Key Points
PPARγ upregulation upon VEN/DEC-induced PPARG promoter hypomethylation resulted in fatty acid oxidation activation and treatment resistance.
Pharmacological inhibition of PPARγ enhanced sensitivity to VEN/DEC in resistant AML, suggesting PPARγ as a potential therapeutic target.
Visual Abstract
The combination of the B-cell lymphoma 2 (BCL2) inhibitor venetoclax (VEN) and the hypomethylating agent decitabine (DEC; VEN/DEC) constitutes a primary therapeutic strategy for treating older adults with acute myeloid leukemia (AML). However, a notable subset of patients exhibits resistance to VEN/DEC, demonstrating either no disease response or relapse after initial remission. This study aimed to elucidate the molecular mechanisms underlying this resistance through analyses of gene expression and DNA methylation profiles. We conducted comprehensive RNA sequencing analysis and DNA methylation profiling on AML samples from 35 patients undergoing VEN/DEC therapy. The RNA sequencing analysis revealed that several genes related to fatty acid metabolism were significantly upregulated in leukemia cells from patients who received VEN/DEC treatment and relapsed or failed to respond. Increased expression of peroxisome proliferator–activated receptor gamma (PPARG) occurred after treatment and correlated with decitabine-induced promoter hypomethylation. Subsequent in vitro validation demonstrated that decitabine treatment results in hypomethylation of the PPARG promoter, elevating PPARG levels and promoting a metabolic environment characterized by enhanced fatty acid oxidation pathways conducive to VEN/DEC resistance. Furthermore, pharmacological inhibition using either a PPARγ antagonist or a fatty acid oxidation inhibitor enhanced the sensitivity of resistant cells to VEN/DEC, underscoring the crucial role of PPARγ in the development of therapeutic resistance. These findings not only shed light on the metabolic adaptation that contributes to VEN/DEC resistance in AML but also identify PPARγ as a potential therapeutic target for overcoming such resistance, providing new opportunities to improve the efficacy of VEN/DEC-based therapy in AML.
Introduction
The therapeutic landscape for acute myeloid leukemia (AML) has been revolutionized by the introduction of combination therapy using the B-cell lymphoma 2 (BCL2) inhibitor venetoclax (VEN) and hypomethylating agents (HMAs) such as azacitidine (AZA) and decitabine (DEC). This treatment regimen, renowned for its efficacy and tolerability, has become the standard of care for older patients with AML who are ineligible for intensive induction chemotherapy.1,2 Despite achieving clinical remission, resistance to VEN/HMA therapy remains a significant challenge, with a considerable fraction of patients experiencing relapse after initial treatment success. Elucidation and targeting of these resistance mechanisms are essential for the advancement of AML therapeutic strategies.
Recent studies have illuminated several aspects of VEN/HMA resistance in AML. Considering the mechanisms of action of VEN, resistance may logically involve alterations in BCL2 family members. Research suggests that the expression patterns of BCL2, BCL-xL, and myeloid cell leukemia 1(MCL1) in AML stem cells could predict the response to the combination of VEN and AZA (VEN/AZA).3 In addition, resistance mechanisms have been associated with monocytic AML cells, which upregulate MCL1 to circumvent BCL2 inhibition, thereby mitigating mitochondrial stress.4 While HMAs are known to suppress MCL1 expression,41 resistance may emerge if this effect is insufficient or if MCL1 dependency is selectively enhanced during therapy.Building on this, a recent study using VEN/AZA-resistant cell lines have demonstrated increased expression of MCL1 and decreased expression of the pro-apoptotic protein BAX40
Alterations in energy metabolism also significantly contribute to resistance against VEN/HMAs. VEN/AZA specifically targets leukemia stem cells by inhibiting mitochondrial amino acid uptake and reducing oxidative phosphorylation (OXPHOS),5 which decreases energy production and induces cell death. However, leukemia cells that relapse enhance their fatty acid metabolism to overcome amino acid depletion.6 Our previous research demonstrated that bone marrow adipocytes support apoptosis evasion in acute monocytic leukemia (AMoL) cells via fatty acid oxidation (FAO) stimulation by upregulation of peroxisome proliferator–activated receptor gamma (PPARG), CD36, and FABP4 (fatty acid binding protein 4).7 In mitochondria, fatty acids are used for FAO, a process that involves mitochondrial uncoupling, leading to reduced formation of mitochondrial reactive oxygen species and decreased intracellular oxidative stress. These intricate networks of transcriptional regulation and fatty acid metabolism maintain AMoL cells in a quiescent state. This quiescent state is associated with activation of adenosine 5'-monophosphate (AMP)-activated protein kinase, p38-mediated autophagy, and upregulation of heat shock protein family members, which function as antiapoptotic chaperone proteins. Together, these adaptations underlie the development of chemoresistance in these cells.7-9
Genomic profiling has enhanced our understanding of AML resistance, revealing that mutations in genes such as NPM1, IDH1/2, and RUNX1 are correlated with better response rates, whereas mutations in TP53 and in genes encoding activating kinases such as FLT3 and RAS are associated with inferior outcomes.4,10 These genomic alterations influence cellular metabolism, including FAO, thereby affecting drug sensitivity.4,11
The transition between drug sensitivity and resistance in AML cells is likely regulated by growth factor signaling, epigenetic modifications,12 and metabolic reprogramming.13 Therefore, our study aimed to identify the transcriptomic and epigenetic alterations associated with the development of resistance to the combination of VEN and DEC (VEN/DEC) therapy. We identified an upregulation of PPARG and its role in promoting fatty acid metabolism in patients with AML unresponsive to VEN/DEC treatment. This finding suggests a direct link between PPARγ-mediated metabolic reprogramming and therapeutic resistance.
Materials and methods
The detailed procedures for cell lines, culture conditions, reagents, cell viability and apoptosis assays, immunoblot analysis, and statistical methods are provided in the supplemental Materials and methods.
Primary samples
Forty-seven primary peripheral blood and bone marrow samples were collected from patients with treatment-naive or nonnaive AML (N = 35) enrolled in the DEC10-VEN clinical trial (NCT03404193). The samples contained at least 58% blasts. Written informed consent for participation was obtained in accordance with the institutional review board regulations of The University of Texas MD Anderson Cancer Center and the Declaration of Helsinki principles. The study protocol was approved by the respective institutional ethics committees. Ficoll-Hypaque density gradient centrifugation was used to separate the mononuclear cells (Sigma-Aldrich, St Louis, MO).
Next-generation sequencing–based mutation analysis
Next-generation sequencing mutation analysis was performed on 250 ng of DNA from unsorted bone marrow or peripheral blood patient samples. The gene panel interrogated either selected hot spot or entire coding regions of 48 cancer-related genes using TruSeq Amplicon Cancer Panel kit (Illumina, San Diego, CA) as described previously.14
Bulk RNA-seq analysis
Sequencing libraries were generated using TruSeq Stranded Total RNA Ribo-Zero Gold Kit for Illumina (New England Biolabs; Ipswich, MA), following the manufacturer’s recommendations. The library fragments were purified with QIAquick PCR Purificaiton kits (Qiagen, Hilden, Germany). The clustering of the index-coded samples was performed on a cBot cluster generation system using HiSeq PE Cluster Kit v4-cBot-HS (Illumina), according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on a NovaSeq 6000 sequencer (Illumina), and 50-base paired-end reads were generated. RNA sequencing (RNA-seq) FASTQ files were processed through FastQC, a quality control tool to evaluate the quality of sequencing reads at both the base and read levels to generate a series of RNA-seq–related quality control metrics. Samples with passed quality control (QC) were taken into subsequent analysis. STAR15 was used to align raw FASTQ reads to human reference genome (hg38) with default parameters to generate BAM files. Aligned reads were then summarized at the gene level by STAR.
Bioconductor package DESeq216 was used to perform read count normalization, dispersion estimation, and identification of differential expression. DESeq2 models the counts using a negative binomial distribution followed by the Wald test. The final P value was adjusted using the Benjamini-Hochberg method. Differentially expressed genes were identified using statistical cutoffs of false discovery rate (FDR) values <5%. Differential expression analysis was further evaluated using the pathway enrichment tool gene set enrichment analysis (GSEA), and gene sets were obtained from published gene signatures.17 To assess statistical significance, we compared the enrichment score to the results from 1000 random permutations of the gene set, calculating FDR values. FDR value <0.25 was considered statistically significant in GSEA.
Genome-wide methylation assay
Genomic DNA was extracted using a QIAamp DNA Mini kit (Qiagen). Genome-wide DNA methylation analysis was performed using Infinium Human Methylation EPIC Bead Chips (Illumina), as described previously.18 The methylation level of a CpG site was represented by a β value, which ranged from 0 (completely unmethylated) to 1 (completely methylated). The CpG probes were grouped into 551 478 genomic blocks, which consisted of probes within 500 base pairs (bp). Unsupervised hierarchical clustering analysis was performed using R version 3.4.4 with the Heatplus package, as previously described.19 The Euclidean distance was used as the distance function both for samples and genes.
Lentivirus preparation and infection
Lentiviral particles used for transduction of human PPARG complementary DNA (VB220915-1538xjj, pLV[Exp]-SFFV> hPPARG[NM_138712.5]:IRES:EGFP) and a control green fluorescent protein/mCherry vector (VB160109-10005, pLV[Exp]-EGFP:T2A:Puro-EF1A.mCherry) were prepared by VectorBuilder (Chicago, IL). Cells were infected with lentiviral particles using RetroNectin (Takara Bio Inc, Shiga, Japan) for use in subsequent experiments. In case of low transduction efficiency, green fluorescent protein–positive cells were isolated on a Moflo Astrios EQ cell sorter (Beckman Coulter, Brea, CA) and used for subsequent experiments.
Bisulfite pyrosequencing
The methylation status of CpG sites in the promoter-proximal regions was determined using pyrosequencing-based analysis as described previously.20 Briefly, bisulfite conversion of genomic DNA was performed using the EZ DNA Methylation Kit (Zymo Research, Irvine, CA). Bisulfite-treated DNA was amplified by polymerase chain reaction (PCR) using the PPARG-F and PPARG-R primer sets. Pyrosequencing reactions were performed using the PyroMark Q24 (Qiagen) and PyroMark Gold-Q24 reagent kits (Qiagen) and the sequencing primer PPARG-S. The primer sequences are listed in supplemental Table 1.
Extracellular flux assays
The Seahorse XFe24 Extracellular Flux analyzer (Agilent, Santa Clara, CA) and the Seahorse XF Cell Mito Stress Kit (catalog no. 103015-100; Agilent) were used to measure the oxygen consumption rates (OCRs) of MV4;11 and HL60 cells according to the manufacturer’s instructions.21 Briefly, AML cells were cultured with or without the indicated concentrations of VEN and/or DEC for 4 hours. AML cells were then counted, and 3 × 105 cells were seeded in Poly-L-lysine (catalog no. P4707; Sigma)–coated 24-well plates. Thirty minutes before analysis, the medium was replaced with Seahorse XF media (catalog no. 103575-100; Agilent), and the plate was incubated at 37°C. Analyses were performed both at basal conditions and after injection of 15 μg/mL oligomycin, 5- or 20-μM FCCP (carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone) for MV4;11 and HL60 cells, respectively, and 5-μM antimycin A/rotenone (all reagents were finally diluted 10×). Basal respiration, maximal respiration, and spare respiratory capacity (SRC) were calculated following the manufacturer’s instructions.
FAO was measured by changes in OCR after FAO inhibition.22,23 Briefly, to measure FAO, cells (HL60 [2 × 106 cells per mL] and MV4 [8 × 105 cells per mL]) were seeded for 3 hours in substrate-limited XF DMEM (catalog no. 103575-100; Agilent). Cells were then reseeded in FAO assay medium on Seahorse cell culture plates and incubated for 25 minutes in a non-CO2 incubator. FAO-dependent and -independent mitochondrial OCRs were measured in cells seeded on Seahorse cell culture plates in XF media (Agilent), followed by treatment with the FAO inhibitor etomoxir24 (40 μM at final concentration) for 15 minutes.
Results
RNA-seq analysis reveals high energy metabolism and low immune response to VEN/DEC treatment in the R/R cohort
To investigate the transcriptomic patterns influencing the variability in response to VEN/DEC therapy in AML, we conducted RNA-seq analysis on 26 primary peripheral blood or bone marrow samples (21 pretreatment and 5 posttreatment samples) from 13 treatment-naive and 12 previously treated (nonnaive) patients with AML treated in the DEC10-VEN clinical trial (ClinicalTrials.gov identifier: NCT03404193). Supplemental Table 2 summarizes the patients' clinical profiles. We categorized the patients into 2 groups based on their response to VEN/DEC treatment. The first group included patients who achieved complete remission (CR) or CR with incomplete count recovery (CRi), henceforth referred to as CR patients. The second group comprised patients who either did not respond or achieved CR/CRi but relapsed, henceforth referred to as relapsed/refractory (R/R) patients. Gene panel sequencing identified multiple genetic variants, with TP53, FLT3-ITD, and NPM1 being the 3 most frequently mutated genes in the entire cohort. Mutations in TP53, FLT3-ITD, and RAS, markers associated with adverse outcomes,10 were observed in 56% of the CR patients and 75% of the R/R patients.
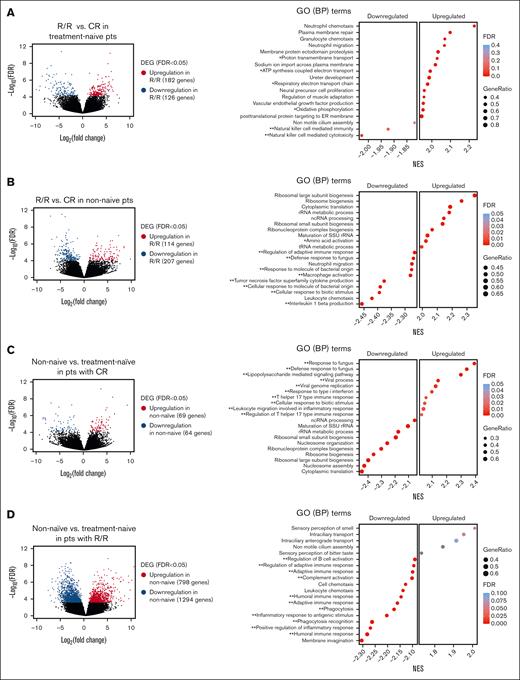
We initially analyzed 21 pretreatment samples from 11 treatment-naive patients and 10 nonnaive patients who failed prior treatments. Within the treatment-naive cohort, 6 patients achieved CR/CRi (CR patients), and 5 did not respond or experienced early relapse (R/R patients). The nonnaive cohort included 3 CR and 7 R/R patients. Initial comparisons of pretreatment samples between the R/R and CR patients in the treatment-naive cohort revealed 182 upregulated and 126 downregulated genes in R/R patients, with an FDR <0.05 (Figure 1A; supplemental Table 3A). GSEA indicated significant enrichment of upregulated genes associated with OXPHOS and cell migration/adhesion processes in R/R patients. In the nonnaive cohort, we identified 114 upregulated and 207 downregulated genes in R/R patients compared to CR patients, demonstrating a decrease in immune-related gene signatures and an increase in ribosomal biogenesis (protein production)–related genes (Figure 1B; supplemental Table 3B).
Transcriptomic differences between R/R and CR pts in pretreatment primary AML samples. (A) Volcano plot profile (left) showing the −log10 FDR value and log2 fold change of DEGs between pts who achieved CR/CRi (CR pts) and pts who did not respond to treatment or relapsed after VEN/DEC treatment (R/R pts) in the treatment-naive AML cohort (R/R, n = 5; CR, n = 6). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in R/R pts. The gene ratio is the percentage of genes that were significantly associated with the R/R group among the total number of genes associated with the process. GO terms are ranked in decreasing order of NES. A single asterisk and a double asterisk indicate GO terms associated with energy production and immune response, respectively. (B) Volcano plot profile (left) of DEGs in R/R pts vs CR pts in the previously treated (nonnaive) group of pts with AML (R/R, n = 7; CR, n = 3). Dot plot (right) of GSEA results illustrating the top of a ranked list of GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in R/R pts. (C) Volcano plot profile (left) of DEGs in nonnaive vs treatment-naive pts in the CR group (nonnaive, n = 3; treatment-naive, n = 6). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in the nonnaive cohort. (D) Volcano plot (left) profile of DEGs in nonnaive vs treatment-naive pts in the R/R group (nonnaive, n = 7; treatment-naive, n = 5). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in the nonnaive cohort. ATP, adenosine triphosphate; BP, biological process; DEGs, differentially expressed genes; ER, endoplasmic reticulum; GO, gene ontology; ncRNA, non-coding RNA; NES, normalized enrichment scores; pts, patients; rRNA, ribosomal RNA; SSU, Small Subunit; tRNA, transfer RNA.
Transcriptomic differences between R/R and CR pts in pretreatment primary AML samples. (A) Volcano plot profile (left) showing the −log10 FDR value and log2 fold change of DEGs between pts who achieved CR/CRi (CR pts) and pts who did not respond to treatment or relapsed after VEN/DEC treatment (R/R pts) in the treatment-naive AML cohort (R/R, n = 5; CR, n = 6). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in R/R pts. The gene ratio is the percentage of genes that were significantly associated with the R/R group among the total number of genes associated with the process. GO terms are ranked in decreasing order of NES. A single asterisk and a double asterisk indicate GO terms associated with energy production and immune response, respectively. (B) Volcano plot profile (left) of DEGs in R/R pts vs CR pts in the previously treated (nonnaive) group of pts with AML (R/R, n = 7; CR, n = 3). Dot plot (right) of GSEA results illustrating the top of a ranked list of GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in R/R pts. (C) Volcano plot profile (left) of DEGs in nonnaive vs treatment-naive pts in the CR group (nonnaive, n = 3; treatment-naive, n = 6). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in the nonnaive cohort. (D) Volcano plot (left) profile of DEGs in nonnaive vs treatment-naive pts in the R/R group (nonnaive, n = 7; treatment-naive, n = 5). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive and negative enrichment scores, respectively, indicate upregulation and downregulation in the nonnaive cohort. ATP, adenosine triphosphate; BP, biological process; DEGs, differentially expressed genes; ER, endoplasmic reticulum; GO, gene ontology; ncRNA, non-coding RNA; NES, normalized enrichment scores; pts, patients; rRNA, ribosomal RNA; SSU, Small Subunit; tRNA, transfer RNA.
Comparative analysis of nonnaive and treatment-naive patients within the CR category revealed 69 upregulated and 64 downregulated genes in nonnaive patients, with significant upregulation in gene ontology biological processes related to immune response and downregulation in ribosomal biogenesis relative to treatment-naive patients (Figure 1C; supplemental Table 3C). Conversely, a comparison of nonnaive and treatment-naive patients within the R/R category exhibited divergent expression patterns. In nonnaive patients, 798 genes were upregulated, and 1294 genes were downregulated, with a substantial decrease in immune response–associated genes relative to treatment-naive patients. (Figure 1D; supplemental Table 3D).
These findings suggest that the efficacy of VEN/DEC treatment might be linked to the expression of immune response–related genes, with the most pronounced attenuation observed in nonnaive patients who did not respond to or relapsed after VEN/DEC treatment (supplemental Figure 1A). In addition, upregulation of metabolism-related genes, particularly those involved in adenosine triphosphate (ATP) and protein production, was observed in R/R patients in both the treatment-naive and nonnaive cohorts.
VEN/DEC treatment upregulates genes associated with activation of energy metabolism and immune response in R/R patients
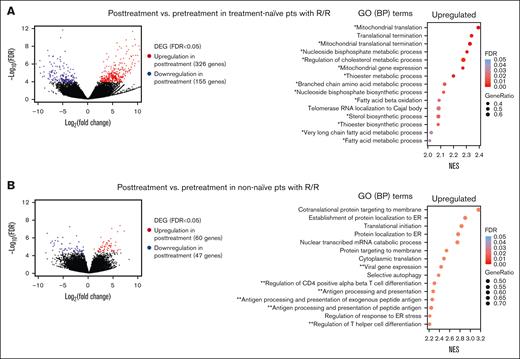
To further assess the impact of VEN/DEC treatment on gene expression, we compared transcriptomic data from 12 pretreatment and 5 posttreatment samples from treatment-naive and nonnaive patients. All samples were obtained from patients who did not respond to or relapsed after VEN/DEC treatment. In treatment-naive patients (posttreatment, n = 2; pretreatment, n = 5), significant transcriptional changes occurred after VEN/DEC treatment, with 326 genes upregulated and 155 genes downregulated relative to their pretreatment levels (Figure 2A; supplemental Table 3E). Furthermore, gene ontology analysis highlighted upregulation of genes involved in metabolic processes, especially those related to fatty acid metabolism, indicating a shift toward increased fatty acid utilization after treatment (supplemental Figure 1B). Similarly, in the nonnaive cohort (posttreatment, n = 3; pretreatment, n = 7), analysis revealed upregulation of 60 genes and downregulation of 47 genes after treatment (Figure 2B; supplemental Table 3F). Posttreatment samples exhibited enhanced expression of genes linked to immune response, indicating activation of immune pathways after treatment (supplemental Figure 1B).
Transcriptomic differences between pretreatment and posttreatment samples obtained from R/R pts. (A) Volcano plot (left) profile showing the –log10 FDR value and log2 fold change of DEGs in posttreatment vs pretreatment samples obtained from pts who did not respond to or relapsed after VEN/DEC treatment (R/R pts) in the treatment-naive AML cohort (posttreatment, n = 2; pretreatment, n = 5). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive enrichment scores indicate upregulation in posttreatment samples. The gene ratio is the percentage of genes that were significantly associated with posttreatment samples among the total number of genes associated with the process. A single asterisk and a double asterisk indicate GO terms associated with energy production and immune response, respectively. (B) Volcano plot profile (left) of DEGs in posttreatment vs pretreatment samples obtained from R/R pts in the non–treatment-naive AML cohort (posttreatment, n = 3; pretreatment, n = 7). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). ER, endoplasmic reticulum.
Transcriptomic differences between pretreatment and posttreatment samples obtained from R/R pts. (A) Volcano plot (left) profile showing the –log10 FDR value and log2 fold change of DEGs in posttreatment vs pretreatment samples obtained from pts who did not respond to or relapsed after VEN/DEC treatment (R/R pts) in the treatment-naive AML cohort (posttreatment, n = 2; pretreatment, n = 5). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). Positive enrichment scores indicate upregulation in posttreatment samples. The gene ratio is the percentage of genes that were significantly associated with posttreatment samples among the total number of genes associated with the process. A single asterisk and a double asterisk indicate GO terms associated with energy production and immune response, respectively. (B) Volcano plot profile (left) of DEGs in posttreatment vs pretreatment samples obtained from R/R pts in the non–treatment-naive AML cohort (posttreatment, n = 3; pretreatment, n = 7). Dot plot (right) of GSEA results illustrating the top-ranked GO BP terms enriched in the DEGs (FDR < 0.25). ER, endoplasmic reticulum.
Considering the apparent correlation between treatment response and alterations in immune response and energy metabolism, our investigation next focused on a set of 116 interferon-stimulated genes (ISGs). Among these ISGs, we identified several differentially expressed genes (supplemental Table 4). Notably, PPARG, a transcription factor known for its role in activating fatty acid metabolism and modulating interferon gamma target genes, was significantly upregulated in posttreatment samples across both treatment-naive and nonnaive patients. Further analysis revealed that several PPARG target genes, including APOE (apolipoprotein E; supplemental Table 5A), and multiple genes associated with FAO (supplemental Table 6A) were significantly upregulated in pretreatment samples from R/R patients compared to CR patients. In addition, the lipid transporter CD36 and FABP4 exhibited significant upregulation after VEN/DEC treatment (supplemental Table 5B), and multiple genes associated with FAO were upregulated in posttreatment samples compared to pretreatment samples in R/R patients (supplemental Table 6B).
Primary or adaptive resistance to the combination of VEN and HMAs has been shown to be primarily associated with TP53 alterations and other mutations in genes involved in cell survival activation.4 We found that the expression of ASS1, a target gene of PPARγ and a key enzyme of the urea cycle, was significantly elevated in patients with TP53 mutations. PPARG also tended to be higher in patients bearing TP53 mutations, although not significantly. A lipoprotein lipase, LPL, a target gene of PPARγ, was significantly elevated in patients with SRSF2 mutations (supplemental Figure 2).
DNA hypomethylation around TSSs is associated with response to VEN/DEC treatment
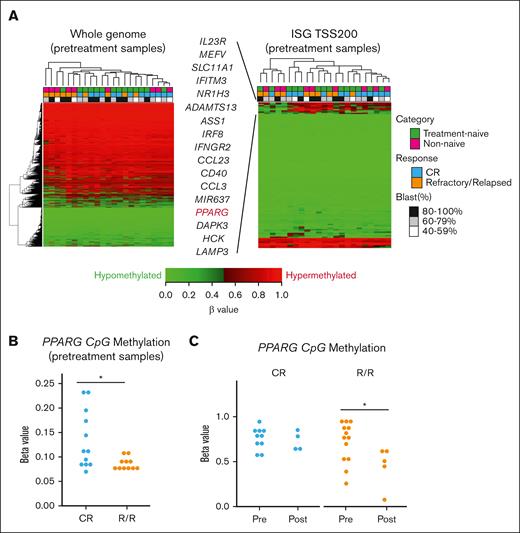
To identify differentially methylated positions related to the response to VEN/DEC treatment, we conducted DNA methylation analysis on 23 pretreatment samples from treatment-naive and nonnaive patients with AML. Because DNA methylation status around transcription start sites (TSSs) can influence gene expression, we evaluated DNA methylation in CpG islands in regions from the TSS to approximately –200 to –1500 bp upstream. We randomly selected 20 000 blocks of CpG probes for whole genome, and no specific clustering was observed (Figure 3A, left). A focused clustering analysis on ISGs identified a cluster of 17 hypomethylated genes compared to other genes. This cluster consisted of some R/R patients (Figure 3A, right). Among the genes, PPARG emerged as a key gene that distinguished between R/R and CR patients. Methylation levels of PPARG in the region from the TSS to –200 bp upstream was significantly lower in pretreatment samples of R/R patients than those of CR patients (Figure 3B). Furthermore, we compared DNA methylation status of pretreatment samples to that of posttreatment samples and found that VEN/DEC treatment significantly promoted hypomethylation of PPARG in regions from TSS to –1500 bp upstream in R/R patients but not in CR patients (Figure 3C). These results align with our RNA-seq analysis, which demonstrated upregulation of PPARG after VEN/DEC treatment in R/R patients.
DNA methylation profiles. (A) Hierarchical clustering analysis of DNA methylation profiles on 23 pretreatment samples (left). Twenty thousand CpG probe blocks for the whole genome were randomly selected. Hierarchical clustering analysis of promoter methylation profiles of 119 gene blocks for 113 ISGs (right). Pretreatment samples were used and methylation (β values) of gene blocks in CpG islands in the region from TSS to −200 bp upstream was analyzed. Seventeen genes were hypomethylated in pts who failed to respond or relapsed after VEN/DEC treatment (R/R pts) compared to pts who achieved CR/CRi (CR pts). (B) Methylation levels (β values) of PPARG in CpG islands within regions from the TSS to −200 bp upstream were compared between R/R and CR pts. (C) Methylation levels (β values) of PPARG in CpG islands within regions from the TSS to −1500 bp upstream were compared between the pretreatment and posttreatment samples obtained from CR and R/R pts. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05.
DNA methylation profiles. (A) Hierarchical clustering analysis of DNA methylation profiles on 23 pretreatment samples (left). Twenty thousand CpG probe blocks for the whole genome were randomly selected. Hierarchical clustering analysis of promoter methylation profiles of 119 gene blocks for 113 ISGs (right). Pretreatment samples were used and methylation (β values) of gene blocks in CpG islands in the region from TSS to −200 bp upstream was analyzed. Seventeen genes were hypomethylated in pts who failed to respond or relapsed after VEN/DEC treatment (R/R pts) compared to pts who achieved CR/CRi (CR pts). (B) Methylation levels (β values) of PPARG in CpG islands within regions from the TSS to −200 bp upstream were compared between R/R and CR pts. (C) Methylation levels (β values) of PPARG in CpG islands within regions from the TSS to −1500 bp upstream were compared between the pretreatment and posttreatment samples obtained from CR and R/R pts. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05.
Because interferon gamma has been reported to be elevated in AML and contribute to VEN resistance,25 we further analyzed the expression and methylation status of IFNG before and after treatment. IFNG was hypomethylated after treatment in both R/R (supplemental Table 7A) and in CR patients (supplemental Table 7B). Although IFNG was hypomethylated after treatment, we did not observe a significant change in RNA expression levels in R/R patients (no posttreatment samples were available in CR group).
PPARG is upregulated by VEN/DEC treatment in cell lines via DNA hypomethylation
To validate these findings, we used 2 cell lines, HL60 and MV4;11, which displayed synergistic susceptibility to the combined VEN/DEC treatment regimen in cell viability assays, as depicted in supplemental Figure 3 (combination index of 0.78 for HL60 and 0.37 for MV4;11). Subsequent analyses confirmed that VEN/DEC treatment upregulated PPARG gene expression in both cell lines (Figure 4A). Furthermore, methylation analyses indicated that this upregulation resulted from DNA hypomethylation processes (Figure 4B). Validation at the protein level also demonstrated that DEC treatment increased PPARγ expression (Figure 4C). Although DEC treatment induced a substantial reduction in CpG methylation, the resulting increase in PPARγ protein levels was modest. This discrepancy suggests that complex posttranscriptional and translational regulatory mechanisms may underline the limited correlation between DNA methylation and protein expression. This upregulation of PPARG coincided with the upregulation of CD36, a gene downstream of PPARG (Figure 4D).
Cellular responses to VEN/DEC in AML cell lines. (A) The indicated cell lines were treated with the indicated doses of VEN and/or DEC. PPARG messenger RNA (mRNA) levels were quantified by quantitative reverse transcription polymerase chain reaction (qRT-PCR), and relative expression was normalized to GAPDH expression. (B) PPARG DNA methylation levels in CpG islands at TSS were determined by bisulfite pyrosequencing. The methylation level of untreated cells was set to 100%. (C) Immunoblots showing the amounts of PPARγ protein in each cell line after treatment with the indicated doses of VEN and/or DEC for 3 days. Relative expression of PPARγ was normalized to that of β-actin and the expression level of untreated cells was set to 1.0. (D) The cell lines were treated with the indicated doses of VEN and/or DEC for 3 days. CD36 mRNA levels were quantified by qRT-PCR. Relative expression of CD36 was normalized to GAPDH expression. Data are expressed as the mean ± standard deviation (SD) of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗ vs control, P < .05.
Cellular responses to VEN/DEC in AML cell lines. (A) The indicated cell lines were treated with the indicated doses of VEN and/or DEC. PPARG messenger RNA (mRNA) levels were quantified by quantitative reverse transcription polymerase chain reaction (qRT-PCR), and relative expression was normalized to GAPDH expression. (B) PPARG DNA methylation levels in CpG islands at TSS were determined by bisulfite pyrosequencing. The methylation level of untreated cells was set to 100%. (C) Immunoblots showing the amounts of PPARγ protein in each cell line after treatment with the indicated doses of VEN and/or DEC for 3 days. Relative expression of PPARγ was normalized to that of β-actin and the expression level of untreated cells was set to 1.0. (D) The cell lines were treated with the indicated doses of VEN and/or DEC for 3 days. CD36 mRNA levels were quantified by qRT-PCR. Relative expression of CD36 was normalized to GAPDH expression. Data are expressed as the mean ± standard deviation (SD) of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗ vs control, P < .05.
Upregulation of PPARG correlates with reduced sensitivity to VEN/DEC treatment
To elucidate the role of PPARG in conferring resistance to VEN/DEC therapy, we used a lentiviral system to overexpress PPARG in HL60 and MV4;11 cells (Figure 5A). Immunoblotting confirmed successful overexpression in transfected cells compared to mock-transfected controls. Intriguingly, PPARG overexpression resulted in reduced proliferative capacity in MV4;11 cells but did not trigger apoptotic pathways (supplemental Figure 4). The cells were then treated with various concentrations of VEN/DEC, and the dose-dependent inhibitory effects on cell proliferation and the apoptogenic effects were determined. As shown in Figure 5B, overexpression of PPARG significantly decreased the inhibitory effects of VEN/DEC on cell proliferation (PPARG-overexpressing [OE] vs control; cell growth inhibition, HL60 [30% vs 54%; P < .05] and MV4;11 [33% vs 56%; P < .05]). In addition, PPARG-OE cells exhibited a lower rate of apoptosis after treatment with VEN/DEC than control vector–transfected cells (apoptosis induction, HL60 [14% vs 38%; P < .05] and MV4;11 [25% vs 36%; P < .05]; Figure 5C).
Overexpression of PPARG decreases susceptibility to VEN/DEC treatment. (A) Experimental protocol for generating VEN/DEC-resistant HL60 and MV4;11 cells OE PPARG and subsequent drug treatment (left). Western blot analysis of PPARγ protein expression in control vector vs PPARG-OE cell lines (right). (B) HL60 and MV4;11 cells transfected with either the control vector or PPARG-OE were treated with the indicated doses of VEN ± DEC for 72 hours after mCherry or GFP sorting, respectively. The number of viable cells (expressed as a percentage of untreated cells) was determined using the trypan blue exclusion method. (C) Apoptosis of treated cells was analyzed by flow cytometry. Cells transfected with the control vector were stained with mCherry/annexin V–APC, and PPARG-OE cells were stained with GFP/annexin V–APC. Representative flow cytometry plots showing GFP or mCherry (x-axis) and APC–annexin V staining (y-axis) are displayed on the left. The percentages of annexin V–positive cells are presented on the right. Data are expressed as the mean ± SD of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05. APC, allophycocyanin; CMV, cytomegalovirus; GFP, green fluorescent protein.
Overexpression of PPARG decreases susceptibility to VEN/DEC treatment. (A) Experimental protocol for generating VEN/DEC-resistant HL60 and MV4;11 cells OE PPARG and subsequent drug treatment (left). Western blot analysis of PPARγ protein expression in control vector vs PPARG-OE cell lines (right). (B) HL60 and MV4;11 cells transfected with either the control vector or PPARG-OE were treated with the indicated doses of VEN ± DEC for 72 hours after mCherry or GFP sorting, respectively. The number of viable cells (expressed as a percentage of untreated cells) was determined using the trypan blue exclusion method. (C) Apoptosis of treated cells was analyzed by flow cytometry. Cells transfected with the control vector were stained with mCherry/annexin V–APC, and PPARG-OE cells were stained with GFP/annexin V–APC. Representative flow cytometry plots showing GFP or mCherry (x-axis) and APC–annexin V staining (y-axis) are displayed on the left. The percentages of annexin V–positive cells are presented on the right. Data are expressed as the mean ± SD of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05. APC, allophycocyanin; CMV, cytomegalovirus; GFP, green fluorescent protein.
PPARγ induces upregulation of fatty acid metabolism
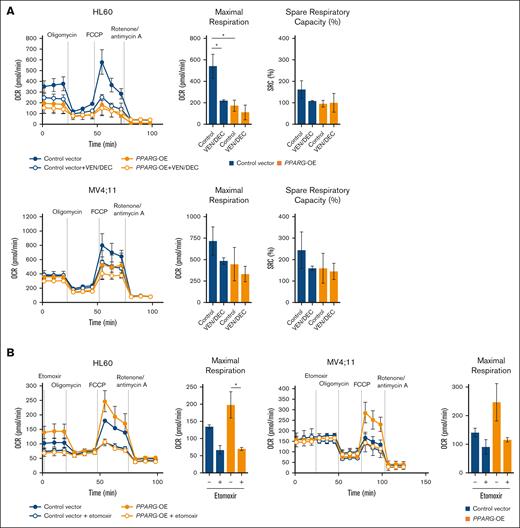
Considering that the antitumor effect of VEN/AZA is associated with OXPHOS inhibition,6 we hypothesized that cells resistant to VEN/DEC treatment might exhibit altered OXPHOS activities compared to their treatment-sensitive counterparts. To investigate this, we used PPARG-OE HL60 and MV4;11 cells and conducted a mitochondrial stress test. As shown in Figure 6A, both HL60 and MV4;11 PPARG-OE cells exhibited lower maximal respiration and SRC, a critical indicator of the mitochondrial flexibility necessary for bioenergetic adaptation under various physiological conditions,26 than mock vector–transfected cells. VEN/DEC treatment significantly reduced maximal respiration in mock HL60 cells, whereas PPARG-OE HL60 cells were not significantly affected. Similarly, in MV4;11 cells, the reduction in maximal respiration after VEN/DEC treatment was diminished in PPARG-OE cells. In both HL60 and MV4;11 cells, VEN/DEC-induced reductions in SRC were only observed in mock vector–transfected cells and not in PPARG-OE cells. Next, to investigate the dependence on FAO metabolism, an FAO assay comparing PPARG-OE cells with mock vector–transfected cells was performed (Figure 6B). After nutrient deprivation, the maximum OCR was increased in PPARG-OE cells compared to mock-transfected cells in both HL60 and MV4;11. This increased OCR in PPARG-OE cells was reduced by treatment with the FAO inhibitor etomoxir to the same level as etomoxir-treated mock cells. These results indicate that metabolic adaptation may constitute a potential resistance mechanism to VEN/DEC treatment. In particular, the utilization of endogenous fatty acids for ATP production by high expression of PPARG may contribute to survival and resistance to VEN/DEC treatment.
Analysis of cellular bioenergetics in PPARG-OE cells. (A) A Seahorse Bioscience XFe24 Extracellular Flux Analyzer was used to evaluate OCR in control vector vs PPARG-OE cells. MV4;11 and HL60 cells transfected with either the control vector or PPARG-OE construct were treated with VEN ± DEC for 4 hours, after which 5 × 105 cells were added per well. Three technical replicates were plated. During the assay, oligonomycin, FCCP, rotenone, and antimycin A were injected at the times indicated by the arrows. SRC percent was calculated as follows: (maximal OCR/basal OCR × 100). (B) Endogenous FAO was measured with or without etomoxir after nutrient starvation. The maximal OCRs are shown. Data are expressed as the mean ± SD of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05.
Analysis of cellular bioenergetics in PPARG-OE cells. (A) A Seahorse Bioscience XFe24 Extracellular Flux Analyzer was used to evaluate OCR in control vector vs PPARG-OE cells. MV4;11 and HL60 cells transfected with either the control vector or PPARG-OE construct were treated with VEN ± DEC for 4 hours, after which 5 × 105 cells were added per well. Three technical replicates were plated. During the assay, oligonomycin, FCCP, rotenone, and antimycin A were injected at the times indicated by the arrows. SRC percent was calculated as follows: (maximal OCR/basal OCR × 100). (B) Endogenous FAO was measured with or without etomoxir after nutrient starvation. The maximal OCRs are shown. Data are expressed as the mean ± SD of 3 independent experiments. Differences between the groups were tested using the 2-tailed Student t test. ∗P < .05.
To further clarify the role of the PPARγ-induced FAO pathway in the observed resistance phenotype, we treated the cells with either the CPT-1α inhibitor etomoxir or the PPARγ inhibitor T0070907 and evaluated their effects on cell proliferation (supplemental Figure 5A-B). Notably, both etomoxir and T0070907 enhanced the sensitivity of resistant cells to VEN/DEC treatment.
Discussion
In this study, we identified mechanisms of AML resistance to VEN/DEC treatment. Our findings emphasize the role of altered expression of genes related to energy metabolism and immune system functions in resistant leukemia cells. Notably, we observed significant upregulation of PPARG after VEN/DEC treatment in R/R patients, suggesting that PPARG upregulation is an important factor in facilitating FAO and, consequently, VEN/DEC resistance. The study elucidated that DNA hypomethylation at the TSS upregulated PPARG expression and that this effect was mediated by DEC. To our knowledge, this is the first study to demonstrate the role of DEC in upregulating PPARG expression and consequently promoting FAO metabolism as a mechanism of resistance to VEN/HMA treatment.
PPARγ is a member of the nuclear receptor superfamily of ligand-inducible transcription factors that regulate adipogenesis, metabolism, and immune cell function. Specifically, PPARγ is involved in fatty acid metabolism; in the nucleus, fatty acids ligate PPARγ, and activated PPAR induces downstream target genes, including fatty acid transporters, CD36 and FABP4.7,27 Its role in cancer is complex and may vary depending on the cancer type and stage.28 Although historically considered a tumor suppressor that inhibits cell proliferation, reduces invasiveness, and lowers proinflammatory cytokine levels,29 recent studies suggest that PPARγ actually promotes cancer progression, including brain metastasis and growth in prostate and lung cancer, through mechanisms involving fatty acid metabolism and mitochondrial biogenesis.30-32
Although increased expression of PPARG has been reported in patients with AML,33,34 the role of PPARγ in leukemia is not well understood. AML stem cells preferentially rely on mitochondrial OXPHOS for survival.35,36 However, AMoL, a specific subgroup of AML cells, can survive through a state of metabolic activation of FAO, which contributes to chemoresistance.37 Pollyea et al5 demonstrated that VEN/AZA targets OXPHOS, effectively eradicating leukemia stem cells, and Stevens and Jones et al11,38 reported that resistance to this therapy emerges through FAO upregulation. Our findings indicate that PPARG-OE AML cells decrease proliferative capacity without inducing apoptosis, adopting a quiescent-like state and developing resistance to VEN/DEC treatment primarily through reliance on FAO. This resistance mechanism is further corroborated by the observed upregulation of PPARG in response to VEN/DEC treatment, accompanied by increased expression of CD36, a known downstream target of PPARG, in both primary samples and in vitro experiments. These results highlight the complex role of PPARG in mediating metabolic adaptations and resistance mechanisms in AML.
Our investigation also highlights the significant role of epigenetic modulation in PPARG regulation, in which DEC-induced DNA hypomethylation at PPARG promoter regions enhances PPARG expression. Epigenetic effectors are well-known regulators of PPARG,39 and the epigenetic action of DNA methyltransferase inhibitors impairs cancer cell survival by renewing the transcription of silenced genes. These observations led us to posit that DEC-mediated hypomethylation of the PPARG promoter region specifically augments PPARG expression, which in turn contributes to the development of therapeutic resistance.
Although our study provides new insights into resistance mechanisms in AML, it has limitations. The generalizability of our findings may be limited by the sample size and the inherent heterogeneity of AML subtypes. The limitations of bulk RNA-seq include the averaging of global gene expression, which results from intrasampling and intersampling biases. Furthermore, some of the in vitro experiments may not fully replicate the complex in vivo tumor microenvironment, which could affect the translational relevance of our results. For instance, additional functional assays, such as xenograft models, would be valuable for investigating the leukemia-initiating potential of therapy-resistant PPARG-OE AML stem cells. Future research should aim to validate these findings in larger and more diverse patient cohorts, as well as in the relevant in vivo models, to gain a better understanding of the clinical implications of targeting PPARG and related metabolic pathways.
In conclusion, we demonstrate that PPARγ-mediated induction of FAO underlies poor response to VEN/DEC treatment. In particular, DEC contributes to the acquisition of VEN/DEC resistance by increasing PPARG expression, and PPARγ antagonist or FAO inhibitor restored the sensitivity of VEN/DEC-resistant cells. These findings indicate that PPARγ may be a potential therapeutic target for patients who experience poor response to VEN/DEC therapy.
Acknowledgments
The authors are grateful to Yamashita Satoshi, Ushijima Toshikazu, Oleg Gusev, and Ruslan Deviatiiarov for useful discussions. The authors thank members of the Laboratory of Cell Biology (Biomedical Research Core Facilities, Juntendo University Graduate School of Medicine, Japan) as well as Intractable Disease Research Center, Juntendo University Graduate School of Medicine for their technical assistance and for the use of the experimental apparatus.
This work was supported, in part, by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (21K20913 and 23K14719 [K.Y.]; 23K24235 and 22H02974 [Y.T.]), International Joint Research Programs (19KK0221 [Y.T.]), Friends of Leukemia Research Fund (Y.T.), National Institutes of Health Specialized Programs of Research Excellence (SPORE) in Leukemia Project 5 MD Anderson Cancer Center (P50 CA100632-16 [M.K.]), the Cancer Prevention and Research Institute of Texas (RP180309 and RP160693 [M.K.]), and the MD Anderson Cancer Center Sister Institution Network Fund 2017 (M.K.).
Authorship
Contribution: M.K. and Y.T. designed the study, discussed experiments, and contributed to data interpretation; K.Y. performed experiments, analyzed data, and wrote the manuscript; T.W. performed experiments and analyzed data; K.S. performed experiments; A.K., S.X., and S.N. were involved in statistical analysis; A.M., Z.Z., K.H., S.K., C.D.D., Y.O., and M.A. discussed experiments and contributed to data interpretation; and all authors critically reviewed and revised the manuscript for content and approved the manuscript for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yoko Tabe, Department of Clinical Laboratory Medicine, Juntendo University Graduate School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan; email: tabe@juntendo.ac.jp.
References
Author notes
The RNA sequencing data generated in this study have been deposited in the Gene Expression Omnibus (accession code GSE270621).
The full-text version of this article contains a data supplement.