotation database. (E) Sashimi plot (left) and reverse transcription polymerase chain reaction (RT-PCR) validation (right) of BCL2L11 SE events observed on HASPIN depletion in Kasumi-1 cells. Sashimi plot depicts change in mean percent spliced in (PSI) levels of target exons between control (blue) and HASPIN knockdown (red) in Kasumi-1 cells. Inclusion and exclusion form depicted in black schematic beneath plots. Data are grouped from 3 biological replicates. RT-PCR analysis of BCL2L11 target exon inclusion in Kasumi-1 cells expressing control (C) or HASPIN-targeting shRNA 1 (1). Schematic of inclusion (larger) and skipping (smaller) isoforms included to left of gel image. Percent exon inclusion between the 2 isoforms after HASPIN depletion is quantified. Data are mean ± SD of 4 independent experiments. Statistical significance determined by unpaired 2-tailed Student t test. ∗∗P < .01. (F) RBP motif enrichment analysis of SRSF1 and HNRNPC in significant SE splicing events on HASPIN depletion in Kasumi-1 cells. Motif enrichment (top) and P value (bottom) are reported at various positions along representative cassette exon for splicing events with significantly promoted exon inclusion (orange, 2641 events) or suppressed exon inclusion (purple, 2498 events) on HASPIN depletion relative to nondifferentially spliced background (green, 7049 events) as determined by rMAPS2. A region of 250 bp was analyzed using a 50 bp sliding window.
HASPIN depletion dysregulates RNA splicing in AML. (A) Bar plot revealing replicate multivariate analysis of transcript sequences quantification of significant splicing events induced on shRNA-mediated HASPIN depletion in Kasumi-1 cells. Major splicing patterns (left) are as follows: A3SS, alternative 3′ splice site; A5SS, alternative 5′ splice site; MXE, mutually exclusive exon; RI, retained intron; SE, skipped exon. Events that had at least 50 junction-spanning reads, FDR ≤0.05, and absolute delta percent spliced in (|dPSI|) ≥0.10 were considered significant. (B) Heat map depicting GO enrichments of significantly spliced events for splicing pattern. Terms and event type were grouped by hierarchical clustering as indicated by column and row dendrograms. Cell color represents –log10(P value) for corresponding GO term enrichment. (C) Venn diagram of genes with differential alternative splicing events (DAS genes) and genes with differential expression (DE genes) on HASPIN depletion in Kasumi-1 cells. Intersection represents genes that are both DAS and DE on HASPIN depletion. Significant splicing events or differentially expressed genes were overlapped based on respective cutoffs. (D) Proportional bar plots reporting changes between transcript functional biotypes in significant splicing events of each pattern on HASPIN depletion in Kasumi-1 cells. Notation is “Initial:Final.” Each color corresponds with a transition between biotypes. Transcript biotypes assigned according to ENSEMBL transcriptome annotation. “Unannotated” indicates that the transcript was not located in the annotation database. (E) Sashimi plot (left) and reverse transcription polymerase chain reaction (RT-PCR) validation (right) of BCL2L11 SE events observed on HASPIN depletion in Kasumi-1 cells. Sashimi plot depicts change in mean percent spliced in (PSI) levels of target exons between control (blue) and HASPIN knockdown (red) in Kasumi-1 cells. Inclusion and exclusion form depicted in black schematic beneath plots. Data are grouped from 3 biological replicates. RT-PCR analysis of BCL2L11 target exon inclusion in Kasumi-1 cells expressing control (C) or HASPIN-targeting shRNA 1 (1). Schematic of inclusion (larger) and skipping (smaller) isoforms included to left of gel image. Percent exon inclusion between the 2 isoforms after HASPIN depletion is quantified. Data are mean ± SD of 4 independent experiments. Statistical significance determined by unpaired 2-tailed Student t test. ∗∗P < .01. (F) RBP motif enrichment analysis of SRSF1 and HNRNPC in significant SE splicing events on HASPIN depletion in Kasumi-1 cells. Motif enrichment (top) and P value (bottom) are reported at various positions along representative cassette exon for splicing events with significantly promoted exon inclusion (orange, 2641 events) or suppressed exon inclusion (purple, 2498 events) on HASPIN depletion relative to nondifferentially spliced background (green, 7049 events) as determined by rMAPS2. A region of 250 bp was analyzed using a 50 bp sliding window.
RBP activity is modulated by regulatory kinases that influence localization, splicing site selection, and kinetics.38 To determine whether HASPIN depletion functionally affects RBP splicing behavior, we performed RBP motif enrichment analysis using RNA Map Analysis and Plotting Server 2 (rMAPS2). Motifs for major splicing factors such as SRSF1 and heterogeneous nuclear ribonulcear protein C (HNRNPC) were strongly enriched throughout differentially spliced events, suggesting deviation from normal behavior (Figure 4F). In addition, RBP motifs for several major splicing factor protein families including potential HASPIN substrates SRSF2, SRSF5, and SRSF6 were significantly enriched in dysregulated events, recapitulating our integrated proteomics analysis (supplemental Figure 3D). Together, our results demonstrate that HASPIN depletion dysregulates splicing in AML-critical pathways potentially through alteration of RBP behavior.
HASPIN is a clinically relevant, general leukemia dependency
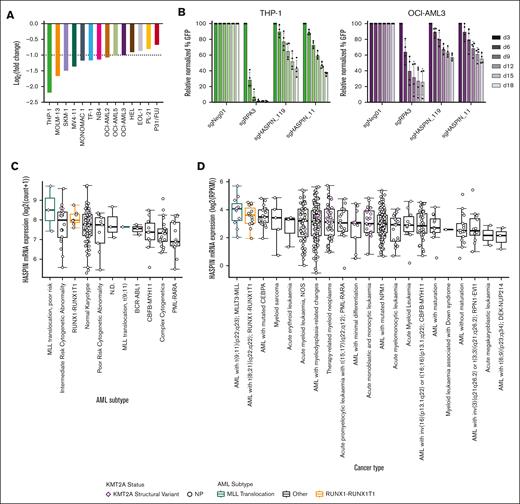
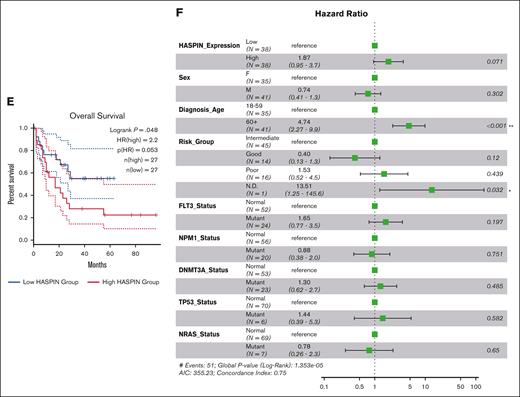
AML is a heterogeneous disease defined by a variety of initiating and supporting chromosomal aberrations and mutations. We initially discovered HASPIN dependency using 2 t(8;21) AML cell lines. To determine whether HASPIN dependency generalizes to other AML subtypes, we queried published CRISPR screens performed in AML cell lines.10,21 HASPIN is a significant dependency in several non-t(8;21) AML cell lines, such as THP-1, OCI-AML3, and MOLM-13, which we independently replicated by competitive growth assay (Figure 5A-B; supplemental Figure 4A). Next, we analyzed HASPIN in AML patient data sets to evaluate its clinical relevance. In both The Cancer Genome Atlas Acute Myeloid Leukemia (TCGA-LAML) (Figure 5C) and BEAT-AML (Figure 5D) data sets, patients with KMT2A lysine methyltransferase (MLL) structural variants or t(8;21) translocations had the highest median HASPIN expression. Furthermore, higher HASPIN expression correlated with worse overall survival in patients with AML (Figure 5E). Multivariate survival analysis of clinical and genetic covariates in high or low HASPIN-expressing patients with AML suggested that advanced diagnosis age, “poor” genetic risk group, and oncogenic mutations increase risk (Figure 5F). However, these covariates did not significantly coincide with either patient cohort (supplemental Figure 4B), suggesting HASPIN expression was the main prognostic factor for survival in our analysis. Together, these findings suggest that HASPIN may be a clinically significant dependency in several non-t(8;21) AML cell types.
HASPIN is a clinically relevant, general leukemia dependency. (A) Bar plot depicting mean log2(fold change) of HASPIN targeting sgRNA genome-wide CRISPR screen performed in several leukemia cell lines as reported by Wang et al.21 Screen data were retrieved from BIOGRID ORCS. Dotted line indicates author-specified significance cutoff. (B) Competitive proliferation assay of THP-1 or OCI-AML3 cells expressing nontargeting negative control, RPA3-targeting positive control, or 1 of 2 HASPIN-targeting sgRNAs derived from CRISPR screen. Relative changes in cell proliferation rate measured by percentage of GFP-positive cells relative to nontargeting control on each day. Data are mean ± SD of 4 independent experiments per cell line. (C) Box plots depicting median HASPIN mRNA expression in the TCGA-LAML patient cohort separated by AML subtype. MLL (KMT2A) or RUNX1-RUNX1T1 t(8;21) translocation cohorts are highlighted in green and orange, respectively. Individuals with KMT2A structural variants are indicated with purple diamonds. (D) Box plots depicting median HASPIN mRNA expression in the BEAT-AML (2022) patient cohort separated by AML subtype. MLL (KMT2A) or RUNX1-RUNX1T1 t(8;21) translocation cohorts are highlighted in green and orange, respectively. Individuals with KMT2A structural variants are indicated with purple diamonds. (E) Kaplan-Meier survival curve depicting comparison of overall survival of patients with TCGA-LAML belonging to the top quartile (red) and bottom quartile (blue) of HASPIN expression. Plot and data derived from GEPIA2. (F) Forest plot of hazard ratios from multivariate Cox proportional hazard analysis of overall survival of patients with TCGA LAML incorporating HASPIN expression level and significant clinical and genetic factors. High and low HASPIN-expressing patients belong to the top and bottom expression quartiles, respectively. Clinical variables include the following: patient sex (Sex), age at first diagnosis (Diagnosis_Age), genetic risk group (Risk_Group), FLT3 mutation status (FLT3_Status), NPM1 mutation status (NPM1_Status), DNMT3A mutation status (DNMT3A_Status), TP53 mutation status (TP53_Status), and NRAS mutation status (NRAS_Status). Clinical metadata and mutation calls derived from the Genomic Data Commons TCGA LAML project patient information.49 N.D., not defined; NOS, not otherwise specified; NP, not profiled.
HASPIN is a clinically relevant, general leukemia dependency. (A) Bar plot depicting mean log2(fold change) of HASPIN targeting sgRNA genome-wide CRISPR screen performed in several leukemia cell lines as reported by Wang et al.21 Screen data were retrieved from BIOGRID ORCS. Dotted line indicates author-specified significance cutoff. (B) Competitive proliferation assay of THP-1 or OCI-AML3 cells expressing nontargeting negative control, RPA3-targeting positive control, or 1 of 2 HASPIN-targeting sgRNAs derived from CRISPR screen. Relative changes in cell proliferation rate measured by percentage of GFP-positive cells relative to nontargeting control on each day. Data are mean ± SD of 4 independent experiments per cell line. (C) Box plots depicting median HASPIN mRNA expression in the TCGA-LAML patient cohort separated by AML subtype. MLL (KMT2A) or RUNX1-RUNX1T1 t(8;21) translocation cohorts are highlighted in green and orange, respectively. Individuals with KMT2A structural variants are indicated with purple diamonds. (D) Box plots depicting median HASPIN mRNA expression in the BEAT-AML (2022) patient cohort separated by AML subtype. MLL (KMT2A) or RUNX1-RUNX1T1 t(8;21) translocation cohorts are highlighted in green and orange, respectively. Individuals with KMT2A structural variants are indicated with purple diamonds. (E) Kaplan-Meier survival curve depicting comparison of overall survival of patients with TCGA-LAML belonging to the top quartile (red) and bottom quartile (blue) of HASPIN expression. Plot and data derived from GEPIA2. (F) Forest plot of hazard ratios from multivariate Cox proportional hazard analysis of overall survival of patients with TCGA LAML incorporating HASPIN expression level and significant clinical and genetic factors. High and low HASPIN-expressing patients belong to the top and bottom expression quartiles, respectively. Clinical variables include the following: patient sex (Sex), age at first diagnosis (Diagnosis_Age), genetic risk group (Risk_Group), FLT3 mutation status (FLT3_Status), NPM1 mutation status (NPM1_Status), DNMT3A mutation status (DNMT3A_Status), TP53 mutation status (TP53_Status), and NRAS mutation status (NRAS_Status). Clinical metadata and mutation calls derived from the Genomic Data Commons TCGA LAML project patient information.49 N.D., not defined; NOS, not otherwise specified; NP, not profiled.
HASPIN inhibitor CHR-6494 effectively targets AML and synergizes with BCL-2 inhibition
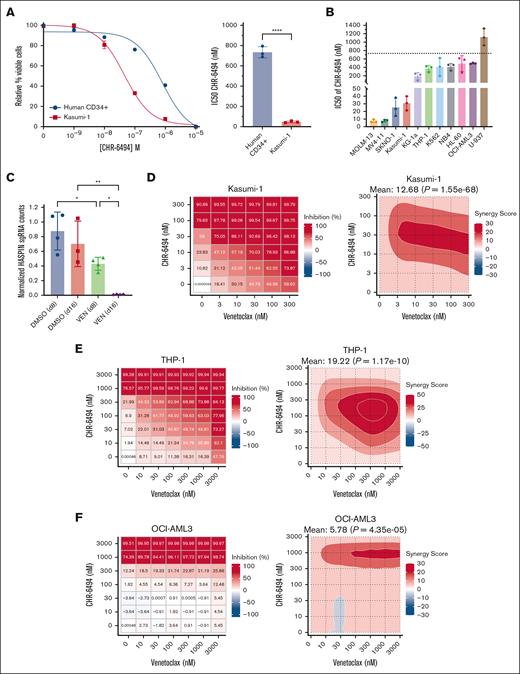
To evaluate the therapeutic potential of HASPIN dependency in leukemia, we performed several in vitro experiments using the specific HASPIN kinase inhibitor, CHR-6494.31 First, we generated CHR-6494 dose-response curves for Kasumi-1 AML cells and healthy human CD34+ progenitor cells derived from patients (Figure 6A). Kasumi-1 AML cells were ∼15-fold more sensitive to HASPIN inhibitor than healthy CD34+ cells by relative CHR-6494 50% inhibitory concentration (IC50) values. To determine whether other AML subtypes were susceptible to CHR-6494, we tested a panel of different leukemia cell lines (Figure 6B). Relative to the 750 nM CHR-6494 IC50 of healthy CD34+ cells, we observed 200 to 600 nM CHR-6494 IC50s in KG1-a, OCI-AML3, THP-1, NB4, K562, and HL-60. CHR-6494 IC50s in Kasumi-1, SKNO-1, MOLM-13, and MV4-11 were 10 to 40 nM, which is substantially lower than other leukemia cell lines and healthy CD34+ cells. Last, we reveal that CHR-6494 reduced splicing factor phosphorylation in AML cells (supplemental Figure 5A-B), suggesting that part of the inhibitor effect is related to the role of HASPIN in splicing. These data demonstrate HASPIN inhibition to be effective in a variety of AML subtypes with especially high efficacy in MLL-translocated and t(8;21) AMLs.
HASPIN inhibitor CHR-6494 effectively targets AML and synergizes with BCL-2 inhibition. (A) Dose-response curves (left) and IC50 comparison (right) of Kasumi-1 and healthy CD34+ hematopoietic progenitor cells treated with CHR-6494. IC50 values determined by nonlinear regression. Data on curve are mean ± SD of technical triplicates. Representative curves of 3 independent experiments revealed. Data on bar plot are mean ± SD of 3 independent experiments. Significance determined by unpaired 2-tailed Student t test. ∗∗∗∗P < .0001. (B) Bar plots comparing CHR-6494 IC50 values in leukemia cell lines. IC50 values determined by dose-response curve with nonlinear regression for each cell line. Data are mean ± SD of 3 independent experiments. Dotted line indicates CHR-6494 IC50 value of healthy CD34+ hematopoietic progenitor cells determined in panel A. (C) Bar plots depicting normalized HASPIN sgRNA counts in a genome-wide CRISPR screen in MOLM-13 cells treated with either DMSO or VEN for 8 or 16 days as performed by Chen et al.50 Screen data were retrieved from BIOGRID ORCS. Counts were normalized to initial time point (d0). One data point was removed from DMSO (d16) as a significant outlier. Data are mean ± SD. Significance determined by 1-way ANOVA with Holm-Sidak multiple comparison correction. ∗P < .05; ∗∗P < .01. (D) Dose-response matrix (left) and corresponding zero interaction potency (ZIP) drug synergy contour plot (right) of Kasumi-1 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. (E) Dose-response matrix (left) and corresponding ZIP drug synergy contour plot (right) of THP-1 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. (F) Dose-response matrix (left) and corresponding ZIP drug synergy contour plot (right) of OCI-AML3 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. DMSO, dimethyl sulfoxide.
HASPIN inhibitor CHR-6494 effectively targets AML and synergizes with BCL-2 inhibition. (A) Dose-response curves (left) and IC50 comparison (right) of Kasumi-1 and healthy CD34+ hematopoietic progenitor cells treated with CHR-6494. IC50 values determined by nonlinear regression. Data on curve are mean ± SD of technical triplicates. Representative curves of 3 independent experiments revealed. Data on bar plot are mean ± SD of 3 independent experiments. Significance determined by unpaired 2-tailed Student t test. ∗∗∗∗P < .0001. (B) Bar plots comparing CHR-6494 IC50 values in leukemia cell lines. IC50 values determined by dose-response curve with nonlinear regression for each cell line. Data are mean ± SD of 3 independent experiments. Dotted line indicates CHR-6494 IC50 value of healthy CD34+ hematopoietic progenitor cells determined in panel A. (C) Bar plots depicting normalized HASPIN sgRNA counts in a genome-wide CRISPR screen in MOLM-13 cells treated with either DMSO or VEN for 8 or 16 days as performed by Chen et al.50 Screen data were retrieved from BIOGRID ORCS. Counts were normalized to initial time point (d0). One data point was removed from DMSO (d16) as a significant outlier. Data are mean ± SD. Significance determined by 1-way ANOVA with Holm-Sidak multiple comparison correction. ∗P < .05; ∗∗P < .01. (D) Dose-response matrix (left) and corresponding zero interaction potency (ZIP) drug synergy contour plot (right) of Kasumi-1 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. (E) Dose-response matrix (left) and corresponding ZIP drug synergy contour plot (right) of THP-1 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. (F) Dose-response matrix (left) and corresponding ZIP drug synergy contour plot (right) of OCI-AML3 cells treated with CHR-6494 and VEN combination for 48 hours. Each cell represents drug combined at indicated concentrations. Treatment response is percent inhibition; higher values indicate lower cell viability. Synergy scores represent ZIP synergy calculations of inhibition effects exceeding values expected between 2 noninteracting agents. Mean synergy scores and significance reported at top of respective contour plot. Representative plots of 3 independent experiments revealed. DMSO, dimethyl sulfoxide.
Next, we wondered whether CHR-6494 could be used in novel combination therapies for AML. We chose to combine CHR-6494 with BCL-2 inhibitor venetoclax as it is US Food and Drug Administration approved and used in combination with current chemotherapeutics. Furthermore, in a published drug-perturbation CRISPR screen, HASPIN KO AML cells experienced dramatic reductions in growth when also treated with venetoclax (Figure 6C).50 To test CHR-6494 and venetoclax in combination, we treated AML cells with dose-response matrices and calculated several synergy scores for each. In Kasumi-1, CHR-6494 and venetoclax dramatically potentiated each other’s effects with maximal zero interaction potency synergy scores at 10 to 100 nM and 3 to 300 nM, respectively (Figure 6D; supplemental Figure 6C). The combination yielded similarly synergistic responses in HL-60 and NB4 and additive responses in MOLM-13 and MV4-11 (supplemental Figure 6A-C). Venetoclax resistance is a significant complication in single-agent venetoclax strategies or in certain de novo primary AML cases.51 Therefore, we next tested whether our combination therapy could be used in venetoclax-resistant AML. THP-1 and OCI-AML3 were chosen to model venetoclax-resistant AML52,53 and exhibited venetoclax IC50s upward of 5 μM (supplemental Figure 6D). Surprisingly, when treated with CHR-6494 and venetoclax together, both OCI-AML3 and THP-1 were discovered to be effectively resensitized to venetoclax (Figure 6E-F; supplemental Figure 6C). In THP-1 cells, 100 to 300 nM of CHR-6494 was sufficient to reach 50% inhibition with 10 nM of venetoclax.
To extend our findings to the patient context, we treated several different primary AML samples (supplemental Table 3) with CHR-6494 alone and in combination with venetoclax. CHR-6494 alone reduced viability of 4 of 5 primary AMLs with IC50 values between 200 and 600 nM (supplemental Figure 7A). To evaluate drug synergy, we focused on PT 1 which was resistant to either single-agent treatment (supplemental Figure 7A-B). Excitingly, CHR-6494 and venetoclax potentiated strong synergistic responses from PT 1 and overcame observed resistances (supplemental Figure 7C-D). Combination treatments yielded mainly additive responses in other primary AMLs (data not revealed). Together, our data emphasize the potential clinical utility of HASPIN inhibition for several AML subtypes alone and in a novel combination with BCL-2 inhibition.
Discussion
In this report, by CRISPR screen, HASPIN was identified as a critical AML dependency where depletion restricted growth and dysregulated transcription and cell cycle. Furthermore, by integrated data mining and further experimental studies, RNA splicing factors were demonstrated as HASPIN kinase substrates. Accordingly, splicing was broadly affected by HASPIN disruption. HASPIN inhibitor treatments were effective and synergized with BCL-2 inhibitors in tested AML cells. These findings establish HASPIN as a valuable dependency in AML, highlight one of its understudied molecular functions, and provide a foundation for continued design of potentially useful therapeutic inhibition strategies.
HASPIN is known as a critical mitotic kinase responsible for recruiting the chromosomal passenger complex to centromeres through phosphorylated histone 3 threonine 3-survivin interactions.24,25 Accordingly, in AML, HASPIN knockdown reduced H3T3 phosphorylation. The consequences of losing this mitotic modification likely contribute to the functional HASPIN dependency in AML. However, HASPIN is active during interphase,25 suggesting additional functionality to explore. Using an atlas of serine/threonine kinase substrate specificities,36 we disambiguated potential off-target effects in the data set of Maiolica et al35 in 2014 and revealed splicing factors such as HNRNPs, RNA-binding motif proteins (RBMs), and, most significantly, SRSFs to be high confidence HASPIN kinase substrates. Both HASPIN-targeting shRNAs and inhibitor induced significant reductions in target SR protein phosphorylation, further supporting this relationship. Structural analyses of HASPIN substrate recognition regions suggest that it prioritizes basic regions comparable to lysine-rich histone tails,54,55 potentially explaining preferences for RBPs with similarly basic arginine-rich regulatory domains. However, how HASPIN phosphorylates RBP substrates is still unclear. Maiolica et al35 initially proposed that HASPIN relies on interactor-substrate complexes to mediate phosphorylation. Using additional substrates and the BIOGRID database, we identified a larger HASPIN interactor-substrate network that supports this adaptor-dependent model. Furthermore, CLK1 and CLK2 are known SR protein kinases. They are also potential HASPIN substrates, making indirect RBP regulation through HASPIN phosphorylating other splicing regulatory kinases, another plausible mechanism. In addition, SR protein phosphorylation can be affected by different stages of the cell cycle,45,56,57 and splicing factor activity is critical for cell cycle regulation.58-60 Because HASPIN depletion induces a cell cycle arrest and causes differential splicing factor phosphorylation, possibly altering behavior, functional crossover may be occurring where both effects feed into each other and contribute to the overall dependency in AML. Further study will necessitate in-depth, specific proteomic analysis of HASPIN and putative splicing-related substrate proteins to characterize these pathways.
RNA splicing is frequently dysregulated in hematological malignancies.61 Mutant RBPs or aberrant regulatory kinase activity establish tumorigenic splicing profiles,62-64 such as splicing factor RBM10 regulating inhibitor of apoptosis protein isoforms in AML to repress proapoptotic responses.20 Because HASPIN likely phosphorylates RBPs and is an AML dependency, we hypothesized that, in addition to mitosis, the role of HASPIN in sustaining AML is regulating splicing factor activity. HASPIN knockdown induced significant differential splicing in transcripts related to DNA repair, cell cycle regulation, and mRNA metabolism and broadly altered transcript coding potentials with predicted functional consequences. Hematopoietic factors implicated in AML with functionally critical splice isoforms, such as PML,65,66 were significantly affected. Splicing factor kinase inhibition alters substrate RBP phosphorylation, which represses AML through subsequent splicing changes.20,64 RBP motif enrichment analysis suggests that HASPIN loss affects RBP activity in AML. Importantly, motifs for putative HASPIN substrates identified in our proteomic analysis, such as SRSF family proteins, were most significantly affected, supporting this model. Together, these results describe an alternative role for HASPIN as a splicing regulator and support further efforts to characterize molecular mechanisms underpinning its role in AML.
Currently, several HASPIN inhibitors exist, including 5-iodotubercidin and CHR-6494.31 Until recently,67,68 none have been tested in leukemia. Our study serves as an exploration of the efficacy and potential therapeutic utility of HASPIN inhibition in AML. Although novel HASPIN inhibitors are being developed,69 we chose CHR-6494 for its increased specificity and successful use in solid tumors. In Kasumi-1, CHR-6494 treatment achieved 50% reduction in cell viability with ∼15-fold less drug compared with healthy CD34+ HSPCs, which support findings that HASPIN is dispensable for normal development and function.29,30 Except for U937, tested AML cell lines were more sensitive to CHR-6494 than healthy CD34+ cells, but t(8;21) (Kasumi-1, SKNO-1) and MLL-translocation (MOLM-13, MV4-11) AMLs were exceptionally responsive. Interestingly, patients with TCGA-LAML and BEAT-AML with these translocations had the highest average HASPIN expression levels, suggesting subtype-specific mechanisms that are more HASPIN reliant than others, but how this might occur is currently unclear. Future studies should consider how primary translocations or mutations in AMLs interact with HASPIN and consequent dependency. Nevertheless, patients with AML with t(8;21) and MLL translocation represent potentially ideal recipients for HASPIN inhibitor treatment.
Venetoclax is a US Food and Drug Administration–approved BCL-2 inhibitor to treat AML.17 Despite this, patients with AML can acquire venetoclax resistance causing disease relapse.70 Analysis of venetoclax-treated CRISPR screens suggested that HASPIN KO contributes to a synthetic lethal relationship in AML. Indeed, in tested AMLs, CHR-6494 and venetoclax increased treatment potency 5- to 10-fold over individual agent treatments when combined. Remarkably, this extended to venetoclax-resistant THP-1 and OCI-AML3, wherein CHR-6494 was sufficient to re-establish venetoclax sensitivity. The opposite was also true, such as in HL-60, where CHR-6494 was significantly potentiated by venetoclax. Importantly, CHR-6494 reduced cell viability in several primary AML samples. Furthermore, combination with venetoclax resensitized resistant primary AML, providing strong preclinical support for the potential utility of HASPIN. In AML, venetoclax resistance hinges on utilization of BCL-2 alternatives, such as MCL-1, metabolic alterations, or TP53 mutation status.51 Kasumi-1, THP-1, HL-60, and NB4 possess TP53 mutations leading to loss of function. However, all 4 responded robustly to the combination treatment. Mechanisms underlying the combined effect of venetoclax and CHR-6494 are unclear. CRISPR screens in venetoclax-resistant AMLs identified RBPs as major mediators of drug resistance.20 In our analysis, BH3-only factors such as BCL2L11 undergo isoform alteration into more active proapoptotic forms after loss of HASPIN and possible accompanying RBP activity. Other BH3-only factors such as PUMA were also upregulated on HASPIN knockdown. Moreover, DNA damage response and cell cycle genes upstream of apoptosis were upregulated despite nonfunctional TP53, suggesting HASPIN may activate TP53-independent pathways to mediate venetoclax response.
In conclusion, we identified HASPIN as a promising, novel kinase dependency in AML, expanded on the underappreciated role of HASPIN in splicing and demonstrated therapeutic efficacy of HASPIN inhibition across several AML subtypes alone or in combination with BCL-2 inhibitors.
Acknowledgments
The authors thank Christopher Vakoc for invaluable advice regarding CRISPR screening. The authors thank the University of California, San Diego Moores Cancer Center Flow Cytometry Core Facility staff for assistance with FACS analysis and the Institute for Genomic Medicine core facility staff for assistance with quality control and associated next-generation sequencing of CRISPR screen library preparations. The visual abstract was created in part with BioRender.com.
This work was supported by the National Institutes of Health (NIH), National Cancer Institute (NCI) Grant R01CA104509 (D.-E.Z.) and the NIH/NCI T32 Contemporary Approaches to Cancer Signaling and Communication Training Grant CA009523 (M.L.). The Flow Cytometry Core Facility is partially supported by NIH/NCI P30CA023100.
Authorship
Contribution: M.L., S.A.S., and D.-E.Z. conceived the project; M.L. performed the research, experiments, and analysis, wrote all necessary code, performed all informatics analysis, and prepared and edited the manuscript; M.Y. and A.G.D. performed wet laboratory experiments; E.D.B. provided patient-derived primary acute myeloid leukemia samples and technical assistance; and D.-E.Z. oversaw the study and the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dong-Er Zhang, University of California San Diego, 9500 Gilman Dr, La Jolla, CA 92037; email: d7zhang@ucsd.edu.
References
Author notes
Raw RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession number GSE281301).
Processed screen and RNA sequencing data are available in the supplemental Data. Other data are available on reasonable request from the corresponding author, Dong-Er Zhang (d7zhang@ucsd.edu).
The full-text version of this article contains a data supplement.