Key Points
Omacetaxine can synergize with VEN to promote loss of viability of myeloblasts.
Responses were seen in patients with MDS, suggesting that dose optimization / combination therapy is necessary in AML.
Visual Abstract
Mutations in RUNX1 (RUNX1mut) occur in 10% to 20% of patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) and are associated with poor outcomes to standard therapy. Omacetaxine mepesuccinate (OM), a semisynthetic analog of homoharringtonine, has been shown to be lethal to RUNX1mut AML cells in vitro through reduction of MCL1 and BCL-XL, and synergizes with venetoclax (VEN) in RUNX1mut AML models. We investigated the safety and efficacy of OM + VEN in relapsed/refractory RUNX1mut MDS/AML in a Bayesian Optimal Interval design. VEN 400 mg daily from days 1 to 14 and OM 1.25 mg/m2 twice daily from days 2 to 4 was selected as the recommended phase 2 dose. Twenty-four patients were treated, 22 with AML and 2 with MDS with excess blasts. There were no dose-limiting toxicities or episodes of tumor lysis syndrome. The most common grade ≥3 toxicity was infection. There were no responses in our heavily pretreated cohort of patients with AML. Both patients with MDS achieved composite complete remission and transitioned to allogeneic stem cell transplant. Treatment-induced downregulation in gene expression in the β-catenin and hedgehog signaling pathway genes were identified in peripheral blood mononuclear cells from patients who responded. As compared to nonresponders, samples from responders also exhibited reduced antiapoptotic and increased proapoptotic protein expression. OM can synergize with VEN to promote loss of viability of myeloid cells. Clinical responses were seen exclusively in patients with MDS, which suggests that dose optimization or combination with cytoreductive agents may be necessary for eliciting clinical activity in AML. This trial was registered at www.ClinicalTrials.gov as #NCT04874194.
Introduction
Myeloid malignancies including acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) are malignant clonal disorders with poor clinical outcomes. Most patients with AML or MDS are older at diagnosis, in whom intensive chemotherapeutic options and allogeneic stem cell transplants (SCTs) are often contraindicated due to advanced age, poor performance status, and/or comorbidities. For most older patients with newly diagnosed AML, treatment with hypomethylating agent (HMA) based therapies are standard of care, alone or in combination with venetoclax (VEN). Although initial response is observed in up to two-thirds of patients with high-risk myeloid malignancies, relapse after HMA-based therapies are nearly universal and outcomes at the time of “HMA failure” are dismal.
Runt-related transcription factor 1 (RUNX1) is a key hematopoietic transcription factor that regulates genes involved in myeloid differentiation.1 Mutations in RUNX1 (RUNX1mut) occur in ∼10% to 20% of AML and ∼15% to 25% of MDS, with an increased frequency in AML with antecedent hematologic disorders or “secondary AML,” older age, and male sex.2,3 The majority of RUNX1mut in MDS or AML are missense, deletions, or truncation mutations, leading to loss of function of the mutated allele. RUNX1mut correlate with poor clinical outcomes, including chemotherapy resistance and higher incidence of refractory disease.2,4 There are no current targeted therapeutic strategies for patients with RUNX1mut malignancies.
We previously reported that knockdown of RUNX1 (wild-type and mutant) leads to increased lethal activity in AML blast progenitor cells expressing mutant vs wild-type RUNX1.5 Using an RNA sequencing (RNA-Seq) signature of RUNX1-depleted (by short hairpin RNA) AML cells, we conducted LINCS (Library of Integrated Network–based Cellular Signatures) Connectivity Mapping analysis and identified several expression mimickers, including the top-ranking protein-translation inhibitor homoharringtonine (HHT) and the semisynthetic analog omacetaxine mepesuccinate (OM).5,6 OM is a highly purified HHT compound (99.7% purity) and acts by inhibiting protein synthesis by ribosomes.7 OM is approved by the US Food and Drug Administration for chronic or accelerated phase chronic myeloid leukemia progressing after at least 2 tyrosine kinase inhibitors. Subsequent preclinical work demonstrated efficacy of HHT and OM against AML cell lines (OCI-AML5 and Mono-Mac-1) and patient-derived primary AML blast progenitor cells, with increased lethality in RUNX1mut samples.8 The differential lethal activity of HHT in the AML cells was associated with marked HHT-mediated depletion of c-Myc, c-Myb, PU.1, mutant and wild-type RUNX1, and MCL1 in a CRISPR-Cas9–mediated knockin cell models of RUNX1mut (AML HL60-R174∗ and OCI-AML2-R174∗) cells. Additionally, cotreatment of HHT with VEN, synergistically induced apoptosis of OCI-AML5, HL60-R174∗, and OCI-AML2-R174∗ cells, and cotreatment of OM and VEN exerted significant reduction in AML burden from the luciferized OCI-AML2-R174∗ cells engrafted into NSG mice. The combination of OM and VEN improved overall survival (OS) of the NSG mice engrafted with OCI-AML2-R174∗ cells, without inducing weight loss or other apparent toxicity.8
Given the poor outcomes in relapsed or refractory patients with RUNX1mut MDS or AML, and our preliminary findings strongly supporting the potential of OM and VEN for RUNX1-mutated myeloid malignancies, we designed a phase 1 and 2 clinical trial of omacetaxine and VEN for patients with relapsed/refractory (R/R) AML or MDS harboring RUNX1mut.
Methods
We designed a single-center, investigator-initiated 2-arm nonrandomized clinical trial. The study was designed to assess the safety, tolerability, and efficacy of the combination of OM and VEN for patients with R/R AML (arm A) or MDS (arm B). The trial was approved by the institutional review board, and the study was performed in accordance with the Declaration of Helsinki. All enrolled patients provided informed consent before initiation of study procedures.
Patients aged ≥18 years with R/R AML or MDS, an Eastern Cooperative Oncology Group performance status of ≤2, adequate organ function and no active uncontrolled clinically significant medical condition, were eligible. Documented RUNX1mut was required and was confirmed locally via next-generation sequencing (sensitivity of variant allele frequency >2%).
Study procedures
The phase 1b portion was designed to evaluate the safety and tolerability of the combination of OM and VEN in cohorts of 3 patients each, for patients with R/R AML (arm A) and MDS (arm B), respectively. The starting dose (dose level 0) in both arms was OM 1.25 mg/m2 subcutaneous injection twice daily on days 2 to 4, with VEN 400 mg orally daily on days 1 to 10 per treatment cycle. The +1-dose level increased the VEN duration to 400 mg orally daily on days 1 to 14 per treatment cycle, if there were no dose-limiting toxicities (DLTs) at dose level 0. A phase 2 portion was planned for both arms (up to 30 patients per arm) once the recommended phase 2 dose (RP2D) for the respective arm was determined in the phase 1b portion.
Treatment could continue for a total of 12 cycles in patients who responded. Dose modifications and/or interruptions were permitted as necessary in the setting of treatment-related toxicity for the benefit of the patient. In the first cycle, the dose of VEN was increased in a standard ramp up over the first 3 days from 100 mg on day 1 to the target dose of 400 mg on day 3 to mitigate the risk of tumor lysis syndrome. VEN doses were adjusted for concomitant administration of CYP3A inhibitors such as azole antifungals.
Bone marrow aspiration and biopsy was performed during screening and at the completion of cycles 1, 3, 5, and every 3 cycles thereafter. Correlative studies were collected on bone marrow samples and from peripheral blood on day 1, 2, 5, 15, end of cycle 1 (EOC1), and at the time of progression.
Outcome measures
The primary safety outcome was DLT of the combination of omacetaxine and VEN. The overall incidence and severity of all adverse events (AEs) were graded according to the Common Terminology Criteria for AEs (version 5.0; National Cancer Institute).
The primary efficacy outcome was the overall response rate (ORR). ORR in patients with AML was defined as the proportion of patients with complete remission (CR), CR with incomplete hematologic recovery (CRh), CR with incomplete count recovery (CRi), partial response (PR) or morphologic leukemia-free state (MLFS) within 3 months of treatment initiation. Response definitions for patients with AML were as outlined in the European LeukemiaNet (ELN) 2022 guidelines.9 ORR in patients with MDS was defined as the proportion of patients with CR, CR with limited count recovery, partial response, hematologic improvement lasting >8 weeks within 3 months of treatment initiation. Response definitions for MDS followed those outlined in the International Working Group consensus proposal.10 OS was defined as the time from treatment initiation to death from any cause or censored at last follow-up.
Statistical considerations
In phase 1b part, the Bayesian Optimal Interval design11 was used to guide dose escalation/de-escalation in each arm, based on a target DLT rate of 0.3. In phase 2 portion, the efficacy and toxicity were monitored simultaneously using the Bayesian optimal phase 29 design in each arm. The combination treatment was deemed inefficacious if the ORR was <20% in patients with AML, or <15% in patients with MDS. A threshold of 20% was used for toxicity monitoring in each arm.
Patient characteristics were summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. Response rates were estimated along with the exact 95% confidence interval (CI). OS was estimated using the Kaplan-Meier method. Statistical analysis was performed with R version 4.3.1 (R Foundation, Vienna, Austria).
Correlatives
Key exploratory objectives included investigation of global gene expression profiles and other potential prognostic markers to explore predictors of antitumor activity and/or resistance to treatment.
Transcriptome analysis
Total RNA was isolated from patient peripheral blood samples using a PureLink RNA Mini kit from Ambion, Inc, following the manufacturer’s protocol (Austin, TX). After quality control and assessment of the total RNA, low-input strand-specific messenger RNA (mRNA) libraries were prepared. Sequencing libraries were prepared with External RNA Controls Consortium (ERCC) spike-in controls in the MD Anderson Cancer Center DNA Sequencing and Microarray Core facility and sequenced on an Illumina NovaSeq 6000 Sequencing System (RRID:SCR_020150). Data were mapped using STAR and SAMtools (STAR [RRID: SCR_004463] and SAMtools [RRID: SCR_002105])12,13 onto the human genome build University of California, Santa Cruz (UCSC) hg38 (National Center for Biotechnology Information [NCBI] 51) for human data. Gene expression was assessed using DESeq214 (DESeq2, RRID: SCR_015687), then variance stabilization and quantile normalization were applied. We considered that significance was achieved for fold changes ≥1.25×, up or down relative to the untreated cells, and P values <.05. The final P values were adjusted using the Benjamini-Hochberg method.15 We inferred enriched pathways using the Gene Set Enrichment Analysis method (RRID: SCR_003199),16 and the gene set collection from the Molecular Signature Database17 (RRID:SCR_016863).
Single-cell next-generation mass cytometry “CyTOF” analysis
Mononuclear cells were isolated from primary, patient-derived mutant RUNX1–expressing MDS and AML cells and cryopreserved in 90% fetal bovine serum and 10% dimethyl sulfoxide until time of analysis. Upon thawing of the sample, cells were washed with phosphate-buffered saline (PBS) and blocked with staining buffer (0.5% bovine serum albumin/PBS) for 30 minutes, then a cocktail of extracellular antibodies (CLEC12A, CD99, CD117, CD86, CD14, CD34, CD244, and CD11b) conjugated to transition element isotopes were added and incubated for 1 hour at room temperature (RT). For viability staining, a 5 μM concentration of cisplatin was added and incubated at the RT for 2 minutes. Cells were washed with the staining buffer, centrifuged at 500g for 5 minutes and the staining buffer was vacuum aspirated. Cells were fixed with 100 μL of 1.6% paraformaldehyde (PFA) for 10 minutes at RT. Following this, cells were permeabilized with 900 μL of ice-cold 100% methanol (90% volume) at −20°C for at least 20 minutes. Next, cells were washed with 1 mL of staining buffer to remove the PFA/methanol solution. Cells were blocked in 50 μL of staining buffer for 30 minutes and a cocktail of intracellular antibodies conjugated to transition element isotopes was added to be used as tags in atomic mass spectrometric analysis of the cells (EVI1, BAX, p21, PU1, PUMA, c-MYC, BFL1/A1, BRD4, MCL1, BCL2, and cl PARP). Cells were incubated for 1 hour at RT, then washed with staining buffer at 500g for 5 minutes. Intercalator was added (500 μL of 1:1000 Ir-intercalator diluted in 1.6% PFA/1× PBS) and cells were incubated overnight at 4°C. Cells were washed twice (500g for 5 minutes per wash) in staining buffer, then counted using a Countess II counting device. Following the last wash, 1 × 106 cells were suspended in 100 μL of deionized water and incubated overnight at 4°C. Time-of-flight mass spectrometry (CyTOF) measured multiple different cellular parameters simultaneously in each cell. The absolute fold change of protein expression changes nonresponder over responder cells within the CLEC12A+, CD99+, CD117+, CD86low, and CD11blow population was analyzed by the Astrolabe Cytometry Platform (Astrolabe, Fort Lee, NJ). In the context of response to therapy (cycle 1 day 5 [C1D5]/C1D1), the absolute fold change of protein expression was determined from the bulk population of cells.
This study was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board (approval no. 2020-0890).
Results
Patients
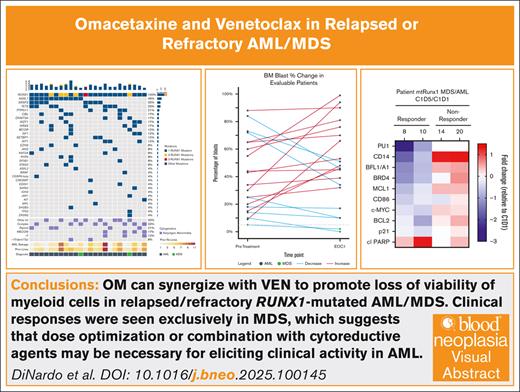
From December 2021 to September 2023, a total of 24 patients were enrolled, 22 with AML and 2 with MDS. Baseline characteristics for all cohorts are shown in Table 1. Median age was 72 years (range, 30-85) with 71% being male. RUNX1 mutations were confirmed in all patients, with a median variant allele frequency of 41% (range, 1-91). Five patients had 2 RUNX1mut, whereas 1 patient had 3 distinct RUNX1mut. Recurring hot spot RUNX1mut were rare, with most mutations at different points along the RUNX1 gene, corresponding to different amino acid changes (Figure 1).
Baseline characteristics
| . | Whole cohort (N = 24), n (%) . |
|---|---|
| Age, y | |
| Median (Min, Max) | 72 (30, 85) |
| ≥60 | 20 (83) |
| Sex | |
| Female | 7 (29) |
| Male | 17 (71) |
| Race | |
| White | 20 (83) |
| Black or African American | 3 (12) |
| Asian | 1 (4) |
| Previous SCT | |
| 2 | 2 (8) |
| 1 | 8 (33) |
| No | 14 (58) |
| Prior venetoclax | 21 (88) |
| No. of AML salvage therapies | |
| Median (Min, Max) | 3 (1, 11) |
| ≥3 | 14 (58) |
| No. of previous lines (including AHN) | |
| Median (Min, Max) | 4 (1, 11) |
| ≥3 | 17 (71) |
| Secondary AML | 14 (58) |
| ELN 2022 risk stratification | |
| Favorable | 0 (0) |
| Intermediate | 0 (0) |
| Adverse | 24 (100) |
| Cytogenetics | |
| Favorable | 0 (0) |
| Diploid | 5 (21) |
| Intermediate nondiploid | 8 (33) |
| Adverse noncomplex | 1 (4) |
| Complex | 8 (33) |
| Insufficient metaphases | 2 (8) |
| Mutations | |
| ASXL1 | 11 (46) |
| BCOR | 3 (12) |
| CBL | 4 (17) |
| DNMT3A | 4 (17) |
| FLT3ITD | 0 (0) |
| NPM1 | 0 (0) |
| NRAS | 4 (17) |
| PTPN11 | 5 (21) |
| RUNX1 | 24 (100) |
| SF3B1 | 2 (8) |
| SRSF2 | 8 (33) |
| TET2 | 8 (33) |
| TP53 | 1 (4) |
| WT1 | 3 (12) |
| ZRSR2 | 1 (4) |
| Splicing | 11 (46) |
| Signaling | 12 (50) |
| Tumor Suppressor | 6 (25) |
| Methylation | 12 (50) |
| Chromatin Modifiers | 12 (50) |
| Cohesin | 2 (8) |
| Median mutation count (Min, Max) | 5 (2, 9) |
| . | Whole cohort (N = 24), n (%) . |
|---|---|
| Age, y | |
| Median (Min, Max) | 72 (30, 85) |
| ≥60 | 20 (83) |
| Sex | |
| Female | 7 (29) |
| Male | 17 (71) |
| Race | |
| White | 20 (83) |
| Black or African American | 3 (12) |
| Asian | 1 (4) |
| Previous SCT | |
| 2 | 2 (8) |
| 1 | 8 (33) |
| No | 14 (58) |
| Prior venetoclax | 21 (88) |
| No. of AML salvage therapies | |
| Median (Min, Max) | 3 (1, 11) |
| ≥3 | 14 (58) |
| No. of previous lines (including AHN) | |
| Median (Min, Max) | 4 (1, 11) |
| ≥3 | 17 (71) |
| Secondary AML | 14 (58) |
| ELN 2022 risk stratification | |
| Favorable | 0 (0) |
| Intermediate | 0 (0) |
| Adverse | 24 (100) |
| Cytogenetics | |
| Favorable | 0 (0) |
| Diploid | 5 (21) |
| Intermediate nondiploid | 8 (33) |
| Adverse noncomplex | 1 (4) |
| Complex | 8 (33) |
| Insufficient metaphases | 2 (8) |
| Mutations | |
| ASXL1 | 11 (46) |
| BCOR | 3 (12) |
| CBL | 4 (17) |
| DNMT3A | 4 (17) |
| FLT3ITD | 0 (0) |
| NPM1 | 0 (0) |
| NRAS | 4 (17) |
| PTPN11 | 5 (21) |
| RUNX1 | 24 (100) |
| SF3B1 | 2 (8) |
| SRSF2 | 8 (33) |
| TET2 | 8 (33) |
| TP53 | 1 (4) |
| WT1 | 3 (12) |
| ZRSR2 | 1 (4) |
| Splicing | 11 (46) |
| Signaling | 12 (50) |
| Tumor Suppressor | 6 (25) |
| Methylation | 12 (50) |
| Chromatin Modifiers | 12 (50) |
| Cohesin | 2 (8) |
| Median mutation count (Min, Max) | 5 (2, 9) |
AHN, antecedent hematologic neoplasm; ELN, European LeukemiaNet; Max, maximum; Min, minimum.
RUNX1 mutations lollipop plot showing distribution and type of RUNX1 mutations.
RUNX1 mutations lollipop plot showing distribution and type of RUNX1 mutations.
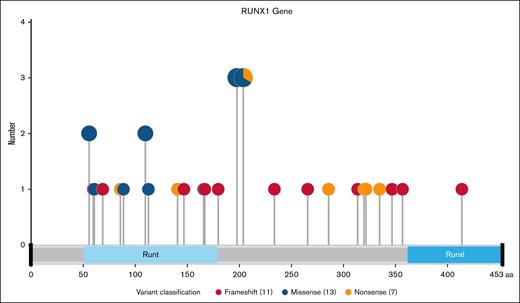
Co-occurring mutations were common, especially in ASXL1 (46%), SRSF2 (33%), TET2 (33%), and PTPN11 (21%). The median number of mutations per patient including RUNX1mut was 5 (range, 2-9). Cytogenetics were most commonly complex or intermediate nondiploid (33% each), with diploid karyotypes in 21%.
Fourteen (64%) of patients with R/R AML were considered secondary AML due to either therapy-related AML in 9 and/or antecedent hematologic neoplasm in 12. The 2 patients with MDS were classified as MDS with excess blasts 1 and 2, respectively. The International Prognostic Scoring System revised and molecular scores were 7.5 and 1.63 for the first patient, and 6.5 and 2.18 for the second patient. All 4 scores correspond to very high-risk disease.
Patients were heavily pretreated, with a median number of 4 (range, 1-11) previous lines of therapy. Disease characteristics are shown in Figure 2. Twenty-one (88%) of patients had previous exposure to VEN, whereas 10 (42%) had a previous allogeneic SCT, including 2 patients who had received 2 previous transplants.
Baseline disease characteristics. The mutational and cytogenetic profile of patients enrolled in this study are shown in the heat map. Previous lines of Rx are divided into salvage number for AML and total number of previous lines, including number of lines of Rx for any antecedent hematologic neoplasm. Of the 24 patients enrolled, 22 had AML and 2 had MDS. Other Int, other intermediate nondiploid karyotypic abnormalities; Rx: treatment.
Baseline disease characteristics. The mutational and cytogenetic profile of patients enrolled in this study are shown in the heat map. Previous lines of Rx are divided into salvage number for AML and total number of previous lines, including number of lines of Rx for any antecedent hematologic neoplasm. Of the 24 patients enrolled, 22 had AML and 2 had MDS. Other Int, other intermediate nondiploid karyotypic abnormalities; Rx: treatment.
Safety
Patients received a median of 1 (range, 1-5) cycle of OM + VEN therapy. Treatment was generally well tolerated. The most common treatment-related AEs of any grade, regardless of attribution, included infections and fatigue. AEs are shown in Table 2. No tumor lysis syndrome events were reported. 30- and 60-day mortality were 8% and 21%, respectively. Of the deaths within 60 days, all were attributable to progressive AML and complications of the disease.
AEs
| Category . | AE . | Grade 1-2, n (%) . | Grade 3-4, n (%) . |
|---|---|---|---|
| Infections and infestations | Febrile neutropenia | 0 (0) | 8 (33) |
| Pneumonia | 0 (0) | 6 (25) | |
| COVID-19 | 0 (0) | 4 (17) | |
| Sepsis | 0 (0) | 3 (13) | |
| Cellulitis | 0 (0) | 1 (4) | |
| Soft tissue infection | 0 (0) | 1 (4) | |
| General disorders and administration site conditions | General disorders and administration site conditions: other | 1 (4) | 4 (17) |
| Fatigue | 0 (0) | 1 (4) | |
| Respiratory, thoracic, and mediastinal disorders | Hypoxia | 0 (0) | 2 (8) |
| Syncope | 0 (0) | 1 (4) | |
| Respiratory, thoracic, and mediastinal disorders: other | 0 (0) | 1 (4) | |
| Investigations | Hypokalemia | 1 (4) | 2 (8) |
| Ejection fraction decreased | 0 (0) | 1 (4) | |
| Renal and urinary disorders | Renal failure | 0 (0) | 1 (4) |
| Cardiac disorders | Atrial fibrillation | 0 (0) | 1 (4) |
| Musculoskeletal and connective tissue disorders | Generalized muscle weakness | 0 (0) | 1 (4) |
| Injury, poisoning, and procedural complications | Fall | 0 (0) | 1 (4) |
| Psychiatric disorders | Confusion | 1 (4) | 1 (4) |
| Category . | AE . | Grade 1-2, n (%) . | Grade 3-4, n (%) . |
|---|---|---|---|
| Infections and infestations | Febrile neutropenia | 0 (0) | 8 (33) |
| Pneumonia | 0 (0) | 6 (25) | |
| COVID-19 | 0 (0) | 4 (17) | |
| Sepsis | 0 (0) | 3 (13) | |
| Cellulitis | 0 (0) | 1 (4) | |
| Soft tissue infection | 0 (0) | 1 (4) | |
| General disorders and administration site conditions | General disorders and administration site conditions: other | 1 (4) | 4 (17) |
| Fatigue | 0 (0) | 1 (4) | |
| Respiratory, thoracic, and mediastinal disorders | Hypoxia | 0 (0) | 2 (8) |
| Syncope | 0 (0) | 1 (4) | |
| Respiratory, thoracic, and mediastinal disorders: other | 0 (0) | 1 (4) | |
| Investigations | Hypokalemia | 1 (4) | 2 (8) |
| Ejection fraction decreased | 0 (0) | 1 (4) | |
| Renal and urinary disorders | Renal failure | 0 (0) | 1 (4) |
| Cardiac disorders | Atrial fibrillation | 0 (0) | 1 (4) |
| Musculoskeletal and connective tissue disorders | Generalized muscle weakness | 0 (0) | 1 (4) |
| Injury, poisoning, and procedural complications | Fall | 0 (0) | 1 (4) |
| Psychiatric disorders | Confusion | 1 (4) | 1 (4) |
Of note, there were no episodes of unexplained hypotension in our study, with the sole grade ≥3 cardiac toxicity being atrial fibrillation in a patient, who also had ongoing sepsis.
Efficacy
In the phase 1b portion of the AML arm, 3 patients were treated at dose level 0 and 9 at dose level +1. No DLTs were reported. Dose level +1 was confirmed as the RP2D for AML and the study moved to the phase 2 portion, with 10 patients with AML treated at the RP2D. Due to lack of efficacy during an interim analysis, the AML arm was closed. In the phase 1b portion of the MDS arm, 2 patients were treated before the study was closed by the sponsor.
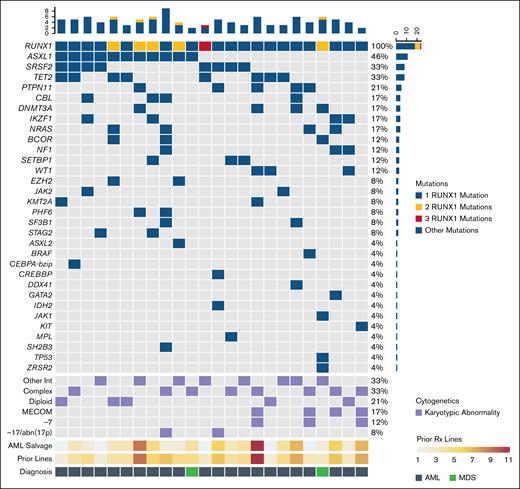
Of the 24 patients enrolled in the study, 3 were not evaluable for response. Two patients died of AML-related complications before EOC1 disease assessment time point, whereas 1 withdrew consent. Of the 21 evaluable patients, 19 had AML and 2 had MDS. None of the 19 (0%; 95% CI, 0-18) patients with AML responded to omacetaxine + VEN. The 2 patients with MDS (100%; 95% CI, 16-100) both responded, achieving CR with unilineage (absolute neutrophil count >1 × 109/L) and bilineage (absolute neutrophil count >1 × 109/L and platelets >100 × 109/L) recovery, respectively, and both patients transitioned successfully to SCT. Changes between pretreatment and EOC1 bone marrow blast percentages are shown in Figure 3. The median OS for the whole cohort of 24 enrolled patients was 4 months (95% CI, 3-9); the 1-year OS was 17% (95% CI, 7-41).
Change in blast percentage in evaluable patients. The change in blast count before and after the first cycle of omacetaxine and VEN is shown. Blast count was quantified by morphology. BM, bone marrow.
Change in blast percentage in evaluable patients. The change in blast count before and after the first cycle of omacetaxine and VEN is shown. Blast count was quantified by morphology. BM, bone marrow.
Translational correlates
First, we determined the differences in expression of the relevant myeloid stem cells related and apoptosis-related proteins in the cells from pretreatment samples (C1D1) of the peripheral blood mononuclear cells (PBMNCs) harvested from patients who responded vs those who did not respond to subsequent treatment with OM and VEN. For this, we used the CD34+ stem/progenitor cells, phenotypically confirmed due to expression of CLEC12A, CD99, and CD117, while concomitantly expressing lower levels of CD86. These cells from 2 nonresponders (patients 14 and 20) were compared to the PBMNCs from the responders (patients 8 and 10). The AML samples were chosen based on availability of biomaterial from the pretreatment and day 5 posttreatment collections. There were no significant differences between white cell differentials on day 1 and day 5 for the 4 patients from whom samples were obtained. White blood cell counts were 1.6 × 109/L, 4.7 × 109/L, 14.9 × 109/L, and 9.5 × 109/L for patients 8, 10, 14, and 20, respectively. Patients 8, 14, and 20 had previous VEN exposure. Both patients with MDS had undergone prior SCT. Cytogenetics were complex in patient 8 and 14. Patient 10 had trisomy 8, whereas patient 20 had diploid cytogenetics. All patients had RUNX1 mutations and it was a criterion for enrollment and were consequently adverse risk. Patients 8, 10, and 14 had additional myelodysplasia-related mutations, whereas patient 20 had mutations in NF1 and WT1.
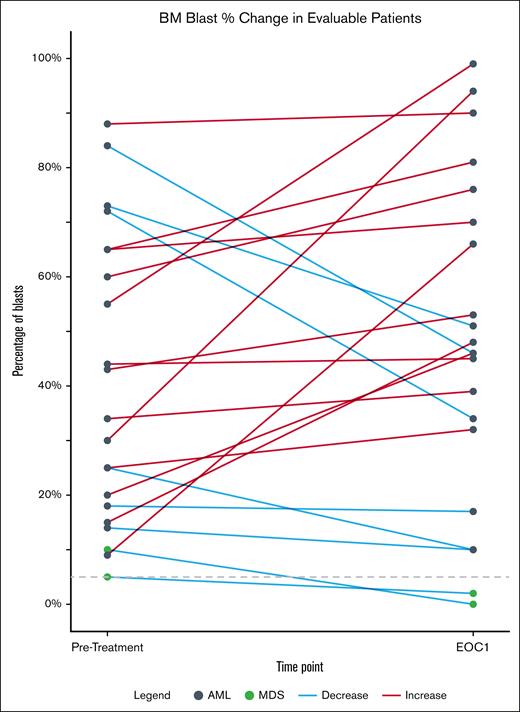
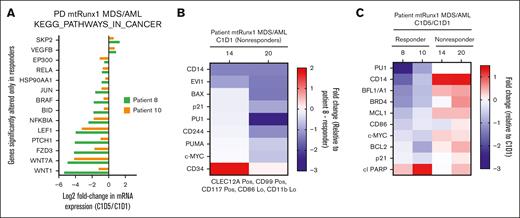
Figure 4A demonstrates that the samples from patient 20 and patient 14, compared to the sample from a responder (patient 8), had significantly lower expression of EVI1, PU.1, and c-Myc, as well as BAX, p21, PUMA, and CD244. We next determined the in vivo effects of OM and VEN treatment on the mRNA expression of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway genes via RNA-Seq analysis in the 2 responders with MDS (8 and 10) vs the 2 nonresponders with AML. Figure 4B demonstrates that, in the PBMNCs obtained at C1D5 after treatment vs C1D1, there was a decline in the mRNA expression exclusively in the PBMNCs from the patients with MDS who responded. This included genes in the WNT–β-catenin pathway genes, including WNT1, WNT7A, FZD3, and LEF1, as well as of the hedgehog pathway gene PTCH1. Using the available, metal-conjugated antibodies followed by CyTOF analysis, we also determined the protein expression alterations in the PBMNCs. Figure 4C demonstrates that, in the PBMNCs from the responders but not the nonresponders, the in vivo OM treatment attenuated c-Myc, PU.1, BFL1, MCL1, and BCL-2 protein expression.
Differential protein and mRNA expression in PBMNCs from nonresponder vs responder with MDS/AML after treatment with omacetaxine and VEN. (A) CyTOF analysis demonstrating myeloid and apoptosis-related proteins differentially expressed at the baseline (C1D1) in phenotypically characterized AML stem/progenitor cells from nonresponders compared to those from patients who responded. (B) mRNA gene expression was exclusively altered in PBMNCs harvested at C1D5 after treatment with omacetaxine and VEN, compared to C1D1, in the patients with MDS who responded, as determined in KEGG pathway genes via RNA-Seq and gene set enrichment analysis. Top downregulated genes include β-catenin members (LEF1, FZD3, WNT7A, and WNT1) and hedgehog signaling pathway member (PTCH1). (C) CyTOF analysis demonstrating log2-fold reduction in expression of proteins in PBMNCs after response to omacetaxine and VEN treatment (C1D5 vs C1D1), occurring only in responders (patients 8 and 10 with MDS) vs nonresponders (patients 14 and 20). Lo, low; mtRux1, mutant RUNX1; PD, patient derived; Pos, positive.
Differential protein and mRNA expression in PBMNCs from nonresponder vs responder with MDS/AML after treatment with omacetaxine and VEN. (A) CyTOF analysis demonstrating myeloid and apoptosis-related proteins differentially expressed at the baseline (C1D1) in phenotypically characterized AML stem/progenitor cells from nonresponders compared to those from patients who responded. (B) mRNA gene expression was exclusively altered in PBMNCs harvested at C1D5 after treatment with omacetaxine and VEN, compared to C1D1, in the patients with MDS who responded, as determined in KEGG pathway genes via RNA-Seq and gene set enrichment analysis. Top downregulated genes include β-catenin members (LEF1, FZD3, WNT7A, and WNT1) and hedgehog signaling pathway member (PTCH1). (C) CyTOF analysis demonstrating log2-fold reduction in expression of proteins in PBMNCs after response to omacetaxine and VEN treatment (C1D5 vs C1D1), occurring only in responders (patients 8 and 10 with MDS) vs nonresponders (patients 14 and 20). Lo, low; mtRux1, mutant RUNX1; PD, patient derived; Pos, positive.
Discussion
We demonstrate that treatment with the combination of OM + VEN is safe and feasible in patients with R/R AML and MDS, with no unexpected AEs. Limited clinical efficacy was observed in the patients with AML treated in the study, which was closed early for lack of efficacy. Only 2 patients with MDS were enrolled, and notably both responded and were able to transition to SCT. RNA-Seq and gene set enrichment analysis demonstrated that, in responders, treatment with OM + VEN altered cancer-related gene expression in the β-catenin and hedgehog signaling pathways.
Our suboptimal results in AML contrast with the favorable reports from Chinese groups. Specifically when HHT was combined with azacitidine and VEN, it produced composite complete remission (CRc) rates of 71% in R/R AML, with a 1-year OS of 62%.18 A multicenter retrospective study affirmed these results, suggesting improved CRc rates of 66% over azacitidine + VEN (44%).19 In children, an open-label, multicenter randomized trial in newly diagnosed AML of HHT-based induction vs etoposide-based induction showed a superior 3-year OS of 69% in the HHT arm.20 Lastly, 2 meta-analyses of HHT/OM-based regimens in newly diagnosed AML have demonstrated possible improved CR in HHT/OM arms.21,22 Differences in the more positive outcomes in these AML studies and the negative results in patients with AML include the use of OM, not HHT, in our study, different chemotherapy backbones, as well as a higher equivalent dose of HHT compared to the OM dose used in our study. Additionally, our OM + VEN study included a more heavily pretreated and adverse risk patient cohort.
In the Chinese studies, HHT was used at doses of 1 mg/m2 on days 1 to 7 in adults and 3 mg/m2 days 1 to 5 in children.18,20,23 Earlier dose-finding studies of HHT noted prolonged myelosuppression when doses of ≥2.5 mg/m2 per day were used.24,25 In particular, dose escalation beyond 5 mg/m2 daily resulted in frequent interruption of HHT infusion because of hypotension.25 Hypotensive episodes occurred in ∼30% of patients; myalgias occurred in 20% of patients and hyperglycemia in 57% of patients. With OM, pharmacokinetic studies suggested that therapeutic plasma concentrations could be achieved at a dose of 1.25 mg/m2 twice daily on days 1 to 14 of a 28-day cycle.26 In the United States, the Food and Drug Administration approved OM at the same dose for the treatment of chronic myeloid leukemia resistant or intolerant to at least 2 tyrosine kinase inhibitors.27,28 At this dose, grade ≥3 thrombocytopenia was seen in 76%, with grade ≥3 neutropenia in 44%. The myelosuppression led to the investigation of attenuated OM dosing in HMA-failure MDS at 1.25 mg/m2 every 12 hours for 3 days, with an ORR of 33%.29 The lower dosing of OM in combination with low-dose cytarabine has also been previously reported, with an ORR of 50% in newly diagnosed AML/MDS.30 In RR AML, a trial investigating a doublet of sorafenib and OM in adults with newly diagnosed or R/R FLT3ITD AML used doses of OM at 2 mg daily on days 1 to 7 in combination with sorafenib (400 mg twice daily) continuously in a 21-day cycle, with OM reduced to 2 mg days 1 to 5 once CRc was obtained.31 In our study, we used a 3-day course of OM at a dose of 1.25 mg/m2 twice daily. It is likely that higher doses of OM, if tolerable, will be required to achieve higher therapeutic effects in patients with AML.
Consistent with the responses seen in the 2 patients with RUNX1mut MDS treated with OM + VEN, our correlative data demonstrate that MDS cells harboring RUNX1mut are preferentially sensitized to the in vivo OM-mediated protein translation inhibition. Further support for the lethal activity of OM + VEN in RUNX1mut MDS cells is provided by the observation that OM treatment consistently and significantly depleted mRNA expression in KEGG cancer-related pathways in the PBMNCs from the 2 responders with MDS, but not in the PBMNCs from nonresponders with AML. Additionally, after in vivo OM treatment, protein expression of c-Myc, PU.1, BFL1, MCL1, and BCL-2 were reduced. Because of its attenuating effects on the stem cell relevant gene expression, that is, WNT–β-catenin and hedgehog pathway genes, the in vivo OM treatment likely also reduced the number of stem/progenitor cells in patients with MDS who responded. Taken together, these OM-mediated effects on the transcriptome and proteome provide evidence to support its clinical efficacy in RUNX1mut MDS, but not in heavily pretreated RUNX1mut AML.
In conclusion, OM + VEN at doses of OM 1.25 mg/m2 daily for 3 days and VEN 400 mg daily for 14 days was safe but did not show activity in our cohort of R/R RUNX1mut AML. The 2 patients with MDS who enrolled in the trial achieved meaningful responses and were able to proceed to SCT. The preclinical basis for response to the combination of OM with VEN-based regimens needs further investigation. Overall, the results of our study should encourage further translational research into OM-based combinations in myeloid malignancies, particularly in RUNX1mut MDS and less heavily pretreated RUNX1mut AML.
Acknowledgments
Omacetaxine was provided free of charge by Teva Pharmaceuticals.
This research is supported, in part, by the MD Anderson Cancer Center Leukemia Support Grant (CA100632). C.D.D. is funded by the Leukemia and Lymphoma Society (grant/award number AWD00006066) and V Foundation Lloyd Family Clinical Scholar Award (grant/award number D2018–013). K.N.B. was supported by National Institutes of Health grants R01 CA262636 and R01 CA255721.
Teva Pharmaceuticals had no role in the data collection, data analysis, data interpretation, or writing of the report.
Authorship
Contribution: C.D.D., W.F., and K.N.B. designed the study; C.D.D., C.P.M., and W.-Y.J. collected and analyzed the data and wrote the manuscript; X.W. provided statistical support; X.S. performed bioinformatics analysis; C.D.D., G.M.-B., N.J.S., K.C., G.C.I., N.P., M.Y., M.A., G.B., T.M.K., N.G.D., and G.G.-M. recruited and treated patients in the study; and all authors reviewed, revised, and approved the final version of the manuscript.
Conflict-of-interest disclosure: C.D.D. reports receiving research support from AbbVie, Astex, BeiGene, Bristol Myers Squibb (BMS), Cleave, Foghorn, Jazz Pharmaceuticals, Loxo, and Servier; and receiving personal fees from AbbVie, Astellas, BMS, GlaxoSmithKline (GSK), Genmab, Genentech, Gilead, Jazz, Loxo, Notable Labs, Servier, and Schrödinger. N.G.D. has received grants or contracts from Hanmi, Cardiff Oncology, Fate Therapeutics, NovImmune, and GlycoMimetics; reports consulting fees from Arog, Novartis, Jazz Pharmaceuticals, Celgene, Syndax Pharmaceuticals, Shattuck Labs, and Agios; and reports grants or contracts and consulting fees from Daiichi Sankyo, BMS, Pfizer, Gilead Sciences Inc, Servier, Genentech, Astellas, AbbVie, ImmunoGen, Amgen, and Trillium. G.C.I. received consultancy or advisory role fees from Novartis, Kura Oncology, Syndax Pharmaceuticals, AbbVie, and NuProbe; and received research funding from Celgene, Novartis, Kura Oncology, Syndax Pharmaceuticals, Merck, Cullinan Oncology, Astex, and NuProbe. N.J.S. has received consulting fees from Pfizer, GSK, Nkarta, Autolus, and Sanofi; research funding from Takeda Oncology, Astellas Pharma, Xencor, Stemline Therapeutics, and NextCure; and honoraria from Adaptive Biotechnologies, Novartis, Amgen, Takeda Oncology, Pfizer, Astellas Pharma, Sanofi, and BeiGene. T.M.K. has received research funding from AbbVie, Amgen, Astex, BMS, Genentech, Jazz Pharmaceuticals, Pfizer, Cellenkos, Ascentage Pharma, GenFleet Therapeutics, Astellas Pharma, AstraZeneca, Amgen, Cyclacel Pharmaceuticals, Delta-Fly Pharma, Iterion Therapeutics, GlycoMimetics, Sellas, and Regeneron Pharmaceuticals; and personal fees from AbbVie, Agios, BMS, Genentech, Hikma, Jazz Pharmaceuticals, Novartis, Servier, Sellas, and PinotBio. S. Loghavi has received honoraria and consulting or advisory role fees from AbbVie, Stemline Therapeutics, BMS, Daiichi Sankyo, ImmunoGen, Arima, Qiagen, GLG, AlphaSights, Recordati, Tempus AI; research grants from Astellas and Amgen; and holds stocks in AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Courtney D. DiNardo, Department of Leukemia, The University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: cdinardo@mdanderson.org.
References
Author notes
The study data are not publicly available to respect participant confidentiality. Deidentified data are available on request from the corresponding author, Courtney D. DiNardo (cdinardo@mdanderson.org).