Key Points
Laser-induced thrombus formation in the Endo-chip provides a humanized model for the ex vivo study of responses to endothelial injury.
The laser-injury Endo-chip model offers a fast tool for testing the efficacy of antithrombotic agents.
Visual Abstract
Mouse models, such as the intravital laser-induced model of thrombus formation, are commonly used for mechanistic and preclinical studies in thrombosis. However, their translational value is limited by species differences. Few in vitro models incorporate laser-induced vascular injury. Here, we developed an endothelialized microfluidic device, the Endo-chip, to model thrombus formation in response to laser injury. Citrated blood was treated with IXA4, an endoplasmic reticulum stress inducer, or with antithrombotic agents including antitissue factor antibody (5G9), antiplatelet drugs (abciximab, aspirin), and anticoagulants (argatroban, heparin). Fluorescently labeled antibodies (to platelets, fibrin, or von Willebrand factor [VWF]), annexin V or the calcium dye Cal520, were added to the blood. After recalcification at 10 mM, blood was perfused through the Endo-chip at a shear rate of 100 s–1 or 800 s–1. Endothelial injury was induced with a 355-nm laser pulse producing a focal 10-μm injury, and phosphatidylserine exposure on endothelial cells within ∼1 cell diameter from the injury site. Annexin V-positive endothelial cells expressed tissue factor and released VWF, supporting localized platelet and fibrin deposition. The thrombus formed a teardrop morphology aligned with flow incorporating VWF with increasing shear. IXA4 enhanced platelet cytoplasmic calcium. Platelet accumulation was inhibited by abciximab but not aspirin, whereas coagulation inhibitors (5G9, argatroban, heparin) markedly reduced thrombus formation. These findings support that the Endo-chip laser-injury model incorporates key features of thrombus formation after endothelial injury, and provides a humanized, in vitro, alternative or auxiliary to mouse models for preclinical studies and antithrombotic drug development.
Introduction
The laser-induced intravital mouse model of thrombus formation was introduced in 2002 by the Furie group, marking a significant milestone in thrombosis/antithrombotic research.1 The Furie model involved the formation of a vascular injury to the cremaster artery by a laser focused through the microscope optics. Thrombus formation was imaged in real time, after injection of fluorescently labeled antibodies to detect platelets and fibrin, using confocal and widefield microscopy. Since then, the laser-induced intravital thrombosis model has been adopted by many labs worldwide, and provided major breakthroughs in our understanding of the mechanisms of thrombosis, including the role of tissue factor (TF),1-3 von Willebrand factor (VWF),4,5 protein disulfide isomerases,6-10 and platelet endoplasmic reticulum (ER) stress,11 among others. It has also found applications in the study of some of the most severe thrombotic disorders, including the antiphospholipid syndrome,12 and heparin-induced thrombocytopenia.13
The laser-induced intravital thrombosis model has been used in preclinical studies of novel antithrombotic agents, such as flavonoids,14 recombinant fusion proteins targeting activated GPIIb/IIIa,15 and “smart” polyanion inhibitors,16 amongst others. In vivo laser-injury models are an outstanding tool to assess the impact of genetic mutations or pharmacologic treatments on platelets and coagulation within the complex milieu of an in vivo environment.
To effectively recapitulate the intravital mouse model of thrombus formation, an in vitro model should illustrate several aspects of thrombus formation. The model should contain physiological components of a blood vessel, including a functional endothelial layer and blood. It should demonstrate platelet adhesion and fibrin formation under shear, and the resultant thrombus should localize to the site of endothelial injury. We hypothesized that this process could be adapted into a microfluidic endothelialized device (the Endo-chip) such that, when perfused with whole blood and pulsed with a laser, a localized thrombus would form that would accurately reproduce features of the intravital laser-induced mouse thrombus. The Endo-chip would thus offer a reliable ex vivo model to study mechanisms of thrombus formation, hemostatic responses, and the efficacy of antithrombotics.
Materials and methods
Human ethics
Human umbilical vein endothelial cells (HUVECs) were isolated from delivered umbilical cords, after informed consent (Sydney Local Health District Ethics, approval HREC X19-0482). Venous blood was collected into tubes, containing 3.2% sodium citrate (BD Biosciences), from healthy donors after informed consent (The University of Sydney Ethics, approval 2014/244). Blood samples were used within 3 hours of collection.
Reagents
Anti-CD41 antibody (clone P2), fluorescently labeled with fluorescein isothiocyanate, was purchased from Beckman Coulter (Brea, CA). Antifibrin antibody, hybridoma clone 59D8, was from Gary Matsueda, Harvard University (Cambridge, MA). Purified antifibrin antibody was fluorescently labeled with Alexa Fluor (AF) 594 kit or AF 649 kit (Thermo Fisher Scientific, Waltham, MA), as described.17 Annexin V AF 647 and wheat germ agglutinin (WGA) AF 647 were from Thermo Fisher Scientific. Annexin V–Pacific Blue was from BioLegend (San Diego, CA). Anti-VWF antibody was from Agilent (Santa Clara, CA), and was labeled with an AF 594 kit. Calcium dye Cal520 AM was from Abcam (Cambridge, United Kingdom). Fibronectin was from Haematologic Technologies (Essex Junction, VT).
IXA4 was from Cayman Chemicals (Ann Arbor, MI). IXA4 is a selective activator of the inositol-requiring enzyme 1, which induces an ER stress response.18 Argatroban 100 mg/mL was from GlaxoSmithKline plc (London, United Kingdom). DBL heparin sodium 5000 IU/0.2 mL was from Pfizer (New York, NY). Anti-TF inhibitory antibody (clone 5G9) was from Absolute Antibody Ltd (Cleveland, United Kingdom). Abciximab (anti-αIIbβ3 inhibitory antibody, clone C7E3) was purchased from MedChemExpress LLC (Monmouth Junction, NJ). Medical grade aspirin for IV use (Aspegic) was from Sanofi Aventis (Paris, France). Polydimethylsiloxane (PDMS) kits (Sylgard 184) were from Dow Corning (Pennant Hills, NSW, Australia).
PDMS device fabrication
PDMS was fabricated from a Sylgard 184 kit according to manufacturer instructions. The kit base material was mixed with the curing agent at a ratio of 10:1. After mixing thoroughly, the final mixture was poured into silicon wafer molds imprinted with cured photoresist SU-8, and placed in a vacuum desiccator to degas. The mold was then left to cure at 60°C for 16 hours to yield a microfluidic channel of 10 000-μm length, 1000-μm width, and 100-μm height. An inlet and outlet were formed by punching holes into opposite ends of the channel using a 6-mm and 1-mm biopsy punch, respectively.
To form the device, the PDMS and a #1 borosilicate coverslip were placed in the vacuum chamber of a plasma cleaner. The chamber was then evacuated to a pressure of 0.05 to 0.07 torr. Pure oxygen was introduced to the chamber to a final pressure of 1.72 torr, and the chamber contents were then exposed to oxygen plasma for 1 minute on the highest setting. The PDMS was placed on the coverslip channel side-down, and incubated at 60°C for 15 minutes.
Device endothelialization
HUVECs were cultured in porcine gelatin-coated T25 culture flasks in M199 media supplemented with 20% volume-to-volume fetal calf serum, 2 mM l-glutamine, 100 μg/mL streptomycin, 100 U/mL penicillin, 1× minimum essential medium nonessential amino acids, 1.34 mM sodium bicarbonate, 1 mM sodium pyruvate, and 0.3% volume-to-volume endothelial cell growth supplement from Cell Applications (San Diego, CA). HUVECs were maintained in culture, and used between passage 2 to 3 at ∼80% confluency. Devices were coated with human fibronectin (50 μg/mL) for 16 hours at 4°C. After washing the fibronectin-coated PDMS channels with phosphate-buffered saline (PBS) 5 times, HUVECs were detached using 1× trypsin/EDTA, adjusted to 20 × 106 cells per mL in M199 media, and perfused through the outlet of the channel. Devices were left to incubate at 37°C, 5% CO2 for 15 to 30 minutes, and then fresh media was gently perfused through the in inlet every hour, allowing for the expansion of a confluent HUVEC monolayer.17 A schematic of the assembled device is shown in Figure 1.
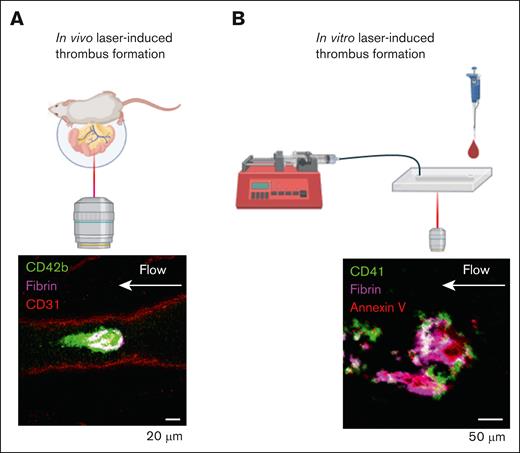
Ablation laser-injury models of thrombus formation. (A) In vivo laser-injury model performed by exteriorizing the mesenteric vein, or artery, and pulsing a high-powered laser. Representative image of platelet (CD42b, green) and fibrin (magenta) accumulation on mesenteric vein endothelium (CD31, red). (B) In vitro laser-injury model performed by pulsing a high-powered laser on the endothelialized surface of a microfluidic chip (Endo-chip). Representative image of platelet (CD41, green), fibrin (magenta), and annexin V (red).
Ablation laser-injury models of thrombus formation. (A) In vivo laser-injury model performed by exteriorizing the mesenteric vein, or artery, and pulsing a high-powered laser. Representative image of platelet (CD42b, green) and fibrin (magenta) accumulation on mesenteric vein endothelium (CD31, red). (B) In vitro laser-injury model performed by pulsing a high-powered laser on the endothelialized surface of a microfluidic chip (Endo-chip). Representative image of platelet (CD41, green), fibrin (magenta), and annexin V (red).
WGA staining
HUVECs were seeded into microfluidic devices, and allowed to form a confluent monolayer. HUVECs were then incubated with AF 647-labeled WGA in serum-free M199 media at 1 μg/mL for 1 hour at 37°C, 5% CO2. After washing 3 times with PBS, HUVECs were fixed with paraformaldehyde at 4% weight-to-volume ratio for 15 minutes, washed 3 times with PBS, stained with Hoechst 33342 at 3 μg/mL for 15 minutes, washed 3 times with PBS, and then imaged.
Blood perfusion
Citrated blood was stained for platelets with anti-CD41 fluorescein isothiocyanate (0.5 μg/mL) or anti-CD42a (0.5 μg/mL), fibrin with antifibrin AF 594 (0.5 μg/mL) or antifibrin AF 647 (0.5 μg/mL), phosphatidylserine (PS) with annexin V AF 647 (1:200), calcium with Cal520 AM (2 μM), or vWF with anti-VWF AF 594 (2 μg/mL), for 15 minutes at room temperature (RT), prior to perfusion. In some experiments, blood was also treated with IXA4 (300 μM for 2 hours at RT), or argatroban (0.5 μg/mL for 10 minutes at RT), or heparin (0.5 U/mL for 10 minutes at RT), or equivalent volume of vehicle (ethanol or PBS, respectively). In other experiments, blood was incubated with aspirin (9 μg/mL for 60 minutes), abciximab (20 μg/mL for 60 minutes), inhibitory anti-TF antibody 5G9 (50 μg/mL for 15 minutes), or equivalent volume of vehicle (NS 0.9%). The endothelialized device was connected to a syringe pump, via the outlet, and perfused with blood, set at a flow rate of 11.7 μL/min and 93.9 μL/min, resulting in a calculated venous shear rate of 100 s–1 and arterial shear rate of 800 s–1, respectively.19 Shear rates of 100 s–1 and 800 s–1 corresponded to shear stress of 3.8 dynes/cm2 and 30.4 dynes/cm2, respectively.
Laser injury
The laser injury was performed using a Rapp OptoElectronic UGA-42 firefly module (Wedel, Germany) coupled to a Zeiss 880 confocal microscope (Oberkochen, Germany) or an Olympus FV3000 (Tokyo, Japan). The Rapp module was set for a 355 nm laser pulse in a 1 μm2 area parfocal to the endothelial monolayer for 300 microseconds at a laser power of 10%, 5 consecutive times (supplemental Figure 1). This resulted in an endothelial damage of ∼10 μm2 (Figure 2A). The focal plane was adjusted to 8 μm above the site of endothelial laser injury to visualize platelet adhesion and fibrin formation. The resultant thrombus formation was imaged on a 40× water objective (NA 1.2).
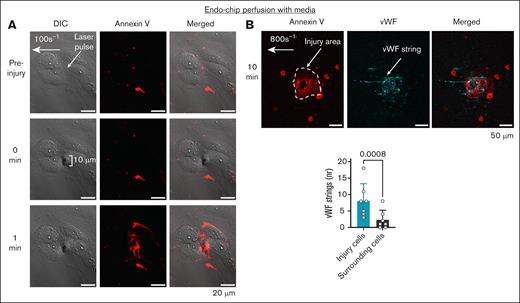
Laser injury of the perfused endothelial monolayer induces a procoagulant surface by PS exposure and secretion of VWF. (A) Laser pulse to the endothelial monolayer (arrow) perfused with media (at shear 100 s–1), induces an endothelial injury of ∼10 μm with subsequent PS exposure (measured by annexin V binding, red) within 1 minute, but does not disrupt endothelial junctions. Representative images. Scale bar, 20 μm. (B) Laser pulse to the endothelial monolayer (arrow) perfused with media under shear 800 s–1, leads to the secretion of VWF (cyan) from the injured endothelium (stained with annexin V, red). Scale bar, 50 μm. Number of strings counted on annexin V-positive cells (injury, cyan) vs surrounding annexin V-negative cells (gray). Mean ± standard deviation (SD), n = 7 injuries from n = 3 independent experiments, paired t test. DIC, differential interference contrast.
Laser injury of the perfused endothelial monolayer induces a procoagulant surface by PS exposure and secretion of VWF. (A) Laser pulse to the endothelial monolayer (arrow) perfused with media (at shear 100 s–1), induces an endothelial injury of ∼10 μm with subsequent PS exposure (measured by annexin V binding, red) within 1 minute, but does not disrupt endothelial junctions. Representative images. Scale bar, 20 μm. (B) Laser pulse to the endothelial monolayer (arrow) perfused with media under shear 800 s–1, leads to the secretion of VWF (cyan) from the injured endothelium (stained with annexin V, red). Scale bar, 50 μm. Number of strings counted on annexin V-positive cells (injury, cyan) vs surrounding annexin V-negative cells (gray). Mean ± standard deviation (SD), n = 7 injuries from n = 3 independent experiments, paired t test. DIC, differential interference contrast.
VWF release after laser injury
Endothelialized microfluidic devices were perfused with serum-free M199 media, supplemented with 1 mM CaCl2 and 1 mM MgCl2, containing 2 μg/mL of polyclonal anti-VWF AF 594 and 0.6 μg/mL of annexin V–Pacific Blue. Media was perfused at 800 s–1. After applying laser injury to the endothelial cells with the abovementioned settings, VWF string formation was imaged over 3 minutes.20 Annexin V-positive cells were counted as injured cells, while cells immediately adjacent to the injured cells were counted as surrounding cells.
Image analysis
Images were acquired every 2 seconds for 15 minutes. After 15 minutes of blood perfusion, z-stacks were taken using a 40× water immersion objective (NA 1.2) over the area of injury. Images were thresholded on ImageJ (https://imagej.net/Fiji) to produce a binarized image, and the pixels above threshold were analyzed to determine the area of coverage. The time series of the formed thrombus was split into the respective fluorescent channels for each fluorescent reagent used. Surface area coverage of platelets, fibrin, and annexin V were quantified using maximum fluorescence intensity projections generated from z-stacks. Values are reported as the calculated surface area coverage for the given field of view. For Cal520 and VWF, a mask was generated by thresholding platelet fluorescence for each frame of the time series. The fluorescence intensity of Cal520 or the AF 594 conjugate was measured within the respective mask of each frame to quantify calcium and VWF, respectively. The ratio of the fluorescence of the Cal520 dye to CD42a-PE was determined to normalize the calcium flux to the growth of the platelet thrombus.
In vivo thrombosis models
Statistical analysis
Normality of data was tested by Shapiro-Wilk and D’Agostino-Pearson tests. Analysis of parametric data between 2 groups was performed by paired Student t test. Comparison of multiple groups was performed by 1-way analysis of variance with Dunnett’s multiple comparisons test. Analysis was performed on GraphPad Prism v10.
Results
Laser injury of the Endo-chip exposes PS and releases VWF from HUVECs
The application of a laser injury to the endothelium in vivo results in PS exposure.22 A similar response was measured in vitro; when the laser injury was applied to the endothelial monolayer of the Endo-chip, there was immediate accumulation of annexin V ∼1 cell diameter from the site of injury. Annexin V accumulation did not occur elsewhere on the Endo-chip. Laser injury induced annexin V binding, but did not disrupt the endothelial junctions (Figure 2A).
The ablation laser produces a precise and localized injury to the endothelial layer. When comparing the injury induced by the same laser settings, with a set target area of 1 μm2 area, we found that the resultant injury size varied from ∼10 μm2 in a fluorescein-coated slide (supplemental Figure 1) and in the Endo-chip, equivalent to approximately half the diameter of a HUVEC (Figure 2A). The same laser settings resulted in an injury of ∼20 μm2 in the mouse mesenteric vein (Figure 3A). This implies limitations in the minimum size of the injury that can be achieved with this laser module.
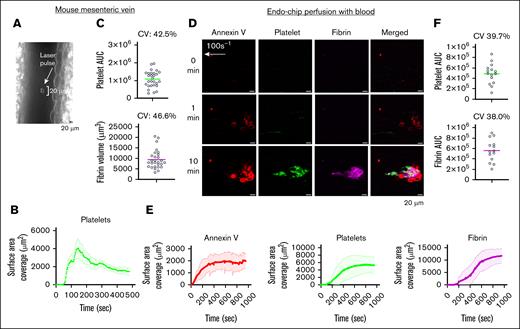
Kinetics of thrombus formation at a venous shear rate. (A) Laser pulse to the mesenteric vein endothelium induces an endothelial injury of ∼20 μm. A representative image is shown. (B) Kinetics of platelet thrombus over time after laser pulse of the mouse mesenteric vein endothelium. Mean platelet surface area coverage over time (line) and standard error of the mean (shaded line) from n = 7 thrombi from n = 3 C57BL/6 mice. (C) Platelet AUC and end point fibrin volume from n = 28 thrombi produced by laser injury of mesenteric veins in n = 9 C57BL/6 mice. Data are presented as individual values (circles), mean (line), and CV. (D) Endo-chip laser-induced thrombus formation model. Blood was incubated with annexin V, anti-CD41 antibody, and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at a venous shear rate of 100 s–1, followed by a laser pulse to the endothelium. Representative images of annexin V (red), platelets (CD41, green), and fibrin (magenta) at 0, 1, and 10 minutes from laser injury are shown. (E) Kinetics of annexin V (red), platelet (green), and fibrin (red) surface area coverage over time in the Endo-chip after laser injury. Mean surface coverage over time (line) and standard error of the mean (shaded line) from n = 3 different blood donors. (F) Platelet and fibrin AUC from 14 different donors over different days. Data are presented as individual values (circles), mean (line), and CV. AUC, area under the curve; CV, coefficient of variation.
Kinetics of thrombus formation at a venous shear rate. (A) Laser pulse to the mesenteric vein endothelium induces an endothelial injury of ∼20 μm. A representative image is shown. (B) Kinetics of platelet thrombus over time after laser pulse of the mouse mesenteric vein endothelium. Mean platelet surface area coverage over time (line) and standard error of the mean (shaded line) from n = 7 thrombi from n = 3 C57BL/6 mice. (C) Platelet AUC and end point fibrin volume from n = 28 thrombi produced by laser injury of mesenteric veins in n = 9 C57BL/6 mice. Data are presented as individual values (circles), mean (line), and CV. (D) Endo-chip laser-induced thrombus formation model. Blood was incubated with annexin V, anti-CD41 antibody, and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at a venous shear rate of 100 s–1, followed by a laser pulse to the endothelium. Representative images of annexin V (red), platelets (CD41, green), and fibrin (magenta) at 0, 1, and 10 minutes from laser injury are shown. (E) Kinetics of annexin V (red), platelet (green), and fibrin (red) surface area coverage over time in the Endo-chip after laser injury. Mean surface coverage over time (line) and standard error of the mean (shaded line) from n = 3 different blood donors. (F) Platelet and fibrin AUC from 14 different donors over different days. Data are presented as individual values (circles), mean (line), and CV. AUC, area under the curve; CV, coefficient of variation.
HUVECs seeded into the Endo-chip maintain their glycocalyx (supplemental Figure 2). Based on WGA staining, the average glycocalyx height was 2.2 ± 0.3 μm, consistent with the previously described glycocalyx height of HUVECs cultured in microfluidic channels.23 Laser injury of the HUVEC monolayer whilst perfused with media led to a rapid release of VWF. With a shear rate of 800 s–1, VWF formed strings in the direction of flow (Figure 2B).
Laser injury of the Endo-chip induces local thrombus formation
In the intravital laser-induced injury mouse model, the application of a laser pulse to a blood vessel results in the formation of a thrombus at the injury site. In our mouse model of mesenteric vein laser-induced thrombosis, peak platelet accumulation occurred at 150 seconds, which was followed by an ∼50% decrease by 400 seconds. This thrombus has a teardrop morphology, with the thrombus tail forming in the direction of flow (Figures 1 and 3B). The coefficient of variation (CV) of platelet and fibrin thrombi induced by laser injury in mouse mesenteric veins was 42.5% and 46.6%, respectively (Figure 3C), consistent with previously described.24 In comparison, in vitro laser-induced injury in the Endo-chip when perfused with blood at a venous shear rate (100 s–1) also resulted in a teardrop-shaped thrombus (Figure 3D; supplemental Video 1). However, the peak platelet accumulation was at 500 seconds, remaining stable until 1000 seconds (Figure 3E). The CV of platelet and fibrin thrombi induced by laser injury in the Endo-chip was 39.7% and 38.0%, respectively (Figure 3F). Testing the same blood donor on the same day had lower variability than when tested on different days, indicating that the preparation of the Endo-chip itself is a source of variability (supplemental Figure 3).
Intravital laser-induced thrombosis of the cremaster artery results in peak platelet accumulation at 100 seconds, followed by an ∼50% decrease by 200 seconds (supplemental Figure 4A). In vitro laser-induced injury in the Endo-chip, perfused with blood at arterial shear rate (800 s–1), resulted in a lag in the accumulation of platelets, with a gradual increase in platelet thrombus from 400 seconds until the end of capture at 1000 seconds (supplemental Figure 4B; supplemental Video 2).
TF localizes to the endothelial injury site, and inhibition of TF decreases thrombus formation in the laser-injury Endo-chip
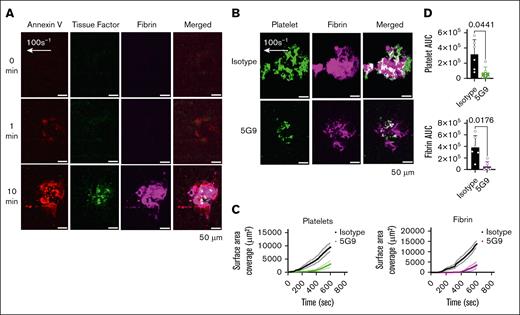
We have previously demonstrated endothelial expression of TF in the Endo-chip after stimulation with an inflammatory stimulus (tumor necrosis factor α [TNF-α]).20 In the laser-injury model, we measured increased binding of fluorescently labeled anti-TF antibody 5G9 over time, supporting the expression of TF within the injury site (Figure 4A). Inclusion of the anti-TF antibody 5G9 at inhibitory doses in the whole blood (50 μg/mL) resulted in significant inhibition of thrombus formation (Figure 4B-D).
TF localizes to the endothelial injury site, and its inhibition reduces thrombus formation in the laser-injury Endo-chip. (A) Blood was labeled with annexin V-AF647, anti-TF antibody 5G9-Atto488 (2 μg/mL, detection dose), and anti-fibrin-594 antibody, recalcified with CaCl2 (10 mM), and then perfused through the Endo-chip at 100 s–1 followed by laser injury to the endothelial layer. Representative images of annexin V (red), TF (green), fibrin (magenta) at 0, 1, and 10 minutes after laser injury. (B) Blood was incubated with isotype control or inhibitory anti-TF antibody 5G9 (50 μg/mL, inhibitory dose) and then labeled with anti-CD41 antibody and antifibrin antibody, recalcified, and immediately perfused through the Endo-chip at 100 s–1 prior to laser injury. Representative images of platelet (CD41, green) and fibrin (magenta) accumulation at the injury site at 10 minutes. (C) Kinetics of platelet (left panel) and fibrin (right panel) surface area coverage over time in response to isotype control or 5G9. (D) Platelet (upper panel) and fibrin (lower panel) AUC after treatment of blood with isotype control or inhibitory anti-TF antibody 5G9. Mean ± SD, n = 6 independent experiments, paired t test. AUC, area under the curve.
TF localizes to the endothelial injury site, and its inhibition reduces thrombus formation in the laser-injury Endo-chip. (A) Blood was labeled with annexin V-AF647, anti-TF antibody 5G9-Atto488 (2 μg/mL, detection dose), and anti-fibrin-594 antibody, recalcified with CaCl2 (10 mM), and then perfused through the Endo-chip at 100 s–1 followed by laser injury to the endothelial layer. Representative images of annexin V (red), TF (green), fibrin (magenta) at 0, 1, and 10 minutes after laser injury. (B) Blood was incubated with isotype control or inhibitory anti-TF antibody 5G9 (50 μg/mL, inhibitory dose) and then labeled with anti-CD41 antibody and antifibrin antibody, recalcified, and immediately perfused through the Endo-chip at 100 s–1 prior to laser injury. Representative images of platelet (CD41, green) and fibrin (magenta) accumulation at the injury site at 10 minutes. (C) Kinetics of platelet (left panel) and fibrin (right panel) surface area coverage over time in response to isotype control or 5G9. (D) Platelet (upper panel) and fibrin (lower panel) AUC after treatment of blood with isotype control or inhibitory anti-TF antibody 5G9. Mean ± SD, n = 6 independent experiments, paired t test. AUC, area under the curve.
Laser injury of the Endo-chip induces platelet calcium mobilization and VWF incorporation
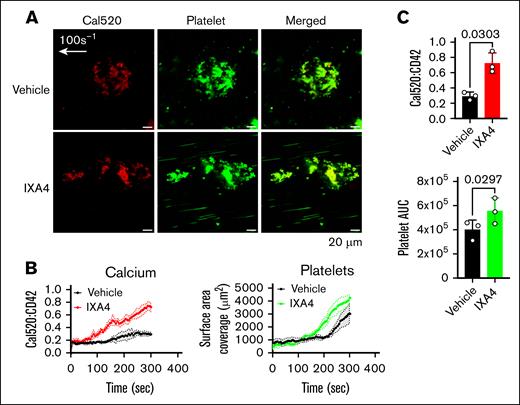
After laser injury, there is an increase in Cal520 fluorescence within platelets over time. Incorporating IXA4,18 an activator of the inositol-requiring enzyme 1 stress pathway, increased calcium mobilization into platelets at the site of endothelial injury. This was accompanied by an increase in the size of the platelet thrombus (Figure 5). Perfusion of blood containing fluorescently labeled anti-VWF antibody, followed by laser pulse to the endothelium, showed increased incorporation of VWF in the growing thrombus with increasing shear rate. This had a punctate morphology (supplemental Figure 5).
Induction of ER stress by IXA4 increases the mobilization of platelet calcium in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle (dimethyl sulfoxide) or the inositol-requiring enzyme 1 activator IXA4 (300 μM) for 2 hours at RT. Blood was then labeled with the calcium dye Cal520 and anti-CD42a, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by a laser pulse to the endothelial layer. Representative images of calcium (red) and platelet (CD42a, red) accumulation at the injury site at 10 minutes. (B) Kinetics of calcium (left panel) and platelet (right panel) surface area coverage over time in response to vehicle or IXA4. (C) Calcium (upper panel) and platelet (lower panel) AUC after treatment of blood with vehicle or IXA4. Mean ± SD, n = 3 independent experiments, paired t test. AUC, area under the curve.
Induction of ER stress by IXA4 increases the mobilization of platelet calcium in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle (dimethyl sulfoxide) or the inositol-requiring enzyme 1 activator IXA4 (300 μM) for 2 hours at RT. Blood was then labeled with the calcium dye Cal520 and anti-CD42a, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by a laser pulse to the endothelial layer. Representative images of calcium (red) and platelet (CD42a, red) accumulation at the injury site at 10 minutes. (B) Kinetics of calcium (left panel) and platelet (right panel) surface area coverage over time in response to vehicle or IXA4. (C) Calcium (upper panel) and platelet (lower panel) AUC after treatment of blood with vehicle or IXA4. Mean ± SD, n = 3 independent experiments, paired t test. AUC, area under the curve.
Thrombus formation is inhibited by argatroban and heparin in the Endo-chip laser-injury model
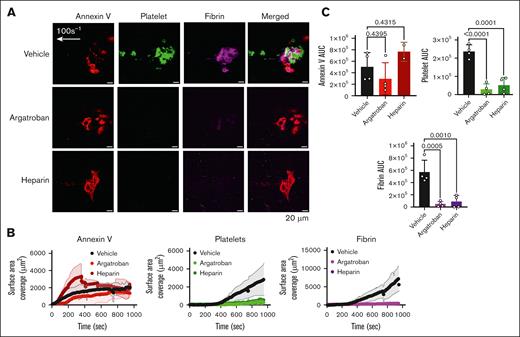
The in vivo laser-induced thrombus formation is thrombin dependent.4 To probe the role of thrombin inhibition in our in vitro Endo-chip, we added the thrombin inhibitor argatroban to blood prior to infusion. Argatroban at 0.5 μg/mL inhibited platelet and fibrin accumulation after laser injury by 87% (Figure 6A-C). Similarly, the inclusion of heparin (Xa and thrombin inhibitor) at 0.5 U/mL in the blood inhibited platelet and fibrin accumulation by 73%. In comparison, the degree of endothelial injury, as assessed by annexin V binding, was similar between Endo-chips used for the perfusion of blood containing vehicle, argatroban, or heparin (Figure 6A-C).
Thrombus formation is inhibited by argatroban (a thrombin inhibitor) and heparin in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle, argatroban (0.5 μg/mL), or heparin (0.5 U/mL) for 10 minutes at RT. Blood was then labeled with annexin V, anti-CD41, and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by laser pulse to the endothelial layer. Representative images of annexin V (red), platelet (CD41, green), and fibrin (magenta) accumulation at the injury site at 10 minutes. (B) Kinetics of annexin V (left panel), platelet (middle panel), and fibrin (right panel) over time in response to vehicle, argatroban, or heparin. (C) Annexin V (upper left panel), platelet (upper right panel), and fibrin (lower panel) AUC after treatment of blood with vehicle, argatroban, or heparin. Mean ± SD, n = 5 independent experiments, 1-way analysis of variance with Dunnett’s multiple comparisons test. AUC, area under the curve.
Thrombus formation is inhibited by argatroban (a thrombin inhibitor) and heparin in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle, argatroban (0.5 μg/mL), or heparin (0.5 U/mL) for 10 minutes at RT. Blood was then labeled with annexin V, anti-CD41, and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by laser pulse to the endothelial layer. Representative images of annexin V (red), platelet (CD41, green), and fibrin (magenta) accumulation at the injury site at 10 minutes. (B) Kinetics of annexin V (left panel), platelet (middle panel), and fibrin (right panel) over time in response to vehicle, argatroban, or heparin. (C) Annexin V (upper left panel), platelet (upper right panel), and fibrin (lower panel) AUC after treatment of blood with vehicle, argatroban, or heparin. Mean ± SD, n = 5 independent experiments, 1-way analysis of variance with Dunnett’s multiple comparisons test. AUC, area under the curve.
Platelet thrombus formation is inhibited by abciximab but not aspirin in the Endo-chip laser-injury model
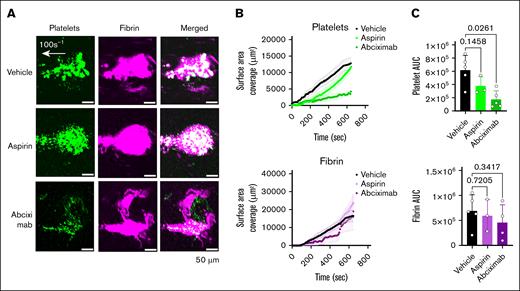
Abciximab is a potent inhibitory antibody directed against human integrin αIIbβ3. It was previously marketed and used as an antithrombotic during coronary artery stenting.25 Aspirin is a cyclooxygenase-1 inhibitor widely used as an antiplatelet agent in the primary and secondary prevention of ischemic heart disease and stroke.26,27 Incubation of blood with abciximab prior to perfusion resulted in significant inhibition of platelet thrombus formation. Fibrin accumulation was not inhibited, except for the central area of the thrombus (Figure 7A-C). Conversely, aspirin did not significantly inhibit platelet or fibrin formation at a clinically relevant low dose (Figure 7A-C).
Inhibition of thrombus formation by inhibitory antiplatelet antibody 7E3 (abciximab) but not by low dose aspirin in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle, aspirin (9 μg/mL), or abciximab (20 μg/mL) for 1 hour at RT. Blood was then labeled with anti-CD41 antibody and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by laser injury to the endothelial layer. Representative images of platelet (CD41, green) and fibrin (magenta) accumulation at the injury site at 10 minutes. (B) Kinetics of platelet (upper panel) and fibrin (lower panel) surface area coverage over time. (C) Platelet (upper panel) and fibrin (lower panel) AUC after treatment of blood with vehicle, aspirin, or abciximab. Mean ± SD, n = 3 to 5, 1-way analysis of variance with Dunnett’s multiple comparisons test. AUC, area under the curve.
Inhibition of thrombus formation by inhibitory antiplatelet antibody 7E3 (abciximab) but not by low dose aspirin in the Endo-chip laser-injury model. (A) Blood was incubated with vehicle, aspirin (9 μg/mL), or abciximab (20 μg/mL) for 1 hour at RT. Blood was then labeled with anti-CD41 antibody and antifibrin antibody, recalcified with CaCl2 (10 mM), and then immediately perfused through the Endo-chip at 100 s–1, followed by laser injury to the endothelial layer. Representative images of platelet (CD41, green) and fibrin (magenta) accumulation at the injury site at 10 minutes. (B) Kinetics of platelet (upper panel) and fibrin (lower panel) surface area coverage over time. (C) Platelet (upper panel) and fibrin (lower panel) AUC after treatment of blood with vehicle, aspirin, or abciximab. Mean ± SD, n = 3 to 5, 1-way analysis of variance with Dunnett’s multiple comparisons test. AUC, area under the curve.
Discussion
Our study presents a convenient model for controlled thrombus formation in an endothelialized microfluidic device simulating venous thrombus formation. This offers a tool for the study of platelet activation and coagulation in response to endothelial injury, which are physiological responses to achieve hemostasis. Platelet activation and fibrin formation also occur in pathological thrombotic responses; however, these involve additional elements such as altered blood composition, flow dynamics, and/or vessel wall pathology (Virchow’s triad).
In the in vivo laser-injury mouse thrombosis model, platelet accumulation is localized to the site of injury followed by the generation of fibrin.1-14,21 This process is not fully captured in current vessel-on-chip models, which typically rely on inflammatory stimuli (eg, TNF-α, phorbol 12-myristate 13-acetate), or photochemical injury to initiate thrombosis producing diffuse thrombi.28-31 For example, Zhao et al used phorbol 12-myristate 13-acetate-treated endothelialized microfluidic devices to study cerebral vein thrombosis,30 whereas we used fixed, TNF-α-treated endothelium to investigate thromboinflammatory responses.17 Qiu et al recently demonstrated the utility of such models to test novel treatments for thromboinflammation.31 In contrast, the laser-injury Endo-chip initiates thrombus formation without preactivating the endothelium. In the absence of laser injury, no thrombus forms during perfusion of recalcified blood. After laser injury, localized PS exposure, platelet accumulation, and fibrin formation are observed, reproducing key features of the in vivo thrombosis model. Although fibrin accumulation on endothelial cells has previously been described in response to laser injury,32 this did not include blood perfusion or defined shear rates.
The Endo-chip laser-injury model can simulate both venous (100 s–1) and arterial (800 s–1) shear rates;19 however, high arterial shear rates in the pathological range were not tested in our system. In addition, the kinetics of the Endo-chip thrombus at 100 s–1 more accurately followed the kinetics of venous thrombus formation in vivo compared with the kinetics of the Endo-chip thrombus at 800 s–1. Consequently, most of our data were acquired under venous shear conditions. A difference observed with the in vivo model was the lack of contraction phase of the platelet clot in the Endo-chip. This may reflect differences in the cytoskeletal contraction of adhered platelets on the endothelial surface compared with adhered platelets on surfaces,33 or in vivo.
Cultured HUVECs in the Endo-chip maintain their glycocalyx supporting the anchorage of VWF and other proteins.34 VWF plays a major role in platelet adhesion and aggregation in physiological and pathological shear conditions. VWF binds to the exposed subendothelium after injury, enabling platelet arrest via interaction of its A1 domain with the platelet glycoprotein Ibα receptor,35 and the formation of membrane tethers.36 Under low-shear conditions (600-900 s–1), aggregation is dominated by αIIbβ3-dependent interactions, while under intermediate shear rates (1000-10 000 s–1), both glycoprotein Ibα and αIIbβ3 play an important role in platelet aggregation. At pathological high shear rates (>10 000 s–1), such as those encountered in stenosed coronary arteries, platelet aggregation to immobilized VWF can occur independently from platelet activation.37,38 Vessel stenosis aggravates thrombus propagation poststenosis in a VWF-dependent fashion.39
At a shear rate of 800 s–1, we observed VWF release from laser-injured endothelial cells, and incorporation into localized thrombi. These findings support that the laser-injury Endo-chip model is suitable for studying the contribution of endothelial VWF to thrombus formation at shear rates up to ∼1000 s–1. At higher shear rates, endothelial detachment becomes a limiting factor, necessitating alternative surface coatings of the channel to support the endothelial layer.40 The model may also be clinically relevant to understand the role of VWF in low shear pathological conditions, such as venous thrombosis,41,42 or thromboinflammation.43,44
Our model has certain limitations, including the use of nonphysiological calcium concentrations, and the absence of normal vasoconstrictive responses due to the lack of the smooth muscle layer. However, the endothelial layer retains key functions, including nitric oxide production45 and PS exposure after injury. We recalcified citrated blood with 10 mM calcium prior to perfusion, consistent with previous studies,28,31 to achieve reproducible clot formation within 15 minutes. Although this results in free calcium (∼6 mM) exceeding physiological levels (∼1.3 mM),46 this standardization ensures consistent thrombus formation in this ex vivo system.
Another limitation is variability in thrombus size. Across 14 healthy donors, thrombus variability (CV ∼39%) reflected interindividual differences in blood parameters (hematocrit, platelet count) and HUVEC batch variation. Variability was slightly higher in the in vivo mouse mesenteric vein model compared with the laser Endo-chip, likely due to additional factors such as animal movement, differences in tissue preparation, and blood flow. Ongoing efforts aim to further standardize laser-induced thrombus models.19,47,48
The Endo-chip laser-injury model offers a platform for studying novel mechanisms of thrombus formation within a short capture time (10 minutes). It also provides a simplified vessel-on-chip model to study the contribution of endothelial cells to thrombus formation independent of other vascular cells (ie, smooth muscle cells). Emerging evidence links ER stress to cardiovascular disease49 and thrombosis,11,50 and this model can detect cytoplasmic calcium elevation, a hallmark of ER stress. Modulators of the ER stress response can therefore be further investigated using this system.
Another application of the Endo-chip laser-injury model is its capacity to assess the effect of antithrombotic agents on both circulating blood components and the endothelial surface. Laser injury produces a defined lesion with reproducible thrombus kinetics. Anticoagulants markedly reduced platelet accumulation and fibrin generation, whereas abciximab and aspirin were ineffective in inhibiting fibrin. These results suggest that antiplatelet agents, at clinically relevant doses, may be insufficient to prevent fibrin thrombus formation in this model, highlighting the need for anticoagulation. As this is a humanized model, it offers for the study of antibodies against human targets, which cannot readily be tested in the mouse model. We demonstrated this by confirming the efficacy of abciximab and inhibitory antihuman TF antibody51 in preventing platelet thrombus formation. Notably, an endothelialized microfluidic model previously uncovered the unexpected thrombogenic effect of a monoclonal antibody against CD40L,52 highlighting the potential of the laser-injury Endo-chip in screening antihuman inhibitory agents.
Other potential applications for this model could be to incorporate blood outgrowth endothelial cells derived from a particular donor. This would personalize this device, as it would allow the use of an individual’s endothelial cells to assess their prothrombotic tendency.53 Further refinements of the model could include other type of cells present in the vessel (eg, smooth muscle cells)54 to more accurately reflect in vivo conditions, as well as features of pathological process such as perfusing blood from patients or including geometries of vessel stenosis.55 The laser-injury Endo-chip thus represents a flexible and powerful platform for advancing our understanding of thrombus formation and optimizing antithrombotic therapies.
Acknowledgments
This research was funded by an Medical Research Future Fund Cardiovascular Grant (APP2017914; F.H.P.), a Sydney Nano Research Scheme (Grand Challenge; L.A.J. and F.H.P.), an MRFF Cardiovascular Health Mission Grant (MRF2023977; L.A.J. and F.H.P.), and a Cardiovascular Research Network Professional Development Award (A.D.). L.A.J. is a National Heart Foundation Future Leader Fellow Level 2 (105863) and a Snow Medical Research Foundation Fellow (2022SF176).
Authorship
Contribution: A.D., M.Q., J.C.L.F., and D.M.Y. designed and performed experiments, analyzed data, and cowrote the manuscript; P.R.C., J.R.G., and L.A.J. supervised research direction, contributed reagents and expertise, and edited the manuscript; F.H.P. conceived the study, designed the experiments, supervised research, and cowrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freda Passam, Haematology Research Lab, Charles Perkins Centre, Central Clinical School, Faculty of Medicine and Health, The University of Sydney, Room 3116, Level 3E, John Hopkins Dr, Camperdown, Sydney, NSW 2050, Australia; email: freda.passam@sydney.edu.au.
References
Author notes
Original data are available from the corresponding author, Freda H. Passam (freda.passam@sydney.edu.au), on request.
The full-text version of this article contains a data supplement.