Key Points
Cerebellar gray matter volume in our HA cohort is disproportionately smaller than in healthy controls.
Smaller cerebellar gray matter volume was associated with measures of executive dysfunction in HA.
Visual Abstract
Children with severe HA compared to healthy controls demonstrated lower cerebellar gray matter volumes which corresponded to executive dysfunction.
Children with severe HA compared to healthy controls demonstrated lower cerebellar gray matter volumes which corresponded to executive dysfunction.
The effect of factor VIII (FVIII) deficiency on neurocognitive outcomes in children with hemophilia A (HA) is not well characterized. This study aimed to examine differences in brain volume and neurocognition between children with severe HA and healthy controls.This single-center study included 32 males aged 6 to 16 years, 9 with severe FVIII deficiency and 23 healthy controls. Volumetric data from magnetic resonance imaging and neurocognitive testing were compared using linear models including age to evaluate the association between regional brain volume and function. Cerebellar gray matter volume was significantly smaller in the HA cohort than in healthy controls (estimate, –0.375; 95% confidence interval [CI], –0.732 to –0.019; t(26) = 2.07; P = .049). A reduction in cerebellar gray matter was associated with neurocognitive executive dysfunction as noted by abnormal scores on 2 executive function assessments: the Delis-Kaplan Executive Function System, total switching accuracy (estimate, 0.549; 95% CI, –0.876 to 0.221; t(25) = –3.28; P = .003) and total correct category switching (estimate, 0.538; 95% CI, –0.868 to 0.207; t(25) = –3.19; P = .004), and the Behavior Rating Inventory of Executive Function, behavioral regulation index score (estimate, 0.531; 95% CI, 0.228-0.835; t(25) = 3.44; P = .002). Our study provides key insights into the lower brain volumes found in patients with HA and the corresponding executive dysfunction. Quantitative brain volume assessment in patients with HA may provide an integrated measure and with further research could be a useful clinical tool when assessing risk for neurocognitive dysfunction.
Introduction
Hemophilia A (HA) is an X-linked bleeding disorder characterized by deficiency in factor VIII (FVIII). Symptoms of hemophilia have been associated with complications, including lower quality of life,1,2 increased risk of neuropsychiatric disorders,3 and impairments in executive function.4
Hemophilia-related sequelae may exert a direct, negative impact on the developing brain. Individuals with hemophilia are at risk for hemorrhages within the brain, and HA is the inherited bleeding disorder most commonly associated with intracranial hemorrhage.5 The presence of severe and chronic joint pain due to hemarthroses may limit a student’s ability to complete assignments and increases the risk of school absence. Given these potential associations, it logically follows that children with hemophilia may be at risk for deficits in executive function. However, it is unclear how FVIII deficiency directly impacts the developing brain and how this might relate to neurocognitive outcomes in children with HA.
There is a paucity of research to understand, treat, or prevent brain disease and its effect on neurocognitive outcomes in hemophilia. To date, no published studies have used quantitative neuroimaging techniques to evaluate brain volume in children with hemophilia. The present study sought to address these gaps in knowledge by using quantitative structural/volumetric magnetic resonance imaging (MRI) to compare neuroanatomical features between children with HA and healthy controls. In addition, we investigated the association between brain morphometry and performance on neurocognitive assessments. Despite research on the social, emotional, and behavioral features of individuals with hemophilia, no study has integrated comprehensive neurocognitive assessments with quantitatively processed neuroimaging. Although this association has been investigated in other diagnostic contexts, such as schizophrenia,6 it remains largely unexplored in individuals with bleeding disorders including hemophilia. Therefore, we hypothesized that FVIII deficiency would result in alterations in brain morphometry relative to healthy controls, and that these differences would correlate with poor neurocognitive outcomes, specifically executive function.
Methods
Study design and population
Data collection and study procedures were conducted at the University of Iowa and approved by the institutional review board. Written informed consent and assent were obtained from legal guardians and participants at the time of enrollment. Enrollees were informed that the results of the MRI and neurocognitive testing would not be disclosed to them.
This pilot study included male children (aged 6-16 years) with a diagnosis of severe HA from the Iowa Hemophilia and Thrombosis Center (IHTC) and healthy male controls of the same age range recruited from the surrounding community. Severe hemophilia rarely affects females; ∼250 females are reported to have severe hemophilia worldwide.7 Our center did not have any females with severe HA, limiting the study to males only.
Participants with HA were identified through the IHTC patient database and electronic medical records. The research team then used the electronic medical records to confirm accuracy of age and clinical diagnosis of HA. Exclusion criteria for the full sample were as follows: diagnosis of traumatic brain injury, brain tumor, major chromosomal anomalies, intellectual disability, or potential participants with contraindication to MRI exposure such as implanted metal, pacemaker, etc. Children with HA were excluded if they had a history of or current intracranial bleeding or FVIII inhibitor. Similarly, aged healthy controls with no known chronic illness were included.
Study procedures
Participants with HA and controls completed identical study protocols between 2015 and 2018. During the initial visit, anthropological measurements including height and weight were collected. Participants completed both neurocognitive assessments and a nonsedated, research-based MRI. Parents or caregivers provided information on the participant’s medical history (including birth history, other medical diagnoses, and medications), participation in educational assistance programs, family medical history, and demographic measures including parental socioeconomic status (SES). The Hollingshead Four Factor Index was collected as a parent-reported survey that uses education, occupation, sex, and marital status to determine a family’s composite SES.8
Disease-related parameters
A comprehensive medication and hemophilia-related history was obtained. Parameters obtained included type of FVIII replacement and frequency of use, age at prophylactic initiation, and annual rate of bleeding, which was quantified by adding the number of bleeding events recorded at annual comprehensive visits, in telephone notes, or in pharmacy notes within each year and then averaging this number over the years the participant was seen at the IHTC.
Neurocognitive assessments
All participants completed age-appropriate, standardized neurocognitive assessments with a focus on executive function. This included the Wechsler Intelligence Scale for Children, fourth edition (WISC-IV; Intelligence), the Wide Range Achievement Test, fourth edition (WRAT4; Academic Achievement), and the Delis-Kaplan Executive Function System (D-KEFS; Executive Functioning). All tests were administered by a trained examiner and supervised by a licensed psychologist (A.L.C.). Parents were also asked to complete the Behavior Rating Inventory of Executive Function (BRIEF).
INTELLIGENCE ASSESSMENT
All participants were administered select subtests from the WISC-IV.9 The General Ability Index (GAI; a composite of the Verbal Comprehension and Perceptual Reasoning indices) was the measure of interest. Each composite uses standard scores that have a mean of 100 and standard deviation of 15. For this study, GAI was calculated on a prorated basis using the Similarities, Vocabulary, Block Design, and Matrix Reasoning subtests. WISC-IV data were evaluated by the research team before data analysis to ensure that participants had no major cognitive impairment (eg, GAI <80) that could negatively influence (confound) academic achievement. GAI is a helpful measure of general cognitive functioning, less sensitive to issues with working memory and processing speed, and therefore useful in ruling out major cognitive impairments.10
ACADEMIC ACHIEVEMENT TEST
All participants were administered select subtests from the WRAT4,11 which is a standardized achievement test that screens academic achievement in word reading, sentence comprehension, math computation, and spelling. Each domain has a mean score of 100 and a standard deviation of 15. Internal consistency and alternate-form reliability measures are high (0.87-0.93 and 0.82-0.90, respectively).12 By study design, only assessments of Word Reading and Math Computation were performed because of time limitations.
EXECUTIVE FUNCTION ASSESSMENTS
The D-KEFS13 measures verbal and nonverbal executive functioning in children and adults. It has demonstrated sensitivity to frontal-lobe dysfunction in several neurological and developmental disorders through studies on clinical populations and neuroimaging research.14 Of the 9 stand-alone subtests, the Color Word Interference and verbal fluency subtests (letter fluency, category fluency, category switching) were administered. These measures provided assessment of automaticity, inhibition, initiation, and set-shifting skills. Each measure is converted into a scaled score, with a mean of 10 and a standard deviation of 3.
The BRIEF15 was administered as an additional measure of executive function. This caregiver-reported assessment contains 86 items that assess behavioral, emotional, and cognitive regulation in children and teens. Reported measures include the Metacognition Index and the Behavioral Regulation Index (BRI), which can be combined into the Global Executive Composite. Scores have demonstrated the ability to discriminate among clinical groups, including attention-deficit hyperactivity disorder.16 Each scale yields a T-score, which has a mean of 50 and a standard deviation of 10. Higher scores indicate worse executive function.
Neuroimaging
Seven of 9 participants with HA and 21 of 23 controls completed a nonsedated, noncontrast-enhanced MRI after safety screening. The Siemens Trio 3T MRI scanner in the research facility obtained anatomical T1-weighted and T2-weighted images with 1-mm isotropic resolution. The T1-weighted images assessed intracranial volume (ICV), and the T2-weighted images measured total brain tissue, total cerebral (temporal, occipital, parietal, and frontal) volume, total cerebellar volume, and total cerebrospinal fluid. Following the completion of the scans, a trained neuroimaging specialist visually inspected and processed all images. Each set of images consists of multiple scans. Scans were flagged and removed from analysis if there were too many artifacts to produce reliable data (2 HA scans and 2 control scans were removed before analysis).
Neuroimages were normalized for intensity, corrected for inhomogeneity, and registered to standard atlases using Advanced Normalization Tools. Following preprocessing, neuroimages were processed using the BRAINSAutoWorkup pipeline, which optimizes tissue classification through an iterative framework and produces robust parcellation of brain regions.17 BRAINSAutoWorkup labels brain regions using a multiatlas, similarity-weighted, majority-vote procedure (joint label fusion)18 with a set of expert-segmented templates adapted from the Desikan-Killiany atlas.19 Brain regions include cortical, subcortical, and cerebellar regions, separated by hemispheres and tissue type (gray or white matter) where appropriate. We examined 17 brain regions of interest (ROIs) defined a priori, representing major regions across the brain.
Statistical analysis
To enhance the statistical power of this case-control study, we used a predetermined ratio of at least 2 controls for every case (2:1 control-to-case ratio). We used independent-samples t tests to compare demographic outcomes between controls and participants with HA, except for parental SES and racial identity, for which χ2 tests were performed because of the categorical nature of the variables.
The neurocognitive analysis included comparisons of standard scores and T-scores derived from standardized measures. Given that all normative data were adjusted for age, we did not include it as a covariate in the model.
For imaging data, relationships between ROIs and ICV follow the power law principle, that is, ROI = α × ICVβ, in which α is the constant and β represents the scaling component of the power function. We used linear regression models with ICV as the predictor variable and each ICV-power–adjusted regional brain volume as the dependent variable. The analysis encompassed 17 brain ROIs representing major regions throughout the brain and examination of the whole brain. Brain volumes were normalized according to ICV, which can be influenced by individual size (weight/height/body mass index); therefore, we used the ICV power proportion as described above. Linear regression models were used separately for each ROI, with age included as a covariate in the model. To explore the relationship between brain ROIs (independent variable) and neurocognitive assessments (dependent variable), ad hoc volume-function analyses were conducted using linear regressions. These volume-function analyses were specifically focused on brain regions for which we observed significant group differences in earlier analyses.
Analyses were performed using R, version 4.2.3 (R Foundation for Statistical Computing). Results were considered significant if the unadjusted P was ≤.05. All authors had access to the primary data. There were no corrections for multiple comparisons given that this was a pilot study.
Results
Demographics
All children with severe HA and no inhibitor aged between 6 and 16 years were screened for eligibility. Of the 13 children identified, 1 patient was ineligible because of a brain tumor. Of the remaining 12 patients with severe HA, 3 declined because of travel distance to the testing location. The final sample included 9 participants with HA and 23 healthy controls. Baseline demographics for the sample are outlined in Table 1. The mean age of the entire sample was 11.4 years. The mean paternal education level of the entire sample was 16.3 years, and the mean maternal education level was 16.4 years. Paternal education was significantly lower for those with HA (t(30) = 2.83; P = .008). No other significant differences between groups were found for baseline demographics, including maternal education.
Comparison of demographic variables between participants with HA and controls
| . | Controls . | HA . | Controls vs HA . |
|---|---|---|---|
| n = 23 . | n = 9 . | P value . | |
| Age, mean (SD), y | 11.8 (2.7) | 10.4 (3.7) | .24 |
| Height (mean), cm | 152.3 (16.3) | 141.8 (23.2) | .16 |
| Weight (mean), kg | 43.7 (14.1) | 47.7 (40.1) | .67 |
| Paternal education, mean (SD), y | 17.0 (2.5) | 14.4 (1.7) | .008 |
| Maternal education, mean (SD), y | 16.7 (2.4) | 15.7 (2.8) | .33 |
| Assistance at school through a 504 plan or Individualized Learning Plan, n (%) | 1 (4.3%) | 2 (22%) | ∗χ2 = 2.43, P = .18 |
| Intellectual disability, n (%) | 0 (0%) | 0 (0%) | >.99 |
| Parental SES, n | |||
| Professional degree or college or advanced degree | 19 | 5 | ∗χ2 = 3.94, P = .14 |
| White collar, semiskilled worker, high school education | 4 | 3 | |
| Semiskilled worker/laborer below secondary education | 0 | 1 | |
| Racial identity, n | |||
| White | 22 | 8 | ∗χ2 = .50, P = .48 |
| Other | 1 | 1 | |
| Family history of psychiatric disease, n | 10 | 6 | .25 |
| Fetal hypoxia, n | 5 | 1 | .50 |
| Hemophilia-related variables | |||
| Use of prophylaxis, n (%) | - | 9 (100%) | |
| Age at prophylaxis initiation, mean (median), mo | - | 26 (17) | |
| Annual rate of bleeding, mean (median) | - | 3.6 (2.0) | - |
| . | Controls . | HA . | Controls vs HA . |
|---|---|---|---|
| n = 23 . | n = 9 . | P value . | |
| Age, mean (SD), y | 11.8 (2.7) | 10.4 (3.7) | .24 |
| Height (mean), cm | 152.3 (16.3) | 141.8 (23.2) | .16 |
| Weight (mean), kg | 43.7 (14.1) | 47.7 (40.1) | .67 |
| Paternal education, mean (SD), y | 17.0 (2.5) | 14.4 (1.7) | .008 |
| Maternal education, mean (SD), y | 16.7 (2.4) | 15.7 (2.8) | .33 |
| Assistance at school through a 504 plan or Individualized Learning Plan, n (%) | 1 (4.3%) | 2 (22%) | ∗χ2 = 2.43, P = .18 |
| Intellectual disability, n (%) | 0 (0%) | 0 (0%) | >.99 |
| Parental SES, n | |||
| Professional degree or college or advanced degree | 19 | 5 | ∗χ2 = 3.94, P = .14 |
| White collar, semiskilled worker, high school education | 4 | 3 | |
| Semiskilled worker/laborer below secondary education | 0 | 1 | |
| Racial identity, n | |||
| White | 22 | 8 | ∗χ2 = .50, P = .48 |
| Other | 1 | 1 | |
| Family history of psychiatric disease, n | 10 | 6 | .25 |
| Fetal hypoxia, n | 5 | 1 | .50 |
| Hemophilia-related variables | |||
| Use of prophylaxis, n (%) | - | 9 (100%) | |
| Age at prophylaxis initiation, mean (median), mo | - | 26 (17) | |
| Annual rate of bleeding, mean (median) | - | 3.6 (2.0) | - |
SD, standard deviation.
χ2 test; values in bold type represent significance at P < .05.
Hemophilia-related variables are outlined in Table 1. Within the hemophilia sample, all children had a diagnosis of severe HA (<1% FVIII activity) and had missed <1 school day per year on average because of a hemophilia-related reason. All (9/9) were prescribed FVIII replacement therapy, and all (9/9) were taking FVIII replacement in a prophylactic manner via home therapy (3 infusions per week of a standard half-life product). At the time of the study, no participants were taking long-acting (extended half-life) FVIII replacement therapy. Of note, the annual rate of bleeding was 3.6 events per year with a range from 0 to 14. The median age at initiation of FVIII prophylactic therapy was 17 months (mean, 26 months).
Neurocognitive outcomes
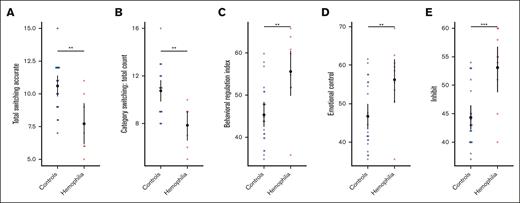
Neurocognitive measures, particularly those assessing executive function, were compared between participants with HA and controls by evaluating 4 neurocognitive assessments: the WISC-IV, WRAT4, D-KEFS, and BRIEF. A summary of all group differences on cognitive, intelligence, and academic tasks is shown in Table 2. No differences were demonstrated between groups on either the WISC-IV or the WRAT4 (supplemental Table 1). Figure 1 illustrates significant differences on cognitive tasks, including 2 verbal fluency (D-KEFS) measures: total switching accuracy (estimate, 0.549; 95% confidence interval [CI], −0.876 to 0.221; t(25) = −3.28; P = .003) (Figure 1A) and total correct category switching (estimate, 0.538; 95% CI, −0.868 to 0.207; t(25) = −3.19; P = .004; Figure 1B), in which healthy controls scored significantly better than the HA cohort. Children with HA also had worse ratings than their peers on the BRIEF, as indicated by higher T-scores. The difference reached significance for the BRI (estimate, 0.531; 95% CI, 0.228-0.835; t(25) = 3.44; P = .002; Figure 1C) and 2 of the included subscales within the BRI: Emotional Control (ability to regulate emotional responses; estimate, 0.453; 95% CI, 0.133-0.772; t(25) = 2.78; P = .009; Figure 1D) and Inhibit (ability to control impulses; estimate, 0.583; 95% CI, 0.292-0.874; t(25) = 3.93; P ≤ .001; Figure 1E).
Group (model) estimates in each domain for participants with HA and controls
| Variable of interest . | Estimate . | Standard error . | Lower . | Upper . | T value . | P value . |
|---|---|---|---|---|---|---|
| WISC-IV | ||||||
| General Abilities Index | 0.271 | 0.176 | −0.616 | 0.073 | −1.543 | .133 |
| Verbal Comprehension Index | 0.230 | 0.178 | −0.578 | 0.118 | −1.294 | .205 |
| Vocabulary | 0.305 | 0.174 | −0.646 | 0.036 | −1.756 | .089 |
| Similarities | 0.114 | 0.181 | −0.469 | 0.242 | −0.627 | .535 |
| Perceptual Reasoning Index | 0.209 | 0.179 | −0.558 | 0.141 | −1.168 | .252 |
| Block design | 0.149 | 0.181 | −0.502 | 0.205 | −0.823 | .417 |
| Matrix reasoning | 0.196 | 0.179 | −0.547 | 0.155 | −1.095 | .282 |
| Processing Speed Index | 0.083 | 0.182 | −0.274 | 0.439 | 0.455 | .652 |
| Coding | 0.045 | 0.182 | −0.403 | 0.312 | −0.249 | .805 |
| Symbol search | 0.172 | 0.180 | −0.181 | 0.524 | 0.955 | .347 |
| Cancellation | 0.143 | 0.181 | −0.498 | 0.211 | −0.794 | .434 |
| WRAT4 | ||||||
| Reading | 0.145 | 0.181 | −0.499 | 0.209 | −0.805 | .427 |
| Arithmetic | 0.088 | 0.182 | −0.445 | 0.268 | −0.486 | .631 |
| D-KEFS | ||||||
| Verbal fluency | ||||||
| Letter fluency | 0.017 | 0.200 | −0.409 | 0.375 | −0.084 | .934 |
| Category fluency | 0.202 | 0.196 | −0.586 | 0.182 | −1.030 | .313 |
| Category switching | 0.538 | 0.169 | −0.868 | 0.207 | −3.190 | .004 |
| Total switching accuracy | 0.549 | 0.167 | −0.876 | 0.221 | −3.282 | .003 |
| Color word interference | ||||||
| Color patch naming | 0.101 | 0.199 | −0.491 | 0.289 | −0.505 | .618 |
| Word naming | 0.075 | 0.199 | −0.466 | 0.316 | −0.375 | .711 |
| Inhibition | 0.216 | 0.195 | −0.599 | 0.167 | −1.107 | .279 |
| BRIEF | ||||||
| Global executive composite | 0.331 | 0.172 | −0.006 | 0.669 | 1.924 | .064 |
| BRI | 0.531 | 0.155 | 0.228 | 0.835 | 3.436 | .002 |
| Shift | 0.329 | 0.172 | −0.009 | 0.667 | 1.909 | .070 |
| Emotional control | 0.453 | 0.163 | 0.133 | 0.772 | 2.780 | .009 |
| Inhibit | 0.583 | 0.148 | 0.292 | 0.874 | 3.928 | <.001 |
| Metacognition | 0.147 | 0.181 | −2.070 | 0.501 | 0.813 | .422 |
| Variable of interest . | Estimate . | Standard error . | Lower . | Upper . | T value . | P value . |
|---|---|---|---|---|---|---|
| WISC-IV | ||||||
| General Abilities Index | 0.271 | 0.176 | −0.616 | 0.073 | −1.543 | .133 |
| Verbal Comprehension Index | 0.230 | 0.178 | −0.578 | 0.118 | −1.294 | .205 |
| Vocabulary | 0.305 | 0.174 | −0.646 | 0.036 | −1.756 | .089 |
| Similarities | 0.114 | 0.181 | −0.469 | 0.242 | −0.627 | .535 |
| Perceptual Reasoning Index | 0.209 | 0.179 | −0.558 | 0.141 | −1.168 | .252 |
| Block design | 0.149 | 0.181 | −0.502 | 0.205 | −0.823 | .417 |
| Matrix reasoning | 0.196 | 0.179 | −0.547 | 0.155 | −1.095 | .282 |
| Processing Speed Index | 0.083 | 0.182 | −0.274 | 0.439 | 0.455 | .652 |
| Coding | 0.045 | 0.182 | −0.403 | 0.312 | −0.249 | .805 |
| Symbol search | 0.172 | 0.180 | −0.181 | 0.524 | 0.955 | .347 |
| Cancellation | 0.143 | 0.181 | −0.498 | 0.211 | −0.794 | .434 |
| WRAT4 | ||||||
| Reading | 0.145 | 0.181 | −0.499 | 0.209 | −0.805 | .427 |
| Arithmetic | 0.088 | 0.182 | −0.445 | 0.268 | −0.486 | .631 |
| D-KEFS | ||||||
| Verbal fluency | ||||||
| Letter fluency | 0.017 | 0.200 | −0.409 | 0.375 | −0.084 | .934 |
| Category fluency | 0.202 | 0.196 | −0.586 | 0.182 | −1.030 | .313 |
| Category switching | 0.538 | 0.169 | −0.868 | 0.207 | −3.190 | .004 |
| Total switching accuracy | 0.549 | 0.167 | −0.876 | 0.221 | −3.282 | .003 |
| Color word interference | ||||||
| Color patch naming | 0.101 | 0.199 | −0.491 | 0.289 | −0.505 | .618 |
| Word naming | 0.075 | 0.199 | −0.466 | 0.316 | −0.375 | .711 |
| Inhibition | 0.216 | 0.195 | −0.599 | 0.167 | −1.107 | .279 |
| BRIEF | ||||||
| Global executive composite | 0.331 | 0.172 | −0.006 | 0.669 | 1.924 | .064 |
| BRI | 0.531 | 0.155 | 0.228 | 0.835 | 3.436 | .002 |
| Shift | 0.329 | 0.172 | −0.009 | 0.667 | 1.909 | .070 |
| Emotional control | 0.453 | 0.163 | 0.133 | 0.772 | 2.780 | .009 |
| Inhibit | 0.583 | 0.148 | 0.292 | 0.874 | 3.928 | <.001 |
| Metacognition | 0.147 | 0.181 | −2.070 | 0.501 | 0.813 | .422 |
Analysis based on 9 participants with HA and 23 controls. “Estimate” refers to the model estimate, and 95% CI indicates the 95% confidence limits of the model estimate. Bold values represent significance at P <.05.
Significant differences in performance on the D-KEFS and BRIEF between participants with HA and controls. (A-B) Two verbal fluency measures from the D-KEFS were significantly worse in the HA cohort than in healthy controls: (A) total switching accuracy (P = .003) and (B) category switching total count (P = .004). Higher scores reflect better performance. (C-E) The BRIEF measure BRI and 2 of its subscales were significantly worse in the HA cohort than in healthy controls: (C) BRI (P = .002) and the (D) emotional control (P = .009) and (E) inhibit (P < .001) subscales. Higher scores reflect more concerns. The open black circle represents the mean and error bars 95% CI. ∗∗P < .01; ∗∗∗P < .001.
Significant differences in performance on the D-KEFS and BRIEF between participants with HA and controls. (A-B) Two verbal fluency measures from the D-KEFS were significantly worse in the HA cohort than in healthy controls: (A) total switching accuracy (P = .003) and (B) category switching total count (P = .004). Higher scores reflect better performance. (C-E) The BRIEF measure BRI and 2 of its subscales were significantly worse in the HA cohort than in healthy controls: (C) BRI (P = .002) and the (D) emotional control (P = .009) and (E) inhibit (P < .001) subscales. Higher scores reflect more concerns. The open black circle represents the mean and error bars 95% CI. ∗∗P < .01; ∗∗∗P < .001.
Neuroanatomy
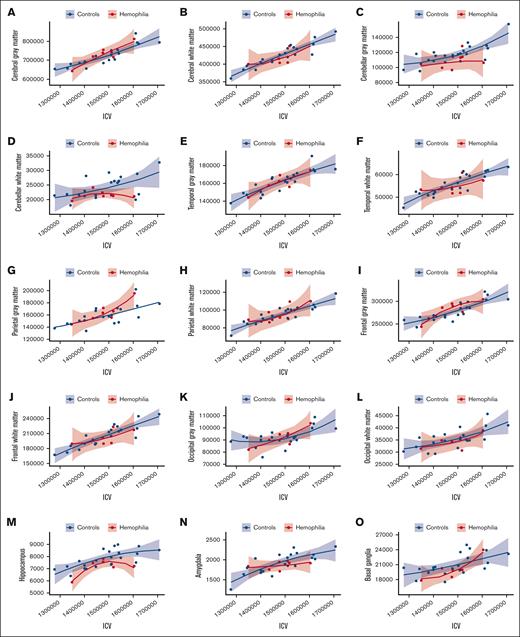
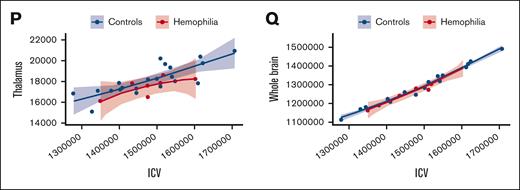
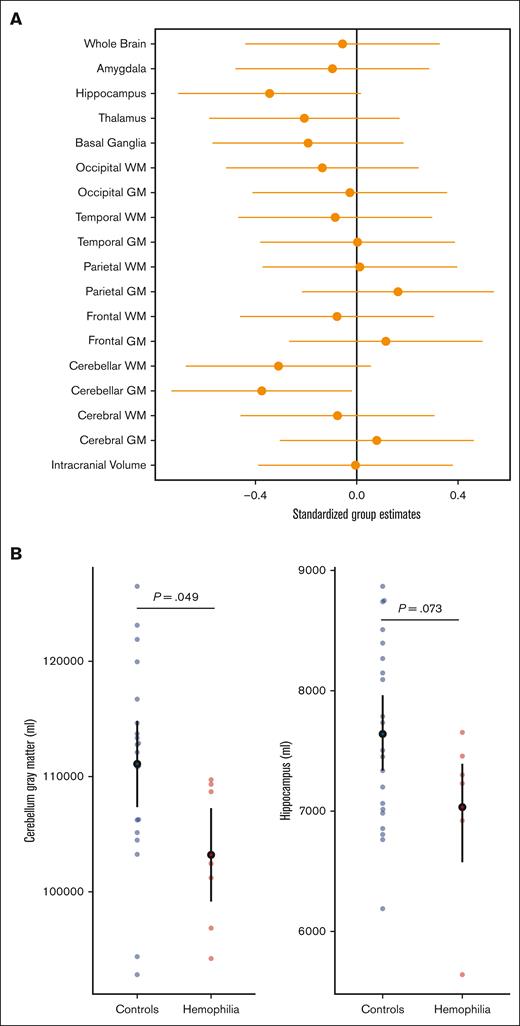
Next, we compared the differences in regional brain volumes between participants with HA and healthy controls. Our results, as demonstrated in Figure 2, illustrate the relationship between ICV and each ROI. ICV-adjusted regional brain volumes were subsequently used in analyses pertaining to brain volume. In Figure 3A, we present the neuroanatomical differences between participants with HA and unaffected controls, including the standardized beta estimates of the difference between groups for all investigated brain volumes. Notably, Figure 3B highlights decreased mean cerebellar and hippocampal volumes in children with HA. Mean cerebellar gray matter volume was significantly lower in the HA group than among controls (estimate, −0.375; 95% CI, −0.732 to −0.019; t(26) = −2.07; P = .049). Hippocampal brain volumes trended lower for the HA cohort than for healthy controls (estimate, −0.344; 95% CI, −0.705 to 0.017; t(26) = −1.87; P = .073); however, this difference was not significant. A summary of all statistics for standardized group estimates for each ROI can be found in supplemental Table 1.
ICV using the power proportion. (A-P) A power-adjusted ROI on the y-axis and the standardized ICV on the x-axis with the HA cohort in red and healthy controls in blue. (Q) The whole brain volume on the y-axis and ICV on the x-axis. (C,M) The results for cerebellar gray matter and hippocampus, respectively.
ICV using the power proportion. (A-P) A power-adjusted ROI on the y-axis and the standardized ICV on the x-axis with the HA cohort in red and healthy controls in blue. (Q) The whole brain volume on the y-axis and ICV on the x-axis. (C,M) The results for cerebellar gray matter and hippocampus, respectively.
Neuroanatomical differences between participants with HA and controls. (A) The model-derived standardized estimates for the group variable (x-axis) for each ROI (y-axis). The error bars represent 95% confidence limits. Estimates were adjusted for age. The black (vertical) line marks 0, or no significant effect of group on ROI. (B) The comparison of cerebellar GM volumes and hippocampal volumes between participants with HA and controls. Cerebellar GM volume (P = .049) and hippocampal volume (P = .073) were compared between the HA cohort and healthy controls, and were both demonstrated to be smaller in children with HA. Controls are shown in blue and participants with HA in red on the x-axis. The power-adjusted ROI (cerebellar GM or hippocampus) is on the y-axis. The open black circle represents the mean and error bars 95% CI. GM, gray matter; WM, white matter.
Neuroanatomical differences between participants with HA and controls. (A) The model-derived standardized estimates for the group variable (x-axis) for each ROI (y-axis). The error bars represent 95% confidence limits. Estimates were adjusted for age. The black (vertical) line marks 0, or no significant effect of group on ROI. (B) The comparison of cerebellar GM volumes and hippocampal volumes between participants with HA and controls. Cerebellar GM volume (P = .049) and hippocampal volume (P = .073) were compared between the HA cohort and healthy controls, and were both demonstrated to be smaller in children with HA. Controls are shown in blue and participants with HA in red on the x-axis. The power-adjusted ROI (cerebellar GM or hippocampus) is on the y-axis. The open black circle represents the mean and error bars 95% CI. GM, gray matter; WM, white matter.
Brain volume-function relationships
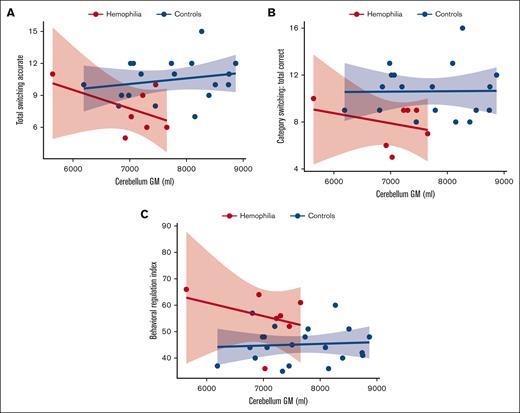
Figure 4A-B illustrates how cerebellar gray matter volume correlates with 2 measures of category switching from the D-KEFS verbal fluency subtest. Smaller cerebellar gray matter volume was associated with lower accuracy of category switching in the HA cohort. Figure 4C illustrates that cerebellar volumes also correlated with scores on the BRI. Smaller cerebellar gray matter volume correlated with higher BRI scores, indicating more problems with inhibition and emotional control.
Brain volume-function relationships between participants with HA and controls. Cerebellar GM is on the x-axis, and the executive function measures total switching accurate (A), category switching: total correct (B), and BRI (C) are on the y-axis. The HA cohort is shown in red and healthy controls in blue. The regression slopes of the HA cohort and healthy controls are significantly different (for panel A, P < .05). In addition, children with HA predominantly fall within the lower left quadrant: smaller cerebellar GM volumes and lower D-KEFS switching scores (for panel A, P < .01 and for panel B, P < .01). Children with HA predominantly fall within the upper left quadrant: smaller cerebellar GM volumes and higher (ie, worse) BRI scores (for panel C, P < .001). The shaded areas represent 95% CIs. Results were adjusted for age. GM, gray matter.
Brain volume-function relationships between participants with HA and controls. Cerebellar GM is on the x-axis, and the executive function measures total switching accurate (A), category switching: total correct (B), and BRI (C) are on the y-axis. The HA cohort is shown in red and healthy controls in blue. The regression slopes of the HA cohort and healthy controls are significantly different (for panel A, P < .05). In addition, children with HA predominantly fall within the lower left quadrant: smaller cerebellar GM volumes and lower D-KEFS switching scores (for panel A, P < .01 and for panel B, P < .01). Children with HA predominantly fall within the upper left quadrant: smaller cerebellar GM volumes and higher (ie, worse) BRI scores (for panel C, P < .001). The shaded areas represent 95% CIs. Results were adjusted for age. GM, gray matter.
Discussion
Differences in brain volumes and the association with neurocognition have not previously been examined in individuals with hemophilia. The purpose of this pilot study was to evaluate brain morphometry in children with HA compared with healthy peers and determine whether brain volumes predicted neurocognitive outcomes. On the basis of the findings in our study, we provide evidence for differences in brain morphometry and executive function between healthy controls and children with severe HA.
Evaluation of brain morphometry demonstrated that cerebellar gray matter volume was disproportionately smaller in the HA cohort compared with healthy controls. It is noteworthy that the cerebellum contains more than half of all neurons in the brain.20 Lesions in the cerebellum have been associated with impairments in learning,21 memory,22 and planning,23 all of which are essential components of executive function.
Analysis of executive function examined by both D-KEFS and BRIEF demonstrated significantly worse scores in children with HA. Our findings support growing evidence that the cerebellum plays a significant role in cognition,24 including executive functioning skills.25 Particular attention has been paid to the cerebellum’s involvement in executive functioning skills such as attention and verbal fluency,26-28 with our analyses confirming a correlation between lower cerebellar gray matter volume in the HA cohort and worse performance in 2 verbal fluency measures from the D-KEFS.13
Higher rates of neuropsychiatric disorders are observed in patients with HA.3 Within the BRIEF, our data revealed a significant impairment on the BRI, highlighting the challenges faced by individuals with HA in shifting cognitive sets and modulating emotions and behaviors via appropriate inhibitory control. This observation is particularly significant when considering that executive function deficiencies, specifically poor inhibition,29 have been identified across a range of psychiatric disorders.30 Our BRIEF results are supported by the recently published eTHINK study (Evolving Treatment of Hemophilia’s Impact on Neurodevelopment, Intelligence, and Other Cognitive Functions),31 which reported worse scores on the global executive function BRIEF among children aged 6 to 18 years with hemophilia compared with normative values. Our battery of neurocognitive assessments included refined measures of clinically relevant executive functioning comparing children with hemophilia with age-matched healthy controls in the same manner/environment.
Similarly, structural brain abnormalities have been linked with deficits in brain function in children with depression,32 attention-deficit hyperactivity disorder,33 and chronic kidney disease.28 Additionally, previous research has noted reductions in cerebellar volume among individuals with schizophrenia compared with neurotypical controls.6,34 These findings not only emphasize the intricate relationship between brain structure and psychiatric pathology, but they may also provide us with valuable insights into the mechanisms underlying the higher prevalence of neuropsychiatric disorders observed in individuals with HA.
Previously, cerebral microbleeds in adults with hemophilia have been correlated with worse cognitive performance and an increased risk of depression, regardless of joint disease.35 There are limitations to solely investigating cerebral microbleeds at a single time point; therefore, our study, which included patients on prophylactic FVIII treatment, aimed to shift the focus away from cerebral microbleeds and toward quantifiable volumetric brain data.
To date, there have been no data evaluating cerebral microbleeds in children with HA. We recognize that it may be possible that our results are due to cerebral microbleeds; however, detection of cerebral microbleeds will often not provide the full picture. Looking for old bleeds in a one-time scan is unhelpful because a single time point is unlikely to capture every previous bleeding event. SWAN (susceptibility-weighted angiography) sequences detect iron deposition; however, iron deposits will not occur after every bleeding event, and if iron deposits do occur, they may resolve with no remaining deposits at the time of scanning. As a post hoc evaluation, we examined SWAN sequences for cerebral microbleeds (using the standard definition of <10 mm) and found 1 microbleed in our HA cohort. It is possible that the decreased brain volumes were caused by microbleeding including single or cumulative events that were not detected individually on these MRI scans. Given that none of our patients had had any intracranial hemorrhage and only 1 of our patients had evidence of a cranial microbleed, exploring the influence of underlying FVIII deficiency on the developing brain and brain volumes is a future direction of this work. To our knowledge, study participants with HA had not used extended half-life FVIII products before these assessments. Therefore, it is unclear how current treatments including extended half-life products and nonfactor products would alter brain volumes and neurocognitive outcomes. These areas warrant future exploration to determine how alternative treatments impact the neurophenotype of individuals with HA.
This study is strengthened by a comprehensively characterized chronic disease cohort and a healthy control population in parallel with a robust assessment of the MRI and neurological outcomes. Although multiple studies have investigated social, emotional, and behavioral functioning in children with hemophilia, no study has combined neurocognitive assessments with quantitatively processed neuroimaging. Strikingly, to the best of our knowledge, there are no published quantitative volumetric neuroimaging data in individuals with hemophilia A or B preceding the present study of children with HA.
Despite notable strengths, our work has limitations. Hemophilia is a rare disease, even more so in females, limiting the population groups to males. These factors restrict the number of available participants at a single center, and thereby eliminate sex-based diversity. Our neurocognitive assessments could be influenced by subtle examiner bias. To mitigate this risk, a standardized interview and assessment process and a double-score and double-data entry methodology applied by 2 trained staff members under the supervision of a licensed psychologist were established. Although our psychometricians were not blinded to case vs control status, the level of HA severity was not revealed to the team members performing cognitive assessment. We recognize that recall bias is a risk when using caregiver-report forms (such as the BRIEF); thus, to mitigate this risk, we used objective (quantitative) data sources and verified caregiver-report forms with the medical record and interviewer-derived objective data.
Our study provides important insights into the previously unrecognized differences in brain volumes in children with HA compared with healthy controls and the corresponding neurocognitive implications. These data suggest that children with HA have neurocognitive deficits and neuropathology/brain injury despite FVIII prophylactic treatment. Of note is that statistically significant differences in brain volumes were found between the healthy controls and the hemophilia sample, even with the small sample size. This highlights an opportunity for future research, which should aim for replication in a larger, longitudinal study with inclusion of all HA severities and younger patients with HA to capture brain morphometry earlier in the disease process. Future research is also necessary to understand how the brain is affected by the new therapies (eg, extended-half-life replacement protein, FVIII mimetics, nonfactor therapies, gene therapy) for individuals with HA given that the standard of care has evolved since the time of our study. It is crucial to continue expanding the currently limited knowledge of the hemophilia–brain relationship (neurohemophilia) to determine the necessary treatments to prevent joint, muscle, and neurological damage, and ensure that individuals with HA are enjoying a quality of life similar to that of their unaffected peers. These results will inform the design of clinical trials in infants and children with the goal of improving neurological outcomes in children with HA.
Acknowledgments
The authors thank all the participants and their families for contributing their time for this study.
This work was supported by the National Hemophilia Foundation Bridge Award (J.M.S.) and the University of Iowa Stead Family Children’s Hospital Children’s Miracle Network Research Fund (A.A.).
Authorship
Contribution: J.M.S. conceived the study; J.M.S. and L.H. designed the study; A.A. and J.M.S. recruited participants and collected data; M.N. collected data; J.M.S., E.v.d.P., L.H., A.L.C., and S.V. analyzed the data; J.M.S., A.A., and S.V. wrote the original draft; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janice M. Staber, 3080J Medical Laboratories, University of Iowa Carver College of Medicine, Iowa City, IA 52242; email: janice-staber@uiowa.edu.
References
Author notes
Data are available on request from the corresponding author, Janice M. Staber (janice-staber@uiowa.edu).
The full-text version of this article contains a data supplement.