Tissue factor (TF) is a transmembrane protein that, in association with its ligand factor VII (FVII)/activated factor VII (FVIIa), activates blood coagulation. TF is highly procoagulant and even very small amounts can activate blood coagulation. Levels of TF–positive extracellular vesicles (EVs) are increased in blood in diseases associated with thrombosis. However, it is challenging to accurately quantify the very low levels of TF in blood. Activity-based assays have higher sensitivity and specificity than antigen-based assays. Many anti-human TF antibodies have been generated but they differ in their affinity for TF and bind to different epitopes. They can be divided into 2 groups: those that compete with FVII/FVIIa binding to TF, and those that bind to both TF and the TF-FVII/VIIa complex. Commercial enzyme-linked immunosorbent assays are commonly used to measure TF antigen in plasma but have low sensitivity and specificity for the detection of TF antigen in plasma. Flow cytometry is used to measure TF antigen on EVs but also has low sensitivity and specificity. Functional TF activity assays should be performed in the presence and absence of an inhibitory anti-TF antibody to distinguish between TF-dependent and TF-independent FXa generation because FVIIa can activate FX in the absence of TF. TF pathway inhibitor inhibits the TF-FVIIa complex and reduces TF activity of isolated EVs. Two commercial assays are available for the measurement of TF activity of EVs isolated from human plasma. Measurement of TF activity of EVs isolated from plasma may be a useful biomarker of thrombotic risk in different diseases.
Introduction
In this review, we describe the different assays used to measure tissue factor (TF) antigen and activity in human plasma, as well as in extracellular vesicles (EVs) isolated from human plasma. We discuss the strengths and weaknesses of the different assays and some of the challenges and pitfalls in measuring TF in plasma and EVs. Most studies use a single method to measure the level of TF and do not independently validate results using a different method.
A fundamental scientific principle is to use negative and positive controls in experiments. However, most investigators do not use these controls when measuring TF in plasma using different assays. Negative and positive controls can be easily made by preparing plasma of blood of healthy volunteers (negative control) or by preparing plasma from whole blood from healthy volunteers stimulated with lipopolysaccharide (LPS) for 5 hours, which induces TF expression in monocytes and the subsequent release of TF-positive EVs.1 In this review, we provide recommendations on the best methods that should be used to measure TF in EVs isolated from human plasma.
TF
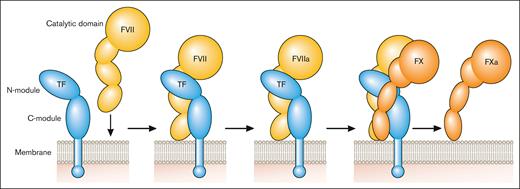
TF is a transmembrane glycoprotein that binds plasma factor VII (FVII)/activated factor VII (FVIIa).2 It was designated CD142.3 FVII/FVIIa binds to TF with high affinity. TF localizes FVII/FVIIa to the membrane and functions as a cofactor for FVIIa by inducing an allosteric change in FVIIa that increases its catalytic activity and by providing a substrate binding site (exosite) for FX (Figure 1). TF initiates the coagulation protease cascade by first binding FVII, which is rapidly converted to the active protease FVIIa to form the extrinsic Xase complex (TF-FVIIa; Figure 1). FX then binds to the TF-FVIIa complex and is activated to FXa (Figure 1). The TF-FVIIa complex also activates FIX.2 Amplification of the coagulation protease cascade is mediated by the intrinsic Xase complex (FVIIIa-FIXa). The TF-FVIIa complex is rapidly inhibited by TF pathway inhibitor (TFPI) after the generation of FXa.4 FXa binds to Kunitz domain 2 of TFPI and then the TFPI-FXa complex binds to FVIIa in the TF-FVIIa-FXa complex via Kunitz domain 1 of TFPI to form a quaternary complex (Figure 2).
Initiation of the coagulation protease cascade. The coagulation protease cascade is initiated by binding of FVII to TF. FVII is rapidly converted to FVIIa that then cleaves FX to FXa. FXa is the favored substrate for the TF-FVIIa complex but this complex can also activate FIX. Professional illustration by Patrick Lane, ScEYEnce Studios.
Initiation of the coagulation protease cascade. The coagulation protease cascade is initiated by binding of FVII to TF. FVII is rapidly converted to FVIIa that then cleaves FX to FXa. FXa is the favored substrate for the TF-FVIIa complex but this complex can also activate FIX. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inhibition of the coagulation protease cascade. TFPI binds to FXa via the Kunitz (K) 2 domain and then this bimolecular complex binds to FVIIa in the TF-FVIIa complex via the K1 domain to inhibit its activity. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inhibition of the coagulation protease cascade. TFPI binds to FXa via the Kunitz (K) 2 domain and then this bimolecular complex binds to FVIIa in the TF-FVIIa complex via the K1 domain to inhibit its activity. Professional illustration by Patrick Lane, ScEYEnce Studios.
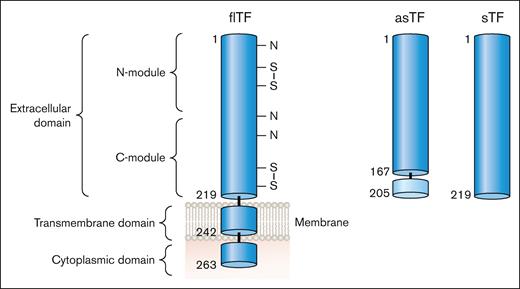
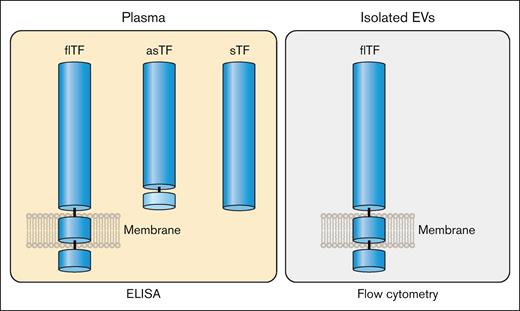
There are 2 naturally occurring isoforms of TF: full-length TF (flTF) and alternatively spliced TF (asTF; Figure 3). Human flTF is a 263 amino acid polypeptide that contains 3 domains: an extracellular domain, a transmembrane domain, and a cytoplasmic domain (Figure 3). The extracellular domain is composed of 2 fibronectin type 3 domains (modules; Figure 3).5 The N-terminal and C-terminal modules bind FVII whereas the C-terminal module binds the substrates FX and FIX (Figure 1).6 asTF is generated by alternative splicing of the TF messenger RNA that results in expression of a protein in which part of the C-terminal module, the transmembrane domain, and the cytoplasmic domain are removed (Figure 3).7 asTF is soluble and has essentially no procoagulant activity because it has lost the substrate binding site in the C-terminal module.8,9 Several groups have expressed the extracellular domain in the absence of the transmembrane and cytoplasmic domains. This protein is referred to as soluble TF (sTF; Figure 3). One study showed that sTF has only 4% of the procoagulant activity of flTF.10 At present, there are no reports of sTF being generated in vivo.
TF isoforms. flTF is a 263–amino acid polypeptide that is divided into 3 domains: an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular domain is composed of 2 fibronectin type 2–like domains. There are 3 N-linked glycosylation sites (N) and 2 disulfide bonds (S-S) in the extracellular domain. asTF contains the first 167 amino acids of TF spliced to 38 different amino acids at the C-terminus. The extracellular domain without the transmembrane domain is a soluble protein (sTF). Professional illustration by Patrick Lane, ScEYEnce Studios.
TF isoforms. flTF is a 263–amino acid polypeptide that is divided into 3 domains: an extracellular domain, a transmembrane domain, and a cytoplasmic domain. The extracellular domain is composed of 2 fibronectin type 2–like domains. There are 3 N-linked glycosylation sites (N) and 2 disulfide bonds (S-S) in the extracellular domain. asTF contains the first 167 amino acids of TF spliced to 38 different amino acids at the C-terminus. The extracellular domain without the transmembrane domain is a soluble protein (sTF). Professional illustration by Patrick Lane, ScEYEnce Studios.
TF expression
High levels of TF are expressed by cells surrounding blood vessels and at body surfaces, such as adventitial fibroblasts, pericytes, and keratinocytes, where it acts as a hemostatic envelope to activate the coagulation protease cascade after vessel injury.11 In 1999, it was reported that human native blood contains a small amount of TF that could drive thrombosis in ex vivo models.12 This TF was called “blood-borne” TF and may be derived, in part, from circulating monocytes, which were reported to express low levels of TF.13 However, a significant issue with measuring TF in blood is the quality of the blood draw and which tube is used for analysis. One study measured levels of fibrinopeptide A in sequential blood samples from healthy volunteers after discarding the first 2.5 mL.14 Levels of fibrinopeptide A serially decreased in 3 sequential samples from 5 healthy volunteers. We recommend discarding the first tube of blood because it is more likely to contain TF from the vessel wall compared with subsequent tubes. At present, it is unclear how much of the apparent TF reported in blood samples is from the blood itself vs TF from the vein wall.
TF-positive EVs
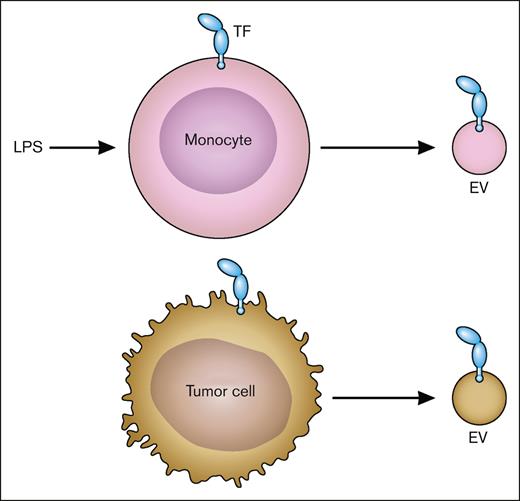
flTF is released from cells on EVs.15 In vitro studies show that TF-positive EVs are released from cancer cells, such as pancreatic cancer cells, and activated nonmalignant cells, such as LPS-stimulated monocytes (Figure 4). Importantly, increased levels of TF-positive EVs are present in the circulation of patients with diseases associated with activation of coagulation, disseminated intravascular coagulation, and thrombosis. These include patients with cancer, endotoxemia and sepsis, and viral infections, including severe acute respiratory syndrome coronavirus 2.15,16 Similar to human diseases, we and others have shown that endotoxemic mice and mice bearing pancreatic tumors have elevated levels of TF-positive EVs in the circulation.17-19 Most, if not all, active TF in plasma is present as flTF on EVs.
TF-positive EVs. LPS-stimulated monocytes express TF and release TF-positive EVs. Tumor cells also constitutively release TF-positive EVs. Professional illustration by Patrick Lane, ScEYEnce Studios.
TF-positive EVs. LPS-stimulated monocytes express TF and release TF-positive EVs. Tumor cells also constitutively release TF-positive EVs. Professional illustration by Patrick Lane, ScEYEnce Studios.
Anti-TF antibodies
Antibodies are useful tools to detect proteins in different biological samples. The guidelines for the use of antibodies from the National Institutes of Health recommend providing the dilution, source, and how the antibodies were validated.20 There are many commercial and noncommercial polyclonal and monoclonal antibodies against human TF. It is important to note that these antibodies have different affinities for TF. In general, those generated by academic laboratories are more carefully characterized compared with those generated by commercial companies. Some monoclonal antibodies, such as HTF-1 and 10H10, were generated in academic laboratories but are sold by commercial companies.
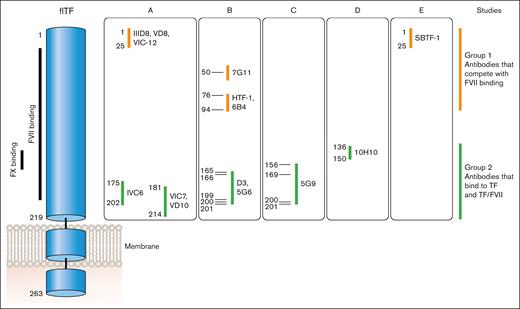
Anti-human TF monoclonal antibodies can be divided into 2 groups based on where they bind to human TF (Figure 5). Antibodies in group 1 bind to the N-terminal module (epitopes amino acids 1-94) and compete with FVII/FVIIa binding to TF. This group includes IIID8, VD8, VIC12, 7G11, HTF-1, 9C3, 6B4, and SBTF-121-24 (F. Dignat-George, written communication, May 2024). Antibodies in group 2 bind to the C-terminal module (epitopes amino acids 136-214) and most compete with binding of the substrates FX and FIX to TF. This group includes IVC6, VIC7, VD10, D3, 5G6, 5G9, and 11D12.21-25 10H10 belongs to group 2 but does not inhibit TF activity.24 Antibodies in this group can bind to either TF or the TF-FVII/FVIIa complex. It was originally thought that antibodies in group 2, such as 5G9, D3, and cH36, could be used to prevent TF-dependent thrombosis because they do not have to compete with FVII/FVIIa for binding to TF.25-28 However, these antibodies were associated with increased bleeding.
Epitopes of different anti-TF antibodies. FVII binds to the N- and C-modules of TF whereas FX binds to the C-module. Anti-TF antibodies can be divided into 2 groups: group 1 antibodies compete with FVII/FVIIa binding to TF via epitopes between amino acids 1 and 94; group 2 antibodies bind to TF (epitopes amino acids 136-214) as well as TF bound with FVII/FVIIa and all except 10H10 inhibit binding of FX and FIX. Professional illustration by Patrick Lane, ScEYEnce Studios.
Epitopes of different anti-TF antibodies. FVII binds to the N- and C-modules of TF whereas FX binds to the C-module. Anti-TF antibodies can be divided into 2 groups: group 1 antibodies compete with FVII/FVIIa binding to TF via epitopes between amino acids 1 and 94; group 2 antibodies bind to TF (epitopes amino acids 136-214) as well as TF bound with FVII/FVIIa and all except 10H10 inhibit binding of FX and FIX. Professional illustration by Patrick Lane, ScEYEnce Studios.
There are very few monoclonal antibodies against mouse TF.29,30 The most commonly used antibody against mouse TF is a rat anti-mouse TF monoclonal antibody from Genentech, called 1H1.30
One method to evaluate an antibody is to determine whether it detects its target on a western blot. However, this does not guarantee that it is suitable for other applications, such as flow cytometry (FCM) and immunohistochemistry, because the epitope may not be preserved in western blotting. In addition, an antibody may detect its target in a western blot but may have off-target effects in other assays. The need for validating antibodies in common research applications was highlighted by the International Working Group for Antibody Validation.31 Several recommendations were made for western blotting, including the elimination or reduction of the target protein and testing multiple antibodies. We found that 4 commercial anti-human TF antibodies, including 2 monoclonal antibodies (HTF-1 and 10H10) detected human TF in a western blot.32 All these antibodies were specific because they only detected TF in a cell lysate from a human pancreatic cancer cell line HPAF-II. Another study found that 5 different anti-human TF monoclonal antibodies (10H0, 9C3, 5G9, 6B4, and VD8) detected TF in a western blot.33 In contrast, only 2 of 5 commercial antibodies detected mouse TF in a western blot using a cell lysate from a mouse pancreatic cancer cell line.32
Measurement of TF antigen in plasma and EVs
There are several major challenges with the immunological detection of TF antigen in plasma. First, TF is present at very low levels compared with other proteins in plasma, such as cytokines. Only very small amounts of TF are needed to initiate the coagulation protease cascade because of amplification of the cascade. Significant amounts of TF are only present in the blood under pathological conditions, such as cancer, sepsis, or viral infections. Furthermore, the level of TF in plasma is often below the detection limit of most assays even under pathological conditions. Secondly, the majority of TF on EVs in plasma will be bound with FVII/FVIIa because of its high affinity and this will interfere with binding of anti-TF antibodies in group 1 (Figure 5). For instance, we find significant TF activity of EVs isolated from LPS-stimulated blood in the absence of exogenous FVIIa, which is consistent with the notion that FVII/FVIIa is already bound to TF (A. Sachetto and N. Mackman, unpublished data, October 2023). This is generally not considered by investigators when choosing antibodies for immunological detection of TF because these antibodies are evaluated in cell-based systems that do not contain FVII/FVIIa. Because FVII/FVIIa binding to TF is calcium dependent, addition of a calcium-chelating agent, such as EDTA, should disrupt binding of FVII/FVIIa to TF and increase binding of group 1 antibodies.34,35 Thirdly, plasma is complex and contains many proteins and multivalent substances, such as heterophilic antibodies, that can generate nonspecific signals independent of the target protein by bridging assay antibodies.36 It is possible that levels of these heterophilic antibodies increase in some disease states.
flTF is the major isoform of TF in plasma but small levels of asTF may also be present (Figure 6). In addition, it is also possible that sTF may be generated under certain disease conditions (Figure 6). In contrast to the multiple forms of TF that may be present in plasma, the only form of TF on isolated EVs is flTF (Figure 6). Therefore, the level of TF antigen in plasma may not correlate with the level of active TF because asTF and sTF have much lower procoagulant activity than flTF. This is important because many studies often use the level of TF antigen in plasma and EVs as a biomarker of thrombotic risk in different diseases.
Isoforms of TF in plasma and EVs. Plasma contains flTF on EVs and asTF. It is possible that plasma may also contain sTF. In contrast, isolated EVs only contain flTF. Professional illustration by Patrick Lane, ScEYEnce Studios.
Isoforms of TF in plasma and EVs. Plasma contains flTF on EVs and asTF. It is possible that plasma may also contain sTF. In contrast, isolated EVs only contain flTF. Professional illustration by Patrick Lane, ScEYEnce Studios.
There are several techniques that have been developed to the measure level of TF antigen in plasma and EVs isolated from plasma.
ELISAs
Enzyme-linked immunosorbent assays (ELISAs) are commonly used to measure proteins in plasma. Although several groups developed in-house ELISAs for human TF,37-40 most investigators use commercial ELISAs. We found 49 different human TF ELISAs sold by 20 different companies. IMUBIND Tissue Factor ELISA (BioMedica Diagnostics, catalog no. 845 [IMUBIND]) and Tissue Factor Quantikine ELISA (R&D Systems, catalog no. DCF300 [Quantikine]) are the most frequently used ELISAs in the literature.36 Concerns have been raised about the ability of commercial assays to accurately quantitate TF antigen levels in human plasma.38,41-43 One study used an in-house double anti-TF monoclonal antibody fluorescent-based immunoassay to detect TF in plasma from 72 healthy individuals.38 They found that 57 (79%) had TF antigen values of <94 pg/mL (the detection limit of the assay), 6 (7%) had values between 94 and 235 pg/mL, and 10 (14%) had a nonspecific signal that was higher than the specific signal.
Table 1 shows some of the recent studies that have used the IMUBIND and Quantikine ELISAs for the measurement of TF antigen in human plasma in healthy individuals and in different diseases. It is striking that IMUBIND gives high values and a large range of values for TF antigen in plasma from healthy individuals (62-215 pg/mL, n = 11; Table 1). The manufacturer of IMUBIND states that “a normal range for human plasma remains to be established” despite being sold for many years. In contrast, Quantikine gives lower values and a smaller range for TF antigen in plasma from healthy individuals (23-110, n = 9); Table 1). Quantikine states that the value for citrated plasma from healthy individuals is 33.3 ± 6.6 pg/mL. The different values reported in the different studies are likely due to differences in preanalytical variables, such as plasma preparation. Although these studies report statistically significant increases in TF antigen in the different diseases, it is a concern that in most of these studies values for the disease group overlap with values from the healthy controls. In addition, no other method was used to measure TF.
Studies measuring TF antigen in plasma using IMUBIND and Quantikine ELISAs
| Disease . | Kit . | Healthy group TF antigen (pg/mL) . | Disease group TF antigen (pg/mL) . | Data type . | P value . | Reference . |
|---|---|---|---|---|---|---|
| Leukemia | IMUBIND | 117 ± 19.2 | 273 ± 90 | Mean ± SD | <.01 | 44 |
| Coronary artery disease | IMUBIND | 187.3 ± 108.7 | 303.6 ± 134.1 | Mean ± SD | <.05 | 45 |
| Peripheral artery disease | IMUBIND | 158 ± 60 | 275 ± 122 | Mean ± SD | <.0001 | 46 |
| Ischemic heart disease | IMUBIND | 142 (18-262) | 179.2 (51.2-380) (stable) 215 (56.8-834.3) (unstable) | Median (IQR) | <.0001 <.0001 | 47 |
| Coronary artery disease | IMUBIND | 76 (32-108) | 116 (92-150) (stable) | Median (IQR) | <.01 | 48 |
| 146 (104-188) (unstable) | <.001 | |||||
| Glomerulonephritis | IMUBIND | 144 ± 73.9 | 283 ± 145 | Mean ± SD | .009 | 49 |
| Non–small cell lung cancer | IMUBIND | 124.1 ± 14.79 | 334.9 ± 95.44 | Mean ± SD | .02 | 50 |
| Obstructive sleep apnea | IMUBIND | 42.83 ± 14.18 | 66.78 ± 41.59 | Mean ± SD | .005 | 51 |
| Aneurysm | IMUBIND | 62 ± 20 | 134 ± 54 (AAA) | Mean ± SD | <.001 | 52 |
| 91 ± 30 (IAA/atherosclerotic lesion) | <.008 | |||||
| Cancer | IMUBIND | 215.6 ± 55.1 | 265.2 ± 124.3 | Mean ± SD | .014 | 53 |
| Breast cancer | IMUBIND | 115.35 (92.65-166.81) | 539.93 (400.94-710.40) | Median (IQR) | .0001 | 54 |
| IMUBIND | 110.9 ± 70.97 | Mean ± SD | 36 | |||
| Leukemia | IMUBIND | 172.2 ± 58.9 | Mean ± SD | 36 | ||
| Pancreatic adenocarcinoma | Quantikine | 52.4 (28.4-72.4) | 63.8 (32.7-112) (stage I-III) 82.8 (45.6-141) (metastatic) | Mean (range) | NS <.001 | 55 |
| Pancreatic adenocarcinoma | Quantikine | 39.1 (16.8-87.3) | 47.2 (0.0-224.8) (without CAT) | Median (range) | .009 | 33 |
| 56.0 (37.6-318.7) (with CAT) | .002 | |||||
| Multiple sclerosis | Quantikine | 110.85 ± 56.31 | 94.06 ± 55.83 (remitting) 45.03 ± 37.66 (relapsing) | Mean ± SD | <.001 | 56 |
| Quantikine | 45.01 ± 12.10 | Mean ± SD | 36 | |||
| Leukemia | Quantikine | 99.30 ± 78.66 | Mean ± SD | 36 |
| Disease . | Kit . | Healthy group TF antigen (pg/mL) . | Disease group TF antigen (pg/mL) . | Data type . | P value . | Reference . |
|---|---|---|---|---|---|---|
| Leukemia | IMUBIND | 117 ± 19.2 | 273 ± 90 | Mean ± SD | <.01 | 44 |
| Coronary artery disease | IMUBIND | 187.3 ± 108.7 | 303.6 ± 134.1 | Mean ± SD | <.05 | 45 |
| Peripheral artery disease | IMUBIND | 158 ± 60 | 275 ± 122 | Mean ± SD | <.0001 | 46 |
| Ischemic heart disease | IMUBIND | 142 (18-262) | 179.2 (51.2-380) (stable) 215 (56.8-834.3) (unstable) | Median (IQR) | <.0001 <.0001 | 47 |
| Coronary artery disease | IMUBIND | 76 (32-108) | 116 (92-150) (stable) | Median (IQR) | <.01 | 48 |
| 146 (104-188) (unstable) | <.001 | |||||
| Glomerulonephritis | IMUBIND | 144 ± 73.9 | 283 ± 145 | Mean ± SD | .009 | 49 |
| Non–small cell lung cancer | IMUBIND | 124.1 ± 14.79 | 334.9 ± 95.44 | Mean ± SD | .02 | 50 |
| Obstructive sleep apnea | IMUBIND | 42.83 ± 14.18 | 66.78 ± 41.59 | Mean ± SD | .005 | 51 |
| Aneurysm | IMUBIND | 62 ± 20 | 134 ± 54 (AAA) | Mean ± SD | <.001 | 52 |
| 91 ± 30 (IAA/atherosclerotic lesion) | <.008 | |||||
| Cancer | IMUBIND | 215.6 ± 55.1 | 265.2 ± 124.3 | Mean ± SD | .014 | 53 |
| Breast cancer | IMUBIND | 115.35 (92.65-166.81) | 539.93 (400.94-710.40) | Median (IQR) | .0001 | 54 |
| IMUBIND | 110.9 ± 70.97 | Mean ± SD | 36 | |||
| Leukemia | IMUBIND | 172.2 ± 58.9 | Mean ± SD | 36 | ||
| Pancreatic adenocarcinoma | Quantikine | 52.4 (28.4-72.4) | 63.8 (32.7-112) (stage I-III) 82.8 (45.6-141) (metastatic) | Mean (range) | NS <.001 | 55 |
| Pancreatic adenocarcinoma | Quantikine | 39.1 (16.8-87.3) | 47.2 (0.0-224.8) (without CAT) | Median (range) | .009 | 33 |
| 56.0 (37.6-318.7) (with CAT) | .002 | |||||
| Multiple sclerosis | Quantikine | 110.85 ± 56.31 | 94.06 ± 55.83 (remitting) 45.03 ± 37.66 (relapsing) | Mean ± SD | <.001 | 56 |
| Quantikine | 45.01 ± 12.10 | Mean ± SD | 36 | |||
| Leukemia | Quantikine | 99.30 ± 78.66 | Mean ± SD | 36 |
AAA, abdominal aortic aneurysm; CAT, cancer-associated thrombosis; IAA, inflammatory aortic aneurysm; IQR, interquartile range; NS, nonsignificant; SD, standard deviation.
A study evaluated the ability of 4 commercial ELISAs (IMUBIND, Quantikine, Zymutest [Hyphen BioMed], and Tissue Factor [CD142] Human ELISA [Abcam, catalog no. ab108903]) to detect TF expressed by a variety of different human cancer cell lines.33 The level of TF detected by all the ELISAs except CD142 correlated with the signal from western blotting. We evaluated the ability of 4 commercial ELISAs (IMUBIND, Quantikine, CD142, and Human Tissue Factor SimpleStep ELISA [Abcam, catalog no. ab220653]) to detect recombinant human TF (12.5-100 pg/mL), recombinant human TF spiked into plasma (12.5-100 pg/mL), low levels of endogenous TF in LPS-stimulated human whole blood, and high levels of endogenous TF in plasma from patients with acute leukemia.36 The level of TF was measured using an EV TF activity assay. All 4 ELISAs stated that they could be used to measure TF in plasma with a limit of detection ranging from 0.69 to 3.6 pg/mL. Consistent with the aforementioned study,33 the CD142 ELISA failed to detect recombinant TF whereas the SimpleStep ELISA detected recombinant TF but failed to detect recombinant TF spiked into plasma. The Quantikine and IMUBIND ELISAs detected recombinant TF spiked into plasma but did not detect low levels of TF in plasma from LPS-treated blood. However, Quantikine but not IMUBIND detected TF in plasma from patients with acute leukemia. We also found that both ELISAs detected TF in EVs isolated from the plasma of patients with leukemia with high levels of EV TF activity, but only Quantikine detected TF in EV-depleted plasma. At present, the source of the TF signal in EV-depleted plasma is unclear.
In conclusion, commercial ELISAs can be used to measure high levels of TF in cell lysates and, in rare cases, of high levels of TF in plasma from patients, such as patients with acute leukemia. However, in general, they were not able to detect low levels of TF antigen in plasma. We do not recommend using commercial ELISAs for measuring TF antigen in human plasma.
We found 37 different mouse TF ELISAs sold by 15 different companies. Mouse Coagulation Factor III/Tissue Factor DuoSet ELISA (R&D Systems, catalog no. Dy3178-05) is the most frequently used ELISA, 5 papers published between 2018 and 2023. We evaluated the ability of 4 commercial mouse TF ELISAs to detect recombinant TF (16-1000 pg/mL), recombinant TF spiked into plasma (16-1000 pg/mL), TF in a cell lysate of a TF-expressing cell line, and endogenous TF-positive EVs in plasma from endotoxemic mice.57,58 The Mouse Tissue Factor ELISA (MyBioSource, catalog no. MBS2512143) and Mouse Tissue Factor Competitive ELISA (MyBioSource, catalog no. MBS722558) failed to detect recombinant mouse TF. The Mouse Tissue Factor SimpleStep ELISA (Abcam, catalog no. ab214091) and the Mouse Coagulation Factor III/Tissue Factor DuoSet ELISA (R&D Systems, catalog no. Dy3178-05) detected recombinant mouse TF and TF in cell lysates but not TF in plasma from endotoxemic mice. These results indicate that, similar to human TF ELISAs, commercial mouse TF ELISAs can detect high levels of TF in cell lysate but cannot detect low levels of TF in plasma.
PLA
The proximity ligation assay (PLA) is a sensitive method to measure levels of proteins in plasma. It uses 2 antibodies against the target protein with attached complementary oligonucleotides that anneal when in close proximity to form double-stranded DNA, which can be amplified by quantitative polymerase chain reaction. One study used a nonconventional PLA strategy that used a single polyclonal anti-human TF antibody that was separately labeled with the complementary oligonucleotides to measure TF in plasma.59 Interestingly, the assay detected recombinant TF spiked into plasma but failed to detect TF-positive EVs in plasma from LPS-stimulated whole blood. OLINK Proteomics (Uppsala, Sweden) uses PLA to measure levels of proteins in plasma, including TF. The advantages of the assay are that it only requires a small amount of plasma and can measure many different proteins in the same sample. However, the disadvantages of the assay are that there is no information on the anti-TF antibodies used in the assay and the results are presented as normalized protein expression rather than absolute values. This means that the results cannot be compared with other antigen-based assays.
FCM
FCM is typically used to analyze cells in solution. TF expression on cells is detected using anti-TF antibodies conjugated to different fluorophores, which include phycoerythrin (PE; yellow-orange), fluorescein isothiocyanate (FITC; green), and allophycocyanin (far-red). However, conjugation of antibodies with fluorophores can decrease their avidity. There are several factors to consider when choosing an anti-TF antibody to detect TF using FCM. It is preferable to know the affinity, the binding epitope on TF, and the specificity of the antibody. One method to validate an anti-TF antibody is to determine whether it binds to LPS-stimulated monocytes. One study analyzed the ability of different anti-TF monoclonal antibodies to bind to unstimulated and LPS-stimulated monocytes.65 As expected, 3 antibodies (HTF-1, VD8, and 10H10) bound to TF on LPS-stimulated monocytes but not to unstimulated monocytes. However, 2 antibodies (VIC7 and CLB/TF-5) bound nonspecifically to unstimulated monocytes. In contrast, another study found that VIC7 bound to LPS-stimulated monocytes but not unstimulated monocytes.3 Recently, we showed that 3 anti-TF monoclonal antibodies (5G9, VIC12, and IIID8) bound to TF on LPS-stimulated monocytes.66
There are 2 major challenges with measuring the level of TF on EVs in plasma or isolated from plasma. First, EVs are very small (∼10 times smaller than cells) and need specialized flow cytometers for detection. Secondly, EVs bind ∼100 times less antibodies compared with a cell. Thirdly, compared with other receptors, such as CD41 that has an abundance of 200 molecules per platelet-derived EV,67 the number of TF molecules per EV is very low, making it difficult to detect. Table 2 shows some of the studies that have used FCM to measure the level of TF-positive EVs in plasma from patients with different diseases. A variety of commercial labeled anti-TF antibodies were used in these studies. Of 17 studies, 53% used HTF-1, 24% used VD8, 6% used VIC7, 6% used IID8, and 11% used other antibodies. VIC7 is the only group 2 antibody used in these studies that binds to both TF and the TF-FVII/FVIIa complex.
Studies measuring TF using FCM
| Disease/treatment . | Sample type . | TF antibody . | Results . | Reference . |
|---|---|---|---|---|
| Cancer | Plasma | CD142-PE (HTF-1), BD | Higher levels of TF-positive EVs in samples from patients with cancer compared with controls. Higher levels of TF-positive EVs in samples from patients with cancer with VTE compared with without VTE | 68 |
| Atorvastatin treatment | Plasma | CD142-PE (HTF-1), BD | Higher levels of TF-positive EVs during active treatment compared with nontreatment | 69 |
| Deep venous thrombosis | EV | CD142-FITC (VIC7), BioMedica Diagnostics | Higher levels of TF-positive EVs in samples from patients with VTE compared with controls | 70 |
| Statin therapy | EV | CD142-FITC (VD8), BioMedica Diagnostics | Statin therapy decreased the levels of TF-positive EVs | 71 |
| Lung cancer | Plasma | CD142-FITC, BioMedica Diagnostics | Levels of TF-positive EVs predicted distant metastasis | 72 |
| Cardiovascular risk | EV | CD142-FITC (VD8), BioMedica Diagnostics | Levels of TF-positive EVs correlated with lipid-rich atherosclerotic plaques | 73 |
| Cardiovascular risk | EV | CD142-FITC (VD8), BioMedica Diagnostics | Levels of TF-positive EVs were higher in patients with high cardiovascular risk than patients without risk | 74 |
| Type 2 diabetes | EV | CD142-PE (HTF-1), BD | Levels of TF-positive EVs were higher in patients with poor glycemic control than patients with good glycemic control | 75 |
| COVID-19 | EV | CD142-BV421 (HTF-1), BD | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 76 |
| HIV | EV | CD142-APC (NY2), BioLegend | No difference in levels of TF-positive EVs between patients infected with HIV and uninfected controls | 77 |
| COVID-19 | Plasma | CD142-PE (HTF-1), BD | No difference in levels of TF-positive EVs between nonpregnant woman, healthy pregnant woman, and pregnant woman with COVID-19 | 78 |
| COVID-19 | Plasma | CD142-FITC, BioMedica Diagnostics | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 79 |
| COVID-19 | EV | CD142-BV421 (HTF-1), BD | Levels of TF-positive EVs are higher in small EVs than in large EVs | 80 |
| COVID-19 | EV | CD142-FITC (VD8), BioMedica Diagnostics and CD142-PE (HTF-1), BD | Comparison of 2 anti-TF antibodies and fluorochrome-to-protein ratio in the detection of TF on EVs by FCM | 81 |
| COVID-19 | Plasma | CD142-PE (HTF-1), eBioscience | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 82 |
| Cirrhosis and bacterial infection | Plasma | CD142 (IIID8), BioMedica Diagnostics and Alexa Fluor 750 (Sigma-Aldrich) | Patients with cirrhosis and bacterial infection had higher levels of TF-positive EVs than patients with cirrhosis without infection | 83 |
| Colorectal cancer | Plasma | CD142-PE (HTF-1), eBioscience | Levels of TF-positive EVs higher in patients with cancer than in controls | 84 |
| Disease/treatment . | Sample type . | TF antibody . | Results . | Reference . |
|---|---|---|---|---|
| Cancer | Plasma | CD142-PE (HTF-1), BD | Higher levels of TF-positive EVs in samples from patients with cancer compared with controls. Higher levels of TF-positive EVs in samples from patients with cancer with VTE compared with without VTE | 68 |
| Atorvastatin treatment | Plasma | CD142-PE (HTF-1), BD | Higher levels of TF-positive EVs during active treatment compared with nontreatment | 69 |
| Deep venous thrombosis | EV | CD142-FITC (VIC7), BioMedica Diagnostics | Higher levels of TF-positive EVs in samples from patients with VTE compared with controls | 70 |
| Statin therapy | EV | CD142-FITC (VD8), BioMedica Diagnostics | Statin therapy decreased the levels of TF-positive EVs | 71 |
| Lung cancer | Plasma | CD142-FITC, BioMedica Diagnostics | Levels of TF-positive EVs predicted distant metastasis | 72 |
| Cardiovascular risk | EV | CD142-FITC (VD8), BioMedica Diagnostics | Levels of TF-positive EVs correlated with lipid-rich atherosclerotic plaques | 73 |
| Cardiovascular risk | EV | CD142-FITC (VD8), BioMedica Diagnostics | Levels of TF-positive EVs were higher in patients with high cardiovascular risk than patients without risk | 74 |
| Type 2 diabetes | EV | CD142-PE (HTF-1), BD | Levels of TF-positive EVs were higher in patients with poor glycemic control than patients with good glycemic control | 75 |
| COVID-19 | EV | CD142-BV421 (HTF-1), BD | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 76 |
| HIV | EV | CD142-APC (NY2), BioLegend | No difference in levels of TF-positive EVs between patients infected with HIV and uninfected controls | 77 |
| COVID-19 | Plasma | CD142-PE (HTF-1), BD | No difference in levels of TF-positive EVs between nonpregnant woman, healthy pregnant woman, and pregnant woman with COVID-19 | 78 |
| COVID-19 | Plasma | CD142-FITC, BioMedica Diagnostics | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 79 |
| COVID-19 | EV | CD142-BV421 (HTF-1), BD | Levels of TF-positive EVs are higher in small EVs than in large EVs | 80 |
| COVID-19 | EV | CD142-FITC (VD8), BioMedica Diagnostics and CD142-PE (HTF-1), BD | Comparison of 2 anti-TF antibodies and fluorochrome-to-protein ratio in the detection of TF on EVs by FCM | 81 |
| COVID-19 | Plasma | CD142-PE (HTF-1), eBioscience | Levels of TF-positive EVs higher in patients with COVID-19 than in healthy controls | 82 |
| Cirrhosis and bacterial infection | Plasma | CD142 (IIID8), BioMedica Diagnostics and Alexa Fluor 750 (Sigma-Aldrich) | Patients with cirrhosis and bacterial infection had higher levels of TF-positive EVs than patients with cirrhosis without infection | 83 |
| Colorectal cancer | Plasma | CD142-PE (HTF-1), eBioscience | Levels of TF-positive EVs higher in patients with cancer than in controls | 84 |
APC, allophycocyanin; VTE, venous thromboembolism.
A recent study evaluated the ability of 2 anti-TF antibodies (VD8-FITC and HTF-1-PE) to detect TF expression on LPS-stimulated monocytes and TF-positive EVs released from LPS-stimulated monocytes, mesenchymal stems cells, and in plasma from patients with COVID-19.81 As expected, both antibodies detected TF expression on LPS-stimulated monocytes. However, the study found that VD8-FITC but not HTF-1-PE detected TF on EVs. VD8 and HTF-1 belong to group 1 anti-TF antibodies and compete with FVII/FVIIa for binding to TF. However, the specific epitopes are different (the epitope for VD8 is amino acids 1-25 whereas the epitope for HTF-1 is amino acid 94 and 76) and VD8 appears to have a higher affinity than HTF-1. Furthermore, the fluorochrome-to-protein ratio for the anti-TF–fluorochrome conjugates affected the ability of the antibody to detect TF on EVs but not TF on LPS-stimulated monocytes. A higher ratio was associated with a higher percentage detection of TF-positive EVs (7.6% vs 1.1% with ratios of 7.7:1 and 5.2:1, respectively, for detection of TF-positive EVs from LPS-stimulated monocytes). These results indicate that the choice of anti-TF antibody and the fluorochrome-to-protein ratio affects the ability of anti-TF antibodies to detect TF on EVs.
The MACSPlex Exosome Kit (Miltenyi Biotec) quantifies EVs by capturing them onto different fluorescently labeled beads using specific antibodies. This system has been used by 1 group to measure levels of TF-positive EVs in plasma from patients with COVID-19.85,86 In contrast, we found that the MACSPlex Exosome Kit failed to detect TF-positive EVs isolated from plasma from LPS-stimulated whole blood.87 We also noted that the TF signal was below the threshold of detection of the assay and >100-times lower than the signal for platelet-derived CD41+ EVs.
Exoview
The ExoView R100 system (Unchained Labs, Pleasanton, CA) is a chip-based system that uses printed antibodies against cell surface proteins to capture EVs.88 We evaluated the ability of ExoView to capture TF-positive EVs from various sources.66 We found that the anti-TF monoclonal antibody 5G9 (group 2) captured more TF-positive EVs from the culture supernatant of the human pancreatic cancer cell line BxPC-3 than HTF-1 (group 1), which suggests that it has a higher affinity for TF because there is no FVII/FVIIa present. Similarly, 5G9 captured more TF-positive EVs present in plasma from LPS-stimulated blood compared with HTF-1, which may also be because of the ability of 5G9 to bind to both TF and the TF-FVII/FVIIa complex. The TF-positive EVs were detected using the anti-TF antibody IIID8 labeled with Alexa Fluor-647. However, the ExoView system was not sensitive enough to detect TF-positive EVs in plasma from patients with ovarian cancer and patients with trauma who had increased levels of EV TF activity.
Measurement of TF activity in plasma and EVs
Activity-based assays are more sensitive than antigen-based assays for the detection of TF. This is important because there are very low levels of TF in plasma. Various methods have been used to detect TF activity in plasma and EVs but the most common method is to measure FXa generation by the TF-FVIIa complex. In this 2-stage assay, FVII or FVIIa and FX are added to the sample containing TF and incubated at 37°C for a period of time to allow the generation of FXa, and then the amount of FXa is determined using a chromogenic substrate.1 However, FVIIa can cleave FX to FXa independently of TF. One study showed that the concentration of FVIIa directly correlates with the TF-independent FXa generation.89 Therefore, we strongly recommend that all TF activity assays are performed with and without an inhibitory anti-TF antibody to distinguish between TF-dependent and TF-independent FXa generation.
Plasma-based TF activity assays
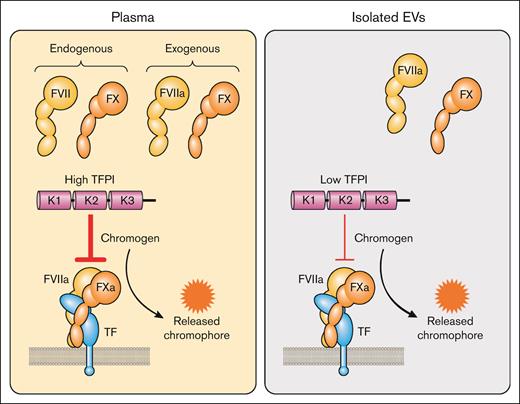
There are 3 commercial assays that claim to measure TF activity in plasma (Actichrome Tissue Factor, catalog no. 846, Sekisui Diagnostics, LLC, Lexington, MA; tissue factor activity assay kit, catalog no. ab109006, Abcam Inc, Boston, MA; and AssaySense human tissue factor chromogenic assay kit, catalog no. CT1002b, AssayPro, St. Charles, MO). These kits use either FVII or FVIIa. There are several concerns with these assays. First, plasma contains endogenous FVII/FVIIa and FX that may interfere with the assay (Figure 7). Second, plasma contains TFPI that will inhibit the TF-FVIIa complex (Figure 7). Third, no anti-TF antibody is used to distinguish between TF-dependent and TF-independent FXa generation.90 Finally, the color of the plasma may interfere with detection of the yellow chromophore. Despite these concerns there are several publications that have used these kits to measure TF activity in plasma.53,91-100 One study measured the level of TF activity in plasma in hospitalized patients with COVID-19 with elevated D-dimer before and after treatment with the TF-FVIIa inhibitor recombinant nematode anticoagulant protein c2.100 Interestingly, there was a 20.3% reduction in TF activity in patients with COVID-19 treated with recombinant nematode anticoagulant protein c2 whereas no decrease was observed in patients treated with heparin. One study measured TF activity in plasma in the presence of an inhibitory anti-TFPI antibody.101 We do not recommend using plasma-based assays to measure the level of TF activity in plasma.
Measurement of TF activity in plasma and isolated EVs. In plasma-based activity assays, exogenous FVIIa and FX are added to plasma that already contains FVII and FX. FVII or FVIIa and FX are added to isolated EVs to determine TF activity. Plasma contains TFPI that can inhibit the TF-FVIIa complex and reduce the amount of FXa generated. Isolated EVs have a small amount of TFPI that is carried over from plasma during the isolation of the EVs. FXa cleavage of a chromogenic peptide substrate leads to the release of a chromophore. Professional illustration by Patrick Lane, ScEYEnce Studios.
Measurement of TF activity in plasma and isolated EVs. In plasma-based activity assays, exogenous FVIIa and FX are added to plasma that already contains FVII and FX. FVII or FVIIa and FX are added to isolated EVs to determine TF activity. Plasma contains TFPI that can inhibit the TF-FVIIa complex and reduce the amount of FXa generated. Isolated EVs have a small amount of TFPI that is carried over from plasma during the isolation of the EVs. FXa cleavage of a chromogenic peptide substrate leads to the release of a chromophore. Professional illustration by Patrick Lane, ScEYEnce Studios.
Measurement of TF activity of isolated EVs using FXa generation
The TF activity of isolated EVs can be measured using different readouts, such as FXa generation, thrombin generation, fibrin generation, and clot formation.39,41,102-106 We, and another group, described EV TF activity assays in the late 2000s that used centrifugation to isolate EVs from plasma.39,102 These assays have been used to show that the level of EV TF activity is increased in a variety of thrombotic diseases, including sepsis, cancer, and viral infections.107 The Chapel Hill assay has been improved by (1) increasing the time and speed of the centrifugation to isolate the EVs, (2) using an inhibitory antibody SBTF-1 that is more potent than HTF-1, and (3) using FVII instead of FVIIa.108 The epitope for the binding of SBTF-1 to TF is the same as VD8 (amino acids 1-25; Dignat-George, personal communication). Finally, a recent study used immunomagnetic separation (IMS) beads that were conjugated with anti-CD29 and anti-CD59 antibodies to capture EVs from plasma instead of isolating them by centrifugation.109 TF activity of the isolated EVs was measured using a 2-stage FXa generation assay. The IMS-based assay had superior reproducibility with no loss of sensitivity or specificity compared with the isolation of EVs using centrifugation. Table 3 shows some of the recent studies that have used a FXa generation assay with an anti-TF antibody to measure the level of TF activity of EVs isolated from plasma. It is notable that elevated levels of EV TF activity were shown to be associated with thrombosis and mortality in patients with COVID-19.16,110
Studies measuring TF activity of EVs using a FXa generation assay
| Disease/treatment . | Sample . | Results . | Reference . |
|---|---|---|---|
| Pancreatic adenocarcinoma | PPP | Levels of TF activity was higher in patients with cancer with VTE than those without VTE, and were associated with metastatic disease and short survival | 111 |
| Ovarian cancer | PPP | No association between levels of EV TF activity and VTE in patients with cancer | 112 |
| Cancer | PPP | Levels of EV TF activity were increased in patients with cancer compared with patients without cancer who were severely ill and healthy controls. Levels of EV TF activity were associated with mortality | 113 |
| Lung cancer | PPP | No difference of levels of EV TF activity between patients with cancer with or without low–molecular weight heparin treatment | 114 |
| Lupus | PPP | No difference in levels of EV TF activity between patients with lupus anticoagulants with history of thrombosis and healthy controls | 115 |
| Hantavirus cardiopulmonary syndrome | Plasma | Levels of EV TF activity were increased in patients with HCPS compared with healthy controls | 116 |
| Multiple myeloma | PFP | Levels of EV TF activity were increased in patients with multiple myeloma compared with controls | 117 |
| Pancreatic and colorectal cancer | PPP | Two of 13 patients with pancreatic cancer had elevated levels of EV TF activity. No patient with colorectal cancer had elevated levels of EV TF activity | 118 |
| Hemorrhagic fever with renal syndrome | Plasma | Levels of EV TF activity were transiently increased during HFRS and patients with DIC showed higher levels of EV TF activity than patients without DIC | 119 |
| HIV | Levels of EV TF activity were associated with carotid artery plaque in patients with HIV | 120 | |
| Cancer | Plasma | No association between the levels of EV TF activity and VTE | 121 |
| Cancer | PPP | Levels of EV TF activity were increased in patients with cancer compared with healthy controls | 122 |
| COVID-19 | PPP | Levels of EV TF activity were increased in patients with COVID-19 compared with controls, and were associated with disease severity and mortality | 110 |
| COVID-19 | PFP | Levels of EV TF activity were increased in patients with severe COVID-19 and were associated with an increased thrombotic risk | 16 |
| COVID-19 and sepsis | PPP | Levels of EV TF activity were higher in ICU patients with COVID-19 than in patients with sepsis | 123 |
| Sepsis | PPP | Levels of EV TF activity were higher in patients with septic DIC than in healthy controls | 124 |
| Leukemia | PFP | Levels of EV TF activity were increased in patients with leukemia compared with controls | 125 |
| Prostate cancer | PPP | Levels of EV TF activity were increased in patients with cancer with DIC compared with patients with cancer without DIC | 126 |
| Lung cancer | PFP | Levels of EV TF activity were increased in patients with cancer compared with healthy controls. | 127 |
| COVID-19 | PFP | Levels of EV TF activity were higher in patients with COVID-19 than in healthy controls | 128 |
| COVID-19 | PFP | Levels of EV TF activity were higher in small EVs than in large EVs from patients with COVID-19 | 80 |
| Sepsis, pancreatic cancer, and COVID-19 | PFP and PPP | Levels of EV TF activity of small or large EVs were different depending on the disease | 129 |
| Sepsis | PPP | Levels of EV TF activity were increased in patients with sepsis with DIC compared with patients with sepsis without DIC. Levels of EV TF activity were associated with inflammatory markers and DIC score | 130 |
| Leukemia | PPP | Levels of EV TF activity were increased in patients with leukemia compared with controls, and were associated with risk of bleeding | 131 |
| Disease/treatment . | Sample . | Results . | Reference . |
|---|---|---|---|
| Pancreatic adenocarcinoma | PPP | Levels of TF activity was higher in patients with cancer with VTE than those without VTE, and were associated with metastatic disease and short survival | 111 |
| Ovarian cancer | PPP | No association between levels of EV TF activity and VTE in patients with cancer | 112 |
| Cancer | PPP | Levels of EV TF activity were increased in patients with cancer compared with patients without cancer who were severely ill and healthy controls. Levels of EV TF activity were associated with mortality | 113 |
| Lung cancer | PPP | No difference of levels of EV TF activity between patients with cancer with or without low–molecular weight heparin treatment | 114 |
| Lupus | PPP | No difference in levels of EV TF activity between patients with lupus anticoagulants with history of thrombosis and healthy controls | 115 |
| Hantavirus cardiopulmonary syndrome | Plasma | Levels of EV TF activity were increased in patients with HCPS compared with healthy controls | 116 |
| Multiple myeloma | PFP | Levels of EV TF activity were increased in patients with multiple myeloma compared with controls | 117 |
| Pancreatic and colorectal cancer | PPP | Two of 13 patients with pancreatic cancer had elevated levels of EV TF activity. No patient with colorectal cancer had elevated levels of EV TF activity | 118 |
| Hemorrhagic fever with renal syndrome | Plasma | Levels of EV TF activity were transiently increased during HFRS and patients with DIC showed higher levels of EV TF activity than patients without DIC | 119 |
| HIV | Levels of EV TF activity were associated with carotid artery plaque in patients with HIV | 120 | |
| Cancer | Plasma | No association between the levels of EV TF activity and VTE | 121 |
| Cancer | PPP | Levels of EV TF activity were increased in patients with cancer compared with healthy controls | 122 |
| COVID-19 | PPP | Levels of EV TF activity were increased in patients with COVID-19 compared with controls, and were associated with disease severity and mortality | 110 |
| COVID-19 | PFP | Levels of EV TF activity were increased in patients with severe COVID-19 and were associated with an increased thrombotic risk | 16 |
| COVID-19 and sepsis | PPP | Levels of EV TF activity were higher in ICU patients with COVID-19 than in patients with sepsis | 123 |
| Sepsis | PPP | Levels of EV TF activity were higher in patients with septic DIC than in healthy controls | 124 |
| Leukemia | PFP | Levels of EV TF activity were increased in patients with leukemia compared with controls | 125 |
| Prostate cancer | PPP | Levels of EV TF activity were increased in patients with cancer with DIC compared with patients with cancer without DIC | 126 |
| Lung cancer | PFP | Levels of EV TF activity were increased in patients with cancer compared with healthy controls. | 127 |
| COVID-19 | PFP | Levels of EV TF activity were higher in patients with COVID-19 than in healthy controls | 128 |
| COVID-19 | PFP | Levels of EV TF activity were higher in small EVs than in large EVs from patients with COVID-19 | 80 |
| Sepsis, pancreatic cancer, and COVID-19 | PFP and PPP | Levels of EV TF activity of small or large EVs were different depending on the disease | 129 |
| Sepsis | PPP | Levels of EV TF activity were increased in patients with sepsis with DIC compared with patients with sepsis without DIC. Levels of EV TF activity were associated with inflammatory markers and DIC score | 130 |
| Leukemia | PPP | Levels of EV TF activity were increased in patients with leukemia compared with controls, and were associated with risk of bleeding | 131 |
DIC, disseminated intravascular coagulation; HCPS, hantavirus cardiopulmonary syndrome; HFRS, hemorrhagic fever with renal syndrome; ICU, intensive care unit; PFP, platelet-free plasma; PPP, platelet-poor plasma; VTE, venous thromboembolism.
Interestingly, incubating IMS-captured EVs from the human pancreatic cancer cell line BxPC-3 with normal plasma reduced TF activity.109 This inhibitory activity was lost with TFPI-deficient plasma or with normal plasma in the presence of an anti-TFPI antibody, indicating that it was due to TFPI. The level of TFPI in EVs isolated from plasma is much lower than the level in plasma (Figure 7). However, we found that addition of an anti-TFPI antibody increased the TF activity of EVs isolated from plasma from LPS-stimulated whole blood. This demonstrated that TFPI present in EVs isolated from plasma by centrifugation inhibited TF-FVIIa–dependent FXa generation.132
There are 2 commercial assays that can be used to measure TF activity of EVs isolated from plasma (ZYMUPHEN MP-TF assay, catalog no. 521196, Aniara Diagnostica, West Chester Township, OH; and CY-QUANT MV-TF, catalog no. 01514, Diagnostica Stago, Parsippany, NJ). The ZYMUPHEN MP-TF assay captures TF-positive EVs onto microtiter plates using the noninhibitory anti-TF antibody 10H10 that binds to both TF and the TF-FVIIa complex. TF activity is then measured using a standard 2-stage FXa generation assay. A strength of the assay is that it uses a plate-based format, which is more convenient than isolating EVs by centrifugation. Table 4 shows the recent studies that have used the ZYMUPHEN MP-TF assay to measure TF activity of EVs isolated from plasma.
Studies measuring TF in EV in plasma using the ZYMUPHEN MP-TF assay
| Disease/treatment . | Sample . | Results . | Reference . |
|---|---|---|---|
| Deep infiltrating endometriosis | PFP | There was no difference between groups of patients with endometriosis with or without deep infiltration endometriosis | 133 |
| Acute leukemia | PFP | 4 of 6 patients who had increased EV TF levels had a thrombotic event | 134 |
| Acute ischemic stroke | PFP | Levels of EV TF activity were higher in patients with stroke than in healthy controls | 135 |
| Cancer | PPP | Patients with cancer had higher levels of EV TF activity than healthy controls | 122 |
| Immune thrombocytopenia | PPP | No difference in EV TF activity between patients with immune thrombocytopenia and controls | 136 |
| Cancer and chemotherapy | PFP | Levels of EV TF activity did not change during chemotherapy but increased after the end of the treatment | 137 |
| Sepsis | PFP | Levels of EV TF activity correlated with levels of interleukin-6 in plasma | 138 |
| COVID-19 | PFP | Patients with COVID-19 had higher levels of EV TF activity than healthy controls | 139 |
| Malaria | PFP | Patients with Plasmodium falciparum infection had increased levels of EV TF activity compared with controls | 140 |
| Disease/treatment . | Sample . | Results . | Reference . |
|---|---|---|---|
| Deep infiltrating endometriosis | PFP | There was no difference between groups of patients with endometriosis with or without deep infiltration endometriosis | 133 |
| Acute leukemia | PFP | 4 of 6 patients who had increased EV TF levels had a thrombotic event | 134 |
| Acute ischemic stroke | PFP | Levels of EV TF activity were higher in patients with stroke than in healthy controls | 135 |
| Cancer | PPP | Patients with cancer had higher levels of EV TF activity than healthy controls | 122 |
| Immune thrombocytopenia | PPP | No difference in EV TF activity between patients with immune thrombocytopenia and controls | 136 |
| Cancer and chemotherapy | PFP | Levels of EV TF activity did not change during chemotherapy but increased after the end of the treatment | 137 |
| Sepsis | PFP | Levels of EV TF activity correlated with levels of interleukin-6 in plasma | 138 |
| COVID-19 | PFP | Patients with COVID-19 had higher levels of EV TF activity than healthy controls | 139 |
| Malaria | PFP | Patients with Plasmodium falciparum infection had increased levels of EV TF activity compared with controls | 140 |
PFP, platelet-free plasma; PPP, platelet-poor plasma.
We found that the ZYMUPHEN MP-TF assay was less sensitive, less specific, and had a higher background compared with our in-house assay.141 We believe that the ZYMUPHEN MP-TF assay is less efficient because it does not capture all the TF-positive EVs in the sample. The lower specificity and higher background are likely because of the use of a higher concentration of FVIIa compared with our in-house assay (12 nM vs 2.5 nM). CY-QUANT MV-TF was recently introduced by Diagnostica Stago, and isolates EVs from plasma using centrifugation. This has been used in 1 study to measure levels of TF activity in EVs isolated from plasma.142
Comparison of different assays that measure TF antigen and activity of EVs isolated from plasma
The International Society for Thrombosis and Hemostasis Scientific Standardization Subcommittee on Vascular Biology recently organized a multicenter study to compare the specificity, sensitivity, and reproducibility of assays that measure TF antigen and activity of EVs in plasma.142 Twenty-one laboratories participated in the study. The study included 8 groups that used conventional FCM assays and 1 group that used the MACSPlex Exosome Kit. Three groups used HTF-1-PE (Miltenyi Biotec/Becton Dickinson), 2 groups used VD8-FITC BioMedica Diagnostics/American Diagnostic [now Sekisui Diagnostics]), 1 group used H9-FITC (Santa Cruz Biotechnology), and 1 group used unconjugated IIID8 (Sekisui Diagnostics) to detect TF on EVs. HTF-1, VD8, and IIID8 are group 1 antibodies and compete with FVII/FVIIa binding to TF. The study also included 18 groups that ran 16 different functional assays (2 laboratories ran the Chapel Hill assay, and 2 laboratories ran the ZYMUPHEN MP-TF assay). Fourteen of the functional assays used FXa generation (9 with an anti-TF antibody and 5 without), 3 used thrombin generation (2 with an anti-TF antibody and 1 without), and 1 used a fibrin generation assay with an anti-TF antibody (Table 5).
Characteristics of functional assays
| Laboratory . | Readout . | Anti-TF antibody or FVIIai . | Isolated EVs . |
|---|---|---|---|
| 1 | FXa | Yes | Yes |
| 2 | FXa | Yes | Yes |
| 3 | FXa | Yes | Yes |
| 4 | FXa | Yes | Yes |
| 5 | FXa | Yes | Yes |
| 6 | FXa | Yes | Yes |
| 7 | FXa | Yes | Yes |
| 8 | FXa | Yes | Yes |
| 9 | FXa | Yes | Yes |
| 10 | FXa | No | Yes |
| 11 | FXa | No | Yes |
| 12 | FXa | No | Yes |
| 13 | FXa | No | Yes |
| 14 | FXa | No | No |
| 15 | Thrombin | Yes | No |
| 16 | Thrombin | Yes | Yes |
| 17 | Thrombin | No | No |
| 18 | Fibrin | Yes | No |
| Laboratory . | Readout . | Anti-TF antibody or FVIIai . | Isolated EVs . |
|---|---|---|---|
| 1 | FXa | Yes | Yes |
| 2 | FXa | Yes | Yes |
| 3 | FXa | Yes | Yes |
| 4 | FXa | Yes | Yes |
| 5 | FXa | Yes | Yes |
| 6 | FXa | Yes | Yes |
| 7 | FXa | Yes | Yes |
| 8 | FXa | Yes | Yes |
| 9 | FXa | Yes | Yes |
| 10 | FXa | No | Yes |
| 11 | FXa | No | Yes |
| 12 | FXa | No | Yes |
| 13 | FXa | No | Yes |
| 14 | FXa | No | No |
| 15 | Thrombin | Yes | No |
| 16 | Thrombin | Yes | Yes |
| 17 | Thrombin | No | No |
| 18 | Fibrin | Yes | No |
Three core laboratories (Lacroix, Nieuwland, and Mackman) prepared 4 paired samples: (1) high and low levels of TF-positive and TF-negative EVs from a cancer cell line spiked into EV-depleted plasma, (2) high and low levels of TF-positive EVs from a cancer cell line spiked into EV-depleted plasma, (3) high and low levels of TF-positive EVs from milk spiked into EV-depleted plasma, and (4) plasma prepared from whole blood with and without LPS stimulation. For reference, high and low TF-positive EVs from the cancer line had 2.6 and 0.48 pg/mL of TF activity, respectively, whereas TF-negative EVs had no TF activity. The samples not stimulated and stimulated with LPS had 0 and 1.40 pg/mL TF activity, respectively. Recombinant TF was used as a common standard for the function assays.
To assess the specificity of the assays, the ability of the assays to discriminate between TF-positive EVs and TF-negative EVs was determined. The median ratio for high TF-positive EVs vs high TF-negative EVs for the 8 FCM assays was 1 compared with 10.1 for the 13 functional assays with a denominator of >0 (Table 6). This indicates that activity assays have a higher specificity than an assay that detects TF protein. In addition, the FXa assays that used an anti-TF antibody to distinguish between TF-dependent and TF-independent FXa generation had a higher ratio (23.3) than FXa generation assays that did not use an anti-TF antibody (2.4; Table 6).
Comparison of TF antigen and activity assays for measuring of TF in EVs in plasma samples
Sample . | Antigen assay . | Functional assays . | ||
|---|---|---|---|---|
| FCM . | Total . | With anti-TF antibody . | Without anti-TF antibody . | |
| High TF-positive EVs/high TF-negative EV (specificity) | 1 (0.5-1.5) n = 8 | 10.1 (0.9-39) n = 13 | 23.3 (6.7-39) n = 5 | 2.4 (1.3-8.9) n = 4 |
| High TF-positive EVs/low TF-positive EVs (sensitivity) | 1.6 (0.7-2.9) n = 8 | 2.4 (0.8-5.9) n = 16 | 2.9 (1.9-5.9) n = 9 | 1.1 (0.9-4.9) n = 5 |
| With LPS stimulation/without LPS stimulation (sensitivity) | 2.3 (1-4.1) n = 8 | 6.2 (0.9-36.6) n = 4 | 6.4 (4-26.1) n = 7 | 2.6 (1.6-32.9) n = 4 |
Sample . | Antigen assay . | Functional assays . | ||
|---|---|---|---|---|
| FCM . | Total . | With anti-TF antibody . | Without anti-TF antibody . | |
| High TF-positive EVs/high TF-negative EV (specificity) | 1 (0.5-1.5) n = 8 | 10.1 (0.9-39) n = 13 | 23.3 (6.7-39) n = 5 | 2.4 (1.3-8.9) n = 4 |
| High TF-positive EVs/low TF-positive EVs (sensitivity) | 1.6 (0.7-2.9) n = 8 | 2.4 (0.8-5.9) n = 16 | 2.9 (1.9-5.9) n = 9 | 1.1 (0.9-4.9) n = 5 |
| With LPS stimulation/without LPS stimulation (sensitivity) | 2.3 (1-4.1) n = 8 | 6.2 (0.9-36.6) n = 4 | 6.4 (4-26.1) n = 7 | 2.6 (1.6-32.9) n = 4 |
Results expressed as median (range); only includes data from assays with a denominator of >0; data are from Bonifay et al.142
To assess the sensitivity of the assays, the ability of the assays to discriminate between samples with high TF-positive EVs and low TF-positive EVs was determined. The ratio for the high TF-positive EVs vs low TF-positive EVs was higher for the functional assays (2.3) than the FCM assays (1.6; Table 6). Similarly, the ratio for LPS-stimulated samples vs unstimulated samples was higher for the functional assays (6.2) than for the FCM assays (2.3; Table 6). This again indicates that activity assays are more sensitive compared with an antigen assay. Inclusion of an anti-TF antibody in the FXa generation assays also increased the sensitivity of the function assay (6.4 vs 2.6; Table 6). Similar results were observed with the thrombin generation assay.142
Conclusions
Anti-TF antibodies have different abilities to detect TF on EVs by FCM that may depend on the affinity of the antibody and the fluorochrome-to-protein ratio. Activity-based assays are more sensitive and specific compared with antigen-based FCM for the measurement of TF on EVs isolated from plasma. Functional assays should use an anti-TF antibody to distinguish TF-dependent and TF-independent activity.
Acknowledgments
The authors thank Yohei Hisada and Sierra Archibald for reading the manuscript and Mac Monroe for helpful suggestions.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant R35HL155657 (N.M.) and the John C. Parker professorship (N.M.).
Authorship
Contribution: N.M. wrote the manuscript; and N.M. and A.T.A.S. edited and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, Department of Medicine, University of North Carolina at Chapel Hill, 116 Manning Dr, 8004B Mary Ellen Jones Bldg, Chapel Hill, NC 27599; email: nmackman@med.unc.edu.
References
Author notes
Data are available on request from the corresponding author, Nigel Mackman (nmackman@med.unc.edu).