Key Points
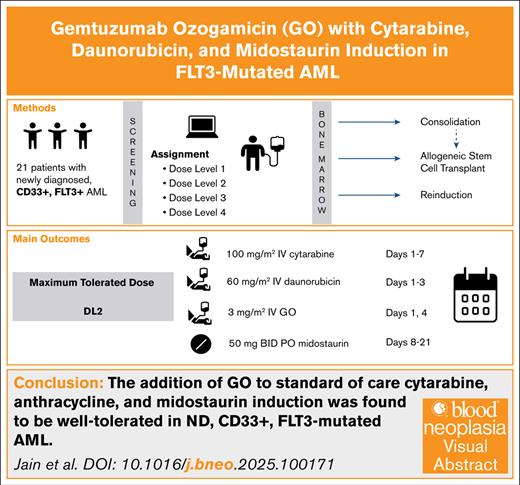
The addition of GO to standard-of-care cytarabine, anthracycline, and midostaurin induction was well tolerated in ND FLT3–mutated AML.
The maximum tolerated dose included 2 doses of GO 3 mg/m2 on days 1 and 4.
Visual Abstract
This phase 1 study investigated the addition of gemtuzumab ozogamicin (GO) to intensive chemotherapy with cytarabine, daunorubicin, and midostaurin in 21 patients with newly diagnosed (ND) FMS-like tyrosine kinase 3 (FLT3)–mutated acute myeloid leukemia (AML). Four dose levels of GO were evaluated. The use of GO was tolerable, with all dose-limiting toxicities similar to those seen in standard-of-care treatment. After induction, the median time to platelet recovery was 26 days, and the median time to absolute neutrophil count (ANC) recovery was 27 days. The maximum tolerated dose was cytarabine 100 mg/m2 on days 1 to 7, midostaurin 50 mg twice daily on days 8 to 21, daunorubicin 60 mg/m2 on days 1 to 3, and GO 3 mg/m2 on days 1 and 4. For the 18 patients who were evaluable for response after induction therapy, 16 patients (76%) achieved a composite complete response (complete remission [CR] + CR with incomplete hematologic recovery), and 2 (10%) had stable disease. Of the 14 patients who proceeded to consolidation, 5 discontinued the study for transplant, 1 for disease progression, and 1 for physician discretion. Seven patients completed consolidation therapy, all of whom achieved a CR. In total, 13 of the 21 patients (62%) received a hematopoietic stem cell transplant. Our results show that GO can safely be combined with intensive chemotherapy with midostaurin in ND, FLT3-mutated AML. This trial was registered at www.clinicaltrials.gov as #NCT03900949.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous disease characterized by distinct genetic alterations. Approximately 30% of patients with AML harbor mutations in the FMS-like tyrosine kinase 3 (FLT3) gene, including internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations. Notably, FLT3-ITD mutations are associated with a poor prognosis, increased relapse risk, and reduced overall survival (OS) compared to wild-type FLT3 AML.1-3
Standard treatment for newly diagnosed (ND) FLT3-mutated AML includes induction therapy with cytarabine and anthracycline (7+3) and a FLT3 inhibitor such as midostaurin or quizartinib, followed by consolidation therapy with or without allogeneic stem cell transplant.4,5 Although survival outcomes for patients with FLT3-mutated AML have significantly improved since the approval of midostaurin in 2017, patients with high allelic ratio of FLT3-ITD continue to have suboptimal outcomes, with a 2-year OS of 58% compared to 85% with low FLT3-ITD.6 Thus, strategies to further improve survival and reduce the risk of relapse in high allele burden FLT3-mutated AML are urgently needed.
Comprehensive flow cytometric analysis has shown that FLT3-mutated AML cells have significantly higher CD33 expression at diagnosis than non-FLT3 AML.7 Moreover, the degree of expression correlates with the FLT3 mutant allele burden, with the highest expression seen in patients with mutant to wild-type FLT3-ITD ratio of >0.78; thus, unraveling a potential role for CD33-targeted therapy in this cohort.
Gemtuzumab ozogamicin (GO) is a humanized anti-CD33 antibody conjugated to calicheamicin, a DNA damaging agent, that is currently approved for ND and relapsed/refractory CD33+ AML. Several studies have illustrated a benefit of adding GO to induction therapy in patients with good and intermediate-risk cytogenetics.8,9 Subgroup analyses from the ALFA-0701 trial demonstrated an event-free survival (EFS) benefit favoring the addition of GO to intensive induction in patients with FLT3-ITD mutations.10 Furthermore, an unplanned subgroup analysis of the UK AML15 and AML17 trials, which evaluated the addition of the FLT3 inhibitor lestaurtinib to induction and consolidation therapy in patients with ND FLT3-mutated AML, revealed a significant clinical benefit only in patients receiving concomitant GO, despite being an overall negative trial. Patients receiving GO, lestaurtinib, and chemotherapy had an impressive 5-year OS of 56% compared to 29% in those receiving GO with chemotherapy alone, implying a potential synergistic effect of treatment intensification using GO in addition to targeted FLT3 inhibition.11 Interestingly, data from the pediatric AML group also demonstrated a significant reduction in relapse risk with the addition of a single dose of GO to induction chemotherapy in patients with high FLT3-ITD ratio (>0.4), although with a higher treatment-related mortality.12
Given the increased CD33 expression on FLT3-mutated blasts and promising early clinical signals from prior studies, we sought to further investigate the potential role of GO in FLT3-mutated AML in this investigator-initiated phase 1 clinical trial. The trial evaluates the safety and preliminary efficacy of a novel frontline combination of GO in addition to standard 7+3 and midostaurin induction therapy in patients with ND FLT3-mutated AML.
Methods
This was a phase 1, open-label, dose modification trial following a Bayesian toxicity probability interval “keyboard” design to explore combination therapy with cytarabine, daunorubicin, midostaurin, and GO (funded by Pfizer, Inc; ClinicalTrials.gov identifier: NCT03900949). Patients who were aged ≥18 years with ND, CD33+, FLT3 (ITD or TKD)–mutated AML with Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and adequate organ function were eligible for enrollment. CD33 positivity and FLT3 mutations were defined per institutional standards. There was not a specific percentage of CD33+ blasts required to qualify. There was not a minimal allelic ratio or variant allele frequency required for FLT3-ITD positivity. Patients with acute promyelocytic leukemia, isolated myeloid sarcoma, or active central nervous system involvement were ineligible.
Eligible participants were administered an induction therapy regimen consisting of GO in combination with standard-of-care cytarabine, daunorubicin, and midostaurin. The induction portion of this study comprised 4 dose levels (DLs). IV cytarabine (100 mg/m2; days 1-7) and oral midostaurin (50 mg twice daily; days 8-21) were the same across all DLs. Daunorubicin 60 mg/m2 was administered on days 1 to 3 for DL1 to DL3. DL4 included daunorubicin 90 mg/m2 on days 1 to 3. GO dosed at 3 mg/m2 IV (capped to one 4.5 mg vial per dose) was given on day 1 for DL1; days 1 and 4 for DL2; and on days 1, 4, and 7 for DL3 and DL4. The use and choice of prophylactic antibacterial, antifungal, and antiviral agents were recommended according to institutional guidelines.
After initiation of this trial, data by Röllig et al presented at the American Society of Hematology Annual Meeting 2022 concluded that the 90 mg/m2 dose of daunorubicin did not lead to higher remission rates or longer survival than the 60 mg/m2 dose.13 Given these findings, we determined that DL3 would be the highest DL moving forward for the trial in progress. The protocol was amended to reflect this decision.
Eligible participants received up to 2 cycles of on-study consolidation therapy, with the trial designed to incorporate transplant. The type of consolidation therapy regimen used in this study was age dependent. For participants aged <60 years, on-study consolidation therapy consisted of standard high-dose cytarabine (3 g/m2 IV twice daily) given on days 1, 3, and 5, GO (3 mg/m2; up to one 4.5-mg vial) given on day 1 of first consolidation course only, and midostaurin (50 mg by mouth, twice daily) administered on days 8 to 21 of the 28-day cycle. On-study consolidation therapy for participants aged ≥60 years consisted of modified intermediate dose cytarabine (cytarabine 1.5-2 g/m2 IV; twice daily) given on days 1, 3, and 5, GO (3 mg/m2; up to one 4.5-mg vial) given on day 1 of the first consolidation course only, and midostaurin (50 mg by mouth; twice daily) administered on days 8 to 21 of a 28-day cycle. In each consolidation regimen, GO administration occurred only within the first cycle of consolidation. If a participant was planned for stem cell transplantation within 60 days of beginning the first cycle of consolidation therapy, omitting the planned GO dose was strongly recommended (but left to the discretion of the treating physician in consultation with the principal investigator).
The primary objective was to assess the maximum tolerated dose (MTD) with combination therapy to determine the recommended phase 2 dose. The MTD was selected based on isotonic regression as specified in Yan et al.14 Specifically, we selected the MTD as the dose for which the isotonic estimate of the toxicity rate is closest to the target toxicity rate, which is 0.2 in this study. Grade ≥3 nonhematologic toxicity related to the study drug was considered a dose-limiting toxicity (DLT), with exceptions for some transient laboratory abnormalities, infection, or constitutional symptoms. A hematologic toxicity was defined as failure to achieve ANC >500/μL or platelet count >50 x 109/L by 6 weeks after the start of a participant’s last induction cycle (including reinduction) in participants without evidence of disease. DLT assessment occurred during the induction and reinduction (if applicable) cycles for nonhematologic toxicities and up to 42 days after the start of a participant’s last induction cycle (which could be reinduction) for hematologic toxicities.
Secondary objectives include incidence of 30-day mortality, preliminary efficacy, and toxicity. Exploratory objectives included CD33 expression and correlation of single nucleotide polymorphism (SNP) status to response and clinical outcomes. All patients were included in the efficacy and safety analyses. Patients were followed for up to 2 years after completing study treatment.
The Ohio State Cancer Institutional Review Board approved the following study: OSU-21190, a phase 1 study to evaluate the safety and tolerability of gemtuzumab ozogamicin and midostaurin when used in combination with standard cytarabine and daunorubicin induction for newly diagnosed FLT3-mutated acute myeloid leukemia.
Results
Patient characteristics
Between 25 July 2020 and 5 July 2024, a total of 21 patients with ND FLT3-mutated AML were enrolled. A summary of baseline patient characteristics can be found in Table 1. The median age was 51 years (range, 20-73); 11 (52.38%) were male. There were 2 patients with favorable-risk disease. One favorable-risk patient had mutations in FLT3-TKD, NPM1, DNMT3A, and PTPN11. The other favorable-risk patient had mutations in FLT3-TKD and NPM1. Overall recurrent mutations included NPM1 (n = 16 [76%]), DNMT3A (n = 9 [43%]), CEBPA (n = 4 [19%]), IDH1 (n = 4 [19%]), and NRAS (n = 3 [14%]). The median percentage of bone marrow blasts at screening was 58% (range, 12%-91%). The degree of CD33 positivity was available for 13 of 21 patients (patients treated at The Ohio State), and CD33 positivity ranged from 93% to 100%.
Baseline patient characteristics
| Parameter . | n (%) . |
|---|---|
| Age group, mean (range), y | 51 (20-73) |
| <65 years | 17 (80.95) |
| ≥65 years | 4 (19.05) |
| Sex | |
| Female | 10 (47.62) |
| Male | 11 (52.38) |
| Race, White | 21 (100) |
| Ethnicity, non-Hispanic | 21 (100) |
| ECOG at screening | |
| 0 | 10 (48) |
| 1 | 8 (38) |
| 2 | 3 (14) |
| FLT3 status | |
| FLT3-ITD+ | 15 (71) |
| FLT3-TKD+ | 4 (19) |
| FLT3-ITD+, FLT3-TKD+ | 2 (10) |
| Comutations | |
| NPM1 | 16 (76) |
| DNMT3A | 10 (48) |
| CEBPA | 4 (19) |
| IDH1 | 3 (14) |
| NRAS | 3 (14) |
| PTPN11 | 3 (14) |
| RUNX1 | 3 (14) |
| IDH2 | 2 (10) |
| KMT2A-PTD | 2 (10) |
| U2AF1 | 2 (10) |
| WT1 | 2 (10) |
| CHEK2 | 1 (5) |
| SRSF2 | 1 (5) |
| Cytogenetics | |
| Normal karyotype | 18 (86) |
| del 20q | 1 (5) |
| +11, del 9q | 1 (5) |
| Not performable | 1 (5) |
| ELN 2022 | |
| Favorable | 2 (10) |
| Intermediate | 15 (71) |
| Adverse | 4 (19) |
| Parameter . | n (%) . |
|---|---|
| Age group, mean (range), y | 51 (20-73) |
| <65 years | 17 (80.95) |
| ≥65 years | 4 (19.05) |
| Sex | |
| Female | 10 (47.62) |
| Male | 11 (52.38) |
| Race, White | 21 (100) |
| Ethnicity, non-Hispanic | 21 (100) |
| ECOG at screening | |
| 0 | 10 (48) |
| 1 | 8 (38) |
| 2 | 3 (14) |
| FLT3 status | |
| FLT3-ITD+ | 15 (71) |
| FLT3-TKD+ | 4 (19) |
| FLT3-ITD+, FLT3-TKD+ | 2 (10) |
| Comutations | |
| NPM1 | 16 (76) |
| DNMT3A | 10 (48) |
| CEBPA | 4 (19) |
| IDH1 | 3 (14) |
| NRAS | 3 (14) |
| PTPN11 | 3 (14) |
| RUNX1 | 3 (14) |
| IDH2 | 2 (10) |
| KMT2A-PTD | 2 (10) |
| U2AF1 | 2 (10) |
| WT1 | 2 (10) |
| CHEK2 | 1 (5) |
| SRSF2 | 1 (5) |
| Cytogenetics | |
| Normal karyotype | 18 (86) |
| del 20q | 1 (5) |
| +11, del 9q | 1 (5) |
| Not performable | 1 (5) |
| ELN 2022 | |
| Favorable | 2 (10) |
| Intermediate | 15 (71) |
| Adverse | 4 (19) |
ELN, European LeukemiaNet, WT1, Wilms Tumor 1.
Dose escalation process
The dose escalation process in this study proceeded as follows: 3 patients were enrolled at each of DL1, DL2, and DL3, with no DLTs. Subsequently, for patients aged <60 years, 3 patients were treated at DL4, with 1 patient dying before completing study drugs. Based on new data from Röllig et al demonstrating a lack of benefit for daunorubicin 90 mg/m2,13 the subsequent 3 patients were enrolled at DL3, with 2 DLTs. Three patients were enrolled at the de-escalated DL2, with 1 DLT. The MTD of DL2 was selected based on the dose for which the isotonic estimate of the toxicity rate was closest to the target toxicity rate of 0.2.14
Safety
As above, 3 patients received DL1, 9 received DL2, 6 received DL3, and 3 received DL4. There were no DLTs observed on DL1 or DL4. One patient on DL2 experienced a hematologic DLT (inadequate count recovery by day 42 in the absence of disease). This patient recovered without intervention by day 49 and proceeded to consolidation. Another patient on DL2 experienced a grade 3 blood bilirubin increase. The bilirubin uptrended to grade 3 on cycle 1 day 27 in the setting of bacteremia. At that time, the patient was on broad spectrum antimicrobials including cefepime, daptomycin, and posaconazole. That same patient had a grade 3 decrease in ejection fraction to 35% to 40% from normal during induction therapy. This patient ultimately proceeded with hospice before completion of induction. Two of 6 patients on DL3 experienced DLTs for gastrointestinal toxicity, resulting in <50% of the planned midostaurin administered (receiving only 4 and 6 days of midostaurin during induction).
The most frequent adverse events (AEs) overall, regardless of attribution, were diarrhea (76%) and nausea (76%). The most frequent grade 3 AEs were hematologic, including white blood cell decrease (62%) and platelet count decrease (57%). The most common nonhematologic grade ≥3 AEs were sepsis (n = 8 [38%]) and dyspnea (n = 4 [19%]). Table 2 shows all AEs that occurred in ≥6 patients (>25%). Table 2 also shows grade 3 and 4 AEs that were reported in ≥10% of patients. There were no grade 5 AEs. As shown in Table 3, 18 patients experienced a grade 3 treatment-emergent AE, and 2 patients experienced a serious AE that was assessed as not treatment related.
Summary of most frequent AEs
| AE . | Frequency of grade 1-2, % . | Frequency of grade ≥3, % . |
|---|---|---|
| Diarrhea | 16 (76) | 1 (5) |
| Nausea | 16 (76) | 0 (0) |
| Mucositis oral | 14 (67) | 3 (14) |
| Vomiting | 13 (62) | 0 (0) |
| White blood cell count decreased | 13 (62) | 13 (62) |
| Fever | 12 (57) | 0 (0) |
| Platelet count decreased | 12 (57) | 12 (57) |
| Dyspnea | 10 (48) | 4 (19) |
| Febrile neutropenia | 10 (48) | 10 (48) |
| Neutrophil count decreased | 10 (48) | 9 (43) |
| Anemia | 8 (38) | 8 (38) |
| Constipation | 8 (38) | 0 (0) |
| Edema limbs | 8 (38) | 0 (0) |
| Fatigue | 8 (38) | 2 (10) |
| Headache | 8 (38) | 0 (0) |
| Sepsis | 8 (38) | 8 (38) |
| Insomnia | 7 (33) | 0 (0) |
| Weight loss | 7 (33) | 2 (10) |
| Abdominal pain | 6 (29) | 0 (0) |
| Cough | 6 (29) | 0 (0) |
| Dizziness | 6 (29) | 0 (0) |
| Lymphocyte count decreased | 6 (29) | 6 (29) |
| Skin and subcutaneous tissue disorder | 6 (29) | 0 (0) |
| Sore throat | 6 (29) | 0 (0) |
| Gastrointestinal disorders, other | 1 (5) | 3 (14) |
| Hypoxia | 1 (5) | 3 (14) |
| Infections and infestations, other | 0 (0) | 3 (14) |
| Lung infection | 0 (0) | 3 (14) |
| Mucositis oral | 0 (0) | 3 (14) |
| Respiratory failure | 0 (0) | 3 (14) |
| Colitis | 2 (10) | 2 (10) |
| Encephalopathy | 0 (0) | 2 (10) |
| Pleural effusion | 1 (5) | 2 (10) |
| Weight loss | 5 (24) | 2 (10) |
| AE . | Frequency of grade 1-2, % . | Frequency of grade ≥3, % . |
|---|---|---|
| Diarrhea | 16 (76) | 1 (5) |
| Nausea | 16 (76) | 0 (0) |
| Mucositis oral | 14 (67) | 3 (14) |
| Vomiting | 13 (62) | 0 (0) |
| White blood cell count decreased | 13 (62) | 13 (62) |
| Fever | 12 (57) | 0 (0) |
| Platelet count decreased | 12 (57) | 12 (57) |
| Dyspnea | 10 (48) | 4 (19) |
| Febrile neutropenia | 10 (48) | 10 (48) |
| Neutrophil count decreased | 10 (48) | 9 (43) |
| Anemia | 8 (38) | 8 (38) |
| Constipation | 8 (38) | 0 (0) |
| Edema limbs | 8 (38) | 0 (0) |
| Fatigue | 8 (38) | 2 (10) |
| Headache | 8 (38) | 0 (0) |
| Sepsis | 8 (38) | 8 (38) |
| Insomnia | 7 (33) | 0 (0) |
| Weight loss | 7 (33) | 2 (10) |
| Abdominal pain | 6 (29) | 0 (0) |
| Cough | 6 (29) | 0 (0) |
| Dizziness | 6 (29) | 0 (0) |
| Lymphocyte count decreased | 6 (29) | 6 (29) |
| Skin and subcutaneous tissue disorder | 6 (29) | 0 (0) |
| Sore throat | 6 (29) | 0 (0) |
| Gastrointestinal disorders, other | 1 (5) | 3 (14) |
| Hypoxia | 1 (5) | 3 (14) |
| Infections and infestations, other | 0 (0) | 3 (14) |
| Lung infection | 0 (0) | 3 (14) |
| Mucositis oral | 0 (0) | 3 (14) |
| Respiratory failure | 0 (0) | 3 (14) |
| Colitis | 2 (10) | 2 (10) |
| Encephalopathy | 0 (0) | 2 (10) |
| Pleural effusion | 1 (5) | 2 (10) |
| Weight loss | 5 (24) | 2 (10) |
Number of patients with AEs
| Outcome . | No. of patients (%) . |
|---|---|
| Treatment-emergent AE | 21 (100) |
| Grade ≥3 treatment-emergent AE | 18 (86) |
| Any SAE | 2 (10) |
| Any treatment-related SAE | 0 (0) |
| Outcome . | No. of patients (%) . |
|---|---|
| Treatment-emergent AE | 21 (100) |
| Grade ≥3 treatment-emergent AE | 18 (86) |
| Any SAE | 2 (10) |
| Any treatment-related SAE | 0 (0) |
SAE, serious AE.
Ten patients (48%) developed grade 3 to 4 neutropenic fever. Among the Ohio State University patients, all grade 3 to 4 neutropenic fever events were associated with bacterial infections. One patient developed fulminant Clostridium difficile colitis. Another patient had legionella with COVID-19 simultaneously. Granulocyte colony-stimulating factor was never given for neutropenic fever during induction. Two patients had grade 3 to 4 noninfectious colitis, in addition to the 2 other patients with grade 2 colitis who required holding of midostaurin as above. Three patients had grade 3 to 4 mucositis. One patient had corrected QT interval (QTc) prolongation that resolved after electrolyte repletion. As noted above, 1 patient developed grade 3 hyperbilirubinemia on DL2. A separate patient on DL2 developed grade 3 elevated alanine aminotransferase (on day 5) and aspartate aminotransferase (on day 6). Posaconazole was switched to micafungin. No study treatment was held due to these events. It resolved to grade 2 within 5 days and thus was not a DLT.
A full list of AEs occurring in the overall population are summarized in supplemental Table 1 in the supplemental Appendix. In the overall population, the 30-day all-cause mortality was 2 of 21 patients (10%). The 60-day mortality rate was 4 of 21 (19%).
Clinical efficacy
After induction therapy, 16 patients (76%) achieved a composite complete response (complete remission [CR] + CR with incomplete hematologic recovery [CRi]), and 2 (9.5%) had stable disease. Three patients (14.3%) were taken off study before the end of induction. Of these 3 patients, 1 elected for hospice, 1 had persistent disease at midcycle bone marrow biopsy, and 1 had grade 4 respiratory complications in the setting of COVID-19 and legionella infection.
Among the 18 patients who completed induction, the median time to platelet recovery was 26 days (range, 19-54). The median number of days to count recovery for each DL are as follows: 27 days for DL1 (n = 2); 25.5 days for DL2 (n = 8); 27 days for DL3 (n = 5); and 22 days for DL4 (n = 3). The median time to ANC recovery for the 18 patients was 27 days (range, 19-68). The median number of days to ANC recovery for each DL are as follows: 46.5 days for DL1 (n = 2); 26.5 days for DL2 (n = 8); 27 days for DL3 (n = 5); and 25.5 days for DL4 (n = 2). Granulocyte colony-stimulating factor was never given during induction.
Two patients proceeded to transplant before consolidation. Two patients transitioned to alternative therapy before consolidation: one came off study at the physician’s discretion after achieving CR during induction and the other for stable disease after induction.
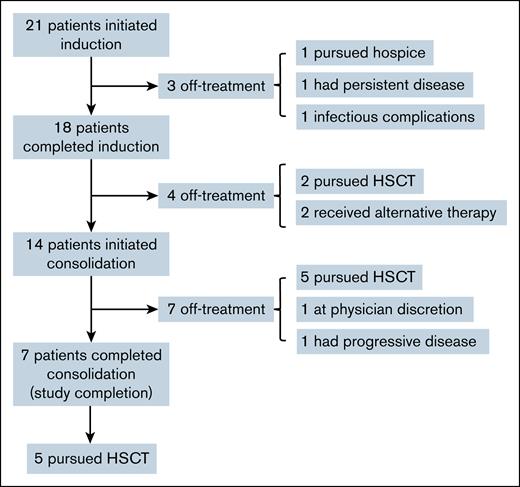
Fourteen patients proceeded to consolidation therapy. One patient came off study during consolidation at the physician’s discretion. One patient had disease progression during consolidation therapy. Five patients proceeded to transplant during consolidation. Of the 7 patients who completed consolidation, all 7 achieved CR. Five of these patients then proceeded to transplant. The CONSORT diagram in Figure 1 summarizes patient dispositions.
CONSORT diagram showing patient dispositions during therapy. In total, 13 of 21 patients (62%) underwent an HSCT. HSCT, hematopoietic stem cell transplant.
CONSORT diagram showing patient dispositions during therapy. In total, 13 of 21 patients (62%) underwent an HSCT. HSCT, hematopoietic stem cell transplant.
The measurable residual disease (MRD) status of 9 patients from OSU with NPM1 mutations was also evaluated, when available, using diagnostic polymerase chain reaction (sensitivity of 1% variant allele frequency). All patients (4/4) evaluated at the end of induction had negative NPM1. Three of these patients were evaluated at the end of cycle 2 and maintained negative MRD. The remaining patient who was NPM1 MRD negative at induction was also negative when evaluated after 4 cycles of consolidation. One additional patient who did not have MRD assessment after induction was found to be MRD negative after 4 cycles of consolidation. During all instances of MRD assessment for NPM1, all assessments were negative.
In total, 13 of 21 patients received a hematopoietic stem cell transplant (62%). Of the 13 patients who proceeded to transplant, 5 received GO during cycle 1 of consolidation. One of these patients only had 1 cycle of consolidation and developed veno-occlusive disease (VOD) after transplant. Four of these patients had a second cycle of consolidation without GO before transplant. The median number of days between the last dose of GO received and the timing of transplant was 137 days (range, 117-359). Nine patients are alive after transplant. Eleven patients have died, 5 of whom received a transplant. Three of the deaths after transplant were due to disease, and 2 were due to nonrelapse mortality.
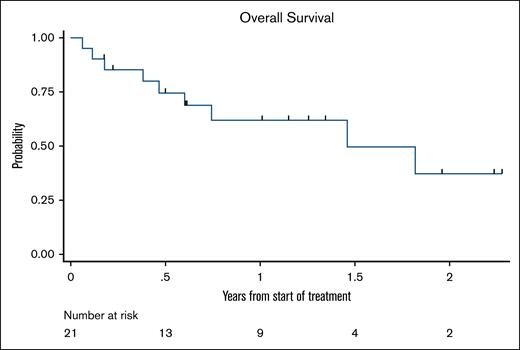
Best overall responses after induction and consolidation are summarized in Table 4. As shown in the Kaplan-Meier curve in Figure 2, OS was 79% at 6 months, 65% at 1 year, 52% at 1.5 years, and 39% at 2 years. The median survival follow-up was 367 days. The median OS was 21.8 months (95% confidence interval, 7.2 to not reached).
Responses to treatment
| Best overall response . | Induction . | Consolidation . |
|---|---|---|
| CR | ||
| Overall | 13/21 | 7/14 |
| DL1 | 0/3 | 2/2 |
| DL2 | 6/9 | 3/6 |
| DL3 | 5/6 | 1/4 |
| DL4 | 2/3 | 1/2 |
| CRi | ||
| Overall | 3/21 | 0/14 |
| DL1 | 2/3 | 0/2 |
| DL2 | 1/9 | 0/6 |
| DL3 | 0/6 | 0/4 |
| DL4 | 0/3 | 0/2 |
| SD | ||
| Overall | 2/21 | 0/14 |
| DL1 | 0/3 | 0/2 |
| DL2 | 1/9 | 0/6 |
| DL3 | 1/6 | 0/4 |
| DL4 | 0/3 | 0/2 |
| PD | ||
| Overall | 0/21 | 1/14 |
| DL1 | 0/3 | 0/2 |
| DL2 | 0/9 | 0/6 |
| DL3 | 0/6 | 0/4 |
| DL4 | 0/3 | 1/2 |
| Nonevaluable for response∗ | ||
| Overall | 3/21 | 6/14 |
| DL1 | 1/3 | 0/2 |
| DL2 | 1/9 | 3/6 |
| DL3 | 0/6 | 3/4 |
| DL4 | 1/3 | 0/2 |
| Best overall response . | Induction . | Consolidation . |
|---|---|---|
| CR | ||
| Overall | 13/21 | 7/14 |
| DL1 | 0/3 | 2/2 |
| DL2 | 6/9 | 3/6 |
| DL3 | 5/6 | 1/4 |
| DL4 | 2/3 | 1/2 |
| CRi | ||
| Overall | 3/21 | 0/14 |
| DL1 | 2/3 | 0/2 |
| DL2 | 1/9 | 0/6 |
| DL3 | 0/6 | 0/4 |
| DL4 | 0/3 | 0/2 |
| SD | ||
| Overall | 2/21 | 0/14 |
| DL1 | 0/3 | 0/2 |
| DL2 | 1/9 | 0/6 |
| DL3 | 1/6 | 0/4 |
| DL4 | 0/3 | 0/2 |
| PD | ||
| Overall | 0/21 | 1/14 |
| DL1 | 0/3 | 0/2 |
| DL2 | 0/9 | 0/6 |
| DL3 | 0/6 | 0/4 |
| DL4 | 0/3 | 1/2 |
| Nonevaluable for response∗ | ||
| Overall | 3/21 | 6/14 |
| DL1 | 1/3 | 0/2 |
| DL2 | 1/9 | 3/6 |
| DL3 | 0/6 | 3/4 |
| DL4 | 1/3 | 0/2 |
PD, progressive disease; SD, stable disease.
Three patients were taken off treatment before completion of induction because they were not candidates for further therapy and did not undergo formal disease assessment. Seven patients were taken off study before completion of consolidation. Bone marrow biopsy was not mandatory for patients discontinuing study treatment to receive a transplant. Five patients discontinued for transplant and 1 at the physician’s discretion and did not undergo formal disease assessment.
Kaplan-Meier survival curve. Of 21 patients, 12 were alive, with a median follow-up time of 367 days.
Kaplan-Meier survival curve. Of 21 patients, 12 were alive, with a median follow-up time of 367 days.
Correlative analyses
All patients were CD33+. CD33 SNP genotype was derived from 19 patient samples. Nine patients (47%) had a CC genotype, 7 patients (37%) had a CT genotype, and 3 patients (16%) had a TT genotype. OS, EFS, and progression-free survival curves for each genotype can be found in the supplemental (supplemental Figure 1).
Discussion
Advances in targeted therapeutics have ushered in a new era of treatment for patients with AML. The RATIFY trial demonstrated an improvement in OS with the addition of midostaurin to intensive induction, and the QuANTUM-First trial additionally supported the use of FLT3 inhibitors with chemotherapy.4,5 A meta-analysis of randomized control trials with GO found a reduction in relapse and improved survival at 5 years in patients with favorable- or intermediate-risk disease.8 The phase 3 ALFA-0701 trial also demonstrated improvement in EFS with the addition of GO to standard induction, and this was more pronounced in patients with FLT3-ITD.10,15 The success of FLT3 inhibition and CD33-directed immunotherapy prompts the question of using these targeted therapies in combination, given the frequent co-occurrence of FLT3 mutations and CD33 expression.
In this study of 21 patients, the MTD was identified as DL2, consisting of IV daunorubicin 60 mg/m2 (days 1-3), GO 3 mg/m2 (days 1 and 4), IV cytarabine 100 mg/m2 (days 1-7), and oral midostaurin (50 mg twice daily; days 8-21). AEs were as expected, with the most common grade 3 or 4 AEs consisting of cytopenias and febrile neutropenia. Notably, elevated bilirubin and liver transaminases were not a common AE, occurring only in 2 patients. No treatment-related deaths were observed. In this trial, 30-day mortality was 10% (2/21), and 4 additional patients (19%) were taken off study before consolidation. This appears overall comparable to patients treated with midostaurin on the RATIFY study, in which 16 of 355 patients (4.5%) died during induction and approximately one-third of patients during induction did not continue therapy or withdrew.
In a retrospective analysis, Weinbergerová et al found good tolerability with 3 doses of GO (days 1,4, and 7), with grade 1 to 2 elevated bilirubin or liver transaminases being the most common AE, followed by bleeding and infection. One patient did have grade 4 sinusoidal obstruction syndrome, although there were no treatment-related deaths in the first 30 days.16 Prospective, randomized studies by Röllig et al supported the use of 2 doses of GO with 14 days of midostaurin 50 mg twice daily, along with 3 days of daunorubicin 60 mg/m2.17,18 In the prospective, randomized NCRI AML19 “MIDOTARG” trial, there was no difference in response rates and survival between single-dose GO and fractionated GO. However, in the MIDOTARG trial, the fractionated GO schedule gave a higher proportion of MRD negativity after course 2 in patients with FLT3mut/NPM1mut, and this was associated with a greatly reduced risk of relapse and death. The results presented in this study provide further supporting evidence that 2 doses of GO is tolerable, which may be the most pertinent for achieving NPM1 MRD negativity, as seen in MIDOTARG.
Rates of CR/CRi after induction were 76% in this study, with a median OS of 21.8 months. Weinbergerová et al reported CR/CRi of 91% in their retrospective analysis.16 In their phase 1 study, Röllig et al found a CR/CRi rate of 92%,17 and the “MIDOTARG” trial reported CR/CRi rates of 88%.18 The lower CR/CRi in our study may be due to differences in patient cohorts and cytogenetic risk, with 90% of patients in this study being intermediate risk or higher based on European LeukemiaNet (ELN) 2022 criteria.
CD33 SNPs and the resulting genotypes are of particular interest, because rs12459419C>T can result in loss of the Immunoglobulin Variable (IgV) domain, which is targeted by GO. Pediatric studies have shown that the CC genotype is a predictor of response to GO, without benefit in CT or TT genotypes.19 However, studies in adults have not redemonstrated this relationship.20 Robust conclusions regarding outcomes based on SNPs cannot be made from this trial’s data given the small sample size and wide confidence interval; yet, the differences in outcomes based on SNPs is an intriguing focus for future studies.
Overall, the combination of standard induction with midostaurin and GO had expected toxicities, which were similar to prior studies of FLT3 inhibition added to intensive chemotherapy. This study supports the use of 2 doses of GO 3 mg/m2 on days 1 and 4 as the MTD. With the recent approval of quizartinib in the frontline setting for AML with FLT3-ITD mutations, trials evaluating quizartinib in combination with GO and chemotherapy are ongoing. There are no current plans to recommend this trial for future development because of several reasons. First, further dose optimization may be necessary given the history of toxicity, with GO requiring dose de-escalation for safety. Additionally, there are now approved targeted agents for FLT3 mutations, and menin inhibitors have demonstrated signs of efficacy in NPM1-mutated disease.21,22 Targeted agents are being further explored as part of combination therapy, including with azacitidine and venetoclax. Although further study is not planned, this study contributes to data exploring whether GO in this patient population is safe and whether there are any hints of efficacy.
Acknowledgment
This work was supported by Pfizer, Inc.
Authorship
Contribution: U.B. and R.B.W. conceived and designed the study; J.L., G.S.L., and R.B.W. performed and curated CD33 SNP analysis; Q.Z. analyzed results and made the figures; and K.P., S.H., and J.J. prepared the manuscript with input from all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Uma Borate, Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, The Ohio State University Comprehensive Cancer Center, 2121 Kenny Rd, Columbus, OH 43210; email: uma.borate@osumc.edu.
References
Author notes
J.J. and K.P. contributed equally to this study.
Original data are available on request from the corresponding author, Uma Borate (uma.borate@osumc.edu). Individual participant data will not be shared. No information concerning the study or the data will be released to any unauthorized third party without prior written approval of the sponsor (Uma Borate/The Ohio State University).
The full-text version of this article contains a data supplement.