Key Points
Continuous uniaxial cyclic stretch at 10% upregulates the antithrombotic TM on primary venous cells.
Interrupted cyclic stretch triggers the increase of the prothrombotic VWF.
Visual Abstract
Deep vein thrombosis (DVT) is the formation of a thrombus in the valvular sinuses of the veins in the lower limbs. It is often associated with blood stasis during prolonged immobilization, however, the triggers for DVT are not well understood. Venous valvular sinuses experience unique blood flow patterns due to the cyclic opening and closing of the valve. We hypothesize that stretching helps maintain vein antithrombotic properties, and its absence could contribute to the onset of DVT. To test this idea, confluent human endothelial cells are cultured on hydrogel-coated elastic membranes subjected to uniaxial cyclic stretching at rates of 60 cycles per minute. We study how different levels and duration of stretching influence the expression of 2 important proteins of the hemostatic system, thrombomodulin (TM) and von Willebrand factor (VWF). Our results show that the cells elongate and align orthogonal to the stretch direction. Stretch amplitude of 10% increases TM levels by 75% within 6 hours and remains high up to 24 hours. Interestingly, no significant change occurred at the 5% stretch even after 24 hours. When 10% stretching is interrupted after 24 hours, VWF levels measured 6 hours after interruption increase significantly. Additionally, we show that stretching flattens the nuclei and aligns them orthogonal to the stretching direction. Notably, the epigenetic acetylation mark histone H3 lysine 27 acetylation, which regulates TM gene expression, increases by 1.6-fold. Taken together, our findings suggest that cyclic stretch contributes to the regulation of endothelium thromboresistance and might prevent thrombosis.
Introduction
Deep vein thrombosis (DVT) is a common and severe health issue due to the formation of a blood thrombus, predominantly beginning in the venous valvular sinuses of the legs. When all or part of such a thrombus is dislodged, it becomes a circulatory tree embolus, which can catastrophically obstruct pulmonary arteries, producing a pulmonary embolism and leading to lethal complications.1-3 The risk factors of this cardiovascular disease have been extensively studied,4 and immobilization, especially in-hospital bedrest is a major threat. Furthermore, long journeys, depression, pregnancy, and malignancy significantly elevate the proneness of developing DVT.5-7 Venous valvular sinuses experience unique blood flow patterns in which the flow is highly modified during each cycle of passive opening and closure of the valves, produced by the heartbeat and by the local peristaltic deformations of the veins by the contraction of the surrounding skeletal muscles of the calves and thighs. Valve closure prevents backflowing of blood, thereby reducing the shear stress to almost 0, whereas the valve's reopening is immediately followed by high shear stress levels on the luminal surface of the valves, with slower but significant recirculation flow in the pockets. Importantly, the endothelium of the valvular sinuses differs from that of the luminal vein, with upregulation of anticoagulant and downregulation of procoagulant proteins,8,9 raising the intriguing question of the reason why DVT occurs more predominantly in these regions. The drastic modification of these highly dynamic flows during immobilization has lead to intense scrutiny of the relationship between resting state changes of blood flow and DVT in vitro, using recent microfluidic technologies to effectively recapitulate blood endothelial-epithelial interactions at the onset of thrombosis in blood vessels.10-12 However, valvular pockets are also highly compliant regions. In standing position, when the valves close, valvular pockets are subjected to cyclic stretch, generated both by the increase in venous blood column hydrostatic pressure and by the deformations induced by leg muscles contractions. Few experiments combining shear and substrate stretching have shown complex morphology cell behavior. At low shear stresses, the cell's alignment is dominated by mechanical stretching, whereas at high shear stresses of the order of 0.5 Pa,13 orientation becomes dominated by flow. However, the respective roles of stretching stresses over viscous stresses in the overall vein homeostasis are yet to be considered. Two important regulators of the coagulation cascade are the proteins thrombomodulin (TM) and von Willebrand factor (VWF). TM is an integral membrane glycoprotein expressed on the plasma membrane of endothelial cells (ECs), which promotes anticoagulant responses by binding circulating thrombin and activating protein C.14-17 TM expression has been studied under flow for cultured venous ECs (human umbilical vein ECs [HUVECs])18 and biaxial cyclic stretch for human aortic endothelial cells, mainly by quantitative reverse transcription polymerase chain reaction and western blot techniques.19 In ECs, VWF is stored in intraluminal vesicles called Weibel-Palade bodies, and in response to procoagulant stimulation it forms elongated, string-like structures known as ultralarge VWF.20,21 It plays a pivotal role during primary hemostasis and coagulation, facilitating platelets adhesion and stabilizing factor VIII in the bloodstream.22,23 VWF expression has mainly been studied under flow at pathological high shear rates to recapitulate arterial thrombosis, whereas the regulation of VWF on HUVECs subjected to stretch remains unexplored. The transcription factor ETS-related gene drives transcription of TM. Low shear stress has been shown to upregulate TM,24 whereas the effect of stretch on the transcriptomic regulation of TM has not been considered. In this work, we raise the hypothesis that the altered hemodynamic conditions of stretch experienced by ECs in the valve sinuses might play a crucial role in venous thrombogenesis. We present the exploration of protein expression changes of a confluent layer of ECs under in-plane uniaxial cyclic stretching using quantitative immunostaining.
We investigate the effect of (1) substrate topography, (2) duration, and (3) magnitude of stretch that we interrogate through the expression levels of TM and VWF.
Materials and methods
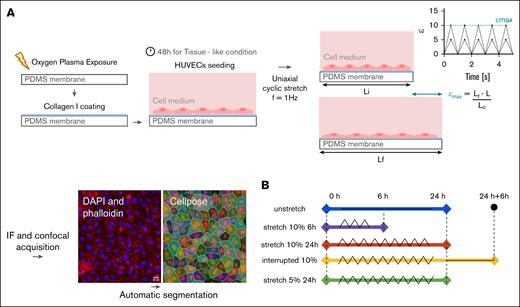
The schematic diagram of the experimental workflow (Figure 1A), briefly describes the surface functionalization, EC seeding, mechanical stimulation, and immunostaining quantified by confocal microscopy. Figure 1B describes the different conditions of stretch applied to the confluent monolayer.
Method: protocol for uniaxial cyclic stretch. (A) The elastomeric membrane of PDMS that serves as a substratum is first exposed to oxygen plasma and then coated with collagen type I. HUVECs are then seeded and cultured for 48 hours. Finally, uniaxial cyclic stretch is applied at a frequency of 1 Hz. IF followed by confocal acquisition. The automatic segmentation Cellpose was performed to quantify the parameters of interest of the individual cell (ROI). A typical confocal image of a HUVEC monolayer, in which DAPI (blue) and phalloidin (F-actin, red) are labeled, is shown. (B) Scheme depicting uniaxial cyclic stretch: triangular wave. Unstretched, 24 hours (blue); stretched at 10%, 6 hours (magenta); stretched at 10%, 24 hours (orange). Interrupted stretch: 24 hours, 10% stretch, followed by 6 hours discharge (yellow). Stretched 5%, 24 hours (green). All membranes have a flat substrate and cyclic stretch frequency set to f = 1 Hz. IF, immunofluorescence.
Method: protocol for uniaxial cyclic stretch. (A) The elastomeric membrane of PDMS that serves as a substratum is first exposed to oxygen plasma and then coated with collagen type I. HUVECs are then seeded and cultured for 48 hours. Finally, uniaxial cyclic stretch is applied at a frequency of 1 Hz. IF followed by confocal acquisition. The automatic segmentation Cellpose was performed to quantify the parameters of interest of the individual cell (ROI). A typical confocal image of a HUVEC monolayer, in which DAPI (blue) and phalloidin (F-actin, red) are labeled, is shown. (B) Scheme depicting uniaxial cyclic stretch: triangular wave. Unstretched, 24 hours (blue); stretched at 10%, 6 hours (magenta); stretched at 10%, 24 hours (orange). Interrupted stretch: 24 hours, 10% stretch, followed by 6 hours discharge (yellow). Stretched 5%, 24 hours (green). All membranes have a flat substrate and cyclic stretch frequency set to f = 1 Hz. IF, immunofluorescence.
Surface functionalization
Polydimethylsiloxane (PDMS) membranes (Curibio, Seattle, WA) were rinsed in ethanol and Milli-Q–purified water, and treated with oxygen plasma at 0.2 to 0.5 mm Hg, 40 W radio frequency power for 2.5 minutes. The membranes were then incubated with 100 μg/mL poly-d-lysine for 15 minutes and coated with collagen I (diluted to 1 mg/mL; catalog no. sc-136157; Santa Cruz Biotechnology). One set of membranes presents a nanopatterned topography (NanoSurface; Curi Bio, Seattle, WA) with grooves of 0.5 μm in depth and 1-μm spacing between the grooves.
Venous ECs confluent culture
HUVECs (HUVEC pool donors; catalog no. C-12250; PromoCell) were cultured with EC growth medium (catalog no. C-22010; PromoCell) complemented with 2.5% serum supplement mix (catalog no. C-39215; PromoCell) and 1% penicillin-streptomycin according to the manufacturer’s protocols. The cells were maintained at 37°C in a humidified incubator under 5% CO2. Cells at passages 2 to 5 were used for stretching experiments. HUVECs were harvested with trypsin-EDTA solution 0.25% (catalog no. MFCD00130286; Sigma-Aldrich), seeded at a density of 300 × 103 cells per cm2 and then cultured for 48 hours to enable tissue condition. In our experiments, we used fully confluent ECs adhering to collagen I substratum (as a tissue support substitute), with cells showing mature tight junctions (supplemental Figure 1C).
Mechanical stimulation of the cells
Uniaxial cyclic strain stimulation is applied to confluent cell layers using a commercial cytostretcher (Curi Bio). The strain is calculated and given as the percentage ϵ(t) = ([Lf(t) – Lo]/Lo) × 100, in which Lo and Lf(t) are the initial and the actual lengths of the PDMS membrane, respectively, during its extension. The cells are stretched with a triangular strain function at a frequency of f = 1 Hz (60 cycles per minutes) for all the experiments with ϵmax defining the maximum strain amplitude. The device is placed inside an incubator to control temperature and CO2 concentration of the atmosphere. Different temporal patterns of cyclic stretch are explored. First, we performed continuous stretching for short timescale runs of 6 hours at ϵmax of 10%, and long timescale runs of 24 hours at ϵmax of 5% and 10%. Second, we applied an “interrupted” pattern of stretching consisting of 24 hours stretching at ϵmax of 10% followed by an interruption of 6 hours to mimic forced stasis (Figure 1B).
Immunostaining
After mechanical stimulation, cells were fixed with 3.2% of paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes, then permeabilized with Triton X-100 (0.1% volume-to-volume ratio; catalog no. 329830772; Merck Sigma-Aldrich) in PBS for 3 minutes. TM was stained with a 1:50 dilution of Dako antibody, overnight, at 4°C (TM Dako catalog no. M0617; monoclonal, mouse nonconjugated, 1009); vascular endothelial (VE) cadherin (Abcam rabbit polyclonal antibody; catalog no. ab33168), diluted 1:100, for 1 hour; and VWF, 1:500 dilution, for 1 hour (Dako Polyclonal Rabbit; catalog no. A0082). Actin filaments were stained with Alexa Fluor phalloidin (catalog no. A34055; Thermo Fisher Scientific) and nuclear DNA with DAPI (4',6-diamidino-2-phenylindole; catalog no. D1306; Thermo Fisher Scientific), all in PBS containing 1% bovine serum albumin as a saturating agent. Histone acetylation mark (catalog no. 39134; Active Motif) was labeled after a second fixation step and a second permeabilization (Triton 0.5% volume-to-volume ratio) in PBS for 5 minutes.
Confocal image acquisition
Images were acquired with an oil objective ×40 magnification and 1.3 NA (confocal microscope, Leica SP8-UV, Montpellier Ressources Imagerie). The cells were imaged with an argon laser, 488 nm for VE cadherins and VWF, a diode-pumped solid-state laser 561 nm for F-actin, a UV light diode (405 nm) for nuclei, and a 647-nm HeNe laser for TM. Each PDMS membrane of the stretcher chambers, in which the cells were deposited, were carefully cut, and the membrane was flipped and deposited with the cell side on a coverslip using a mounting medium (Antifade medium catalog no. ab-104135; refractive index 1.47; Abcam).
Image and data analysis
The automated algorithm Cellpose25 was used for segmentation. Every cell was considered the region of interest (ROI). The intensity of fluorescence was considered as the average gray value, defined by the total fluorescence intensity of a ROI divided by the area of the ROI. The z-stack acquired was analyzed by ImageJ. A sum projection of 15 planes (z-step, 1 μm) was performed. DAPI and F-actin signals were used as masks. The errors in the automatic segmentation were manually adjusted using the ROI manager in ImageJ. Measurements of areas, mean gray values, the major and minor axis, and angle were computed for each cell. With Matlab, the frequency distributions were displayed using the “hist” function. In the graphs, the frequency distributions show 1 representative experiment, whereas the bar and dots graphs represent the total number of experiments performed. Every black dot represents 1 independent experiment with a minimum number of cells per experiment of >500. The relative fold change was normalized by the corresponding unstretched condition. The aspect ratio of the individual cells was calculated as r = a/b, with a and b being the major and the minor axes of the projected cell, respectively, approximated to an ellipsoid. The extent of cell orientation is defined as the angle between the longest axis a, and the horizontal axis of the image.
Statistical analysis
Unpaired Student t tests were conducted on the original raw data using GraphPad (La Jolla, CA) software, available as an online calculator. Values of P values >.05 are considered nonsignificant. Specifically, the level of significant difference is expressed as ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. Subsequently, the relative fold changes were normalized to the corresponding unstretched condition and presented in bar graphs. Mean and error bars indicate the standard deviation of the total number of experiments and have been calculated from the acquired raw data. Pearson r coefficient was calculated with correlation analysis using GraphPad software.
Nucleus morphology quantification
Each nucleus was segmented and analyzed with the software, Imaris (Oxford Instruments), which quantifies each nucleus volume, height, and projected area. The function BoundingBoxAA was used to calculate the main axis of inertia of the cell in 3 dimensions. Noteworthily, this function identifies an object by considering the minimal rectangular box, which fully encloses the object (supplemental Material). Finally, nucleus orientation was measured with Cellpose segmentation of the DAPI channel. The order parameter26,27 S, which measures the degree of alignment, was calculated from the cosine squared of angle χ (in radians) and then averaged as S = 2くcos2(χ)⟩– 1. For S = −1, all the nuclei were aligned perpendicular to the stretch direction, and S = 0, were randomly oriented.
Ethics approval
After specific referral by Elsa Faure, the University Hospital of Nîmes' advisory board gave a favorable opinion on the use, for ex vivo research purposes, of segments of superficial veins from the lower limbs taken during venous surgery.
Results
HUVECs spread and deform orthogonally to the stretching direction
Figure 2 shows the cells' actin cytoskeleton and the corresponding segmentation of cell boundaries. The frequency distribution of individual cells’ projected area, aspect ratio, and long axis orientation are shown. When ϵmax = 0, the cells have polygonal shapes without particular orientations. Actin fibers follow mostly cell boundaries with their neighbors. On nanogrooves, the cells present no significant deformation nor spreading, but the actin bundles are slightly more apparent in the direction of the groove, inducing a weaker peak in the orientation distribution. Under stretching conditions of ϵmax = 10%, cells elongate strongly and spread on the substrate, showing an increase in projected area and aspect ratio. The elongation is perpendicular to the stretching direction and the frequency distribution is highly peaked compared with the flat static distribution.
Cell alignment. Cells align orthogonally to the stretch direction. (A) Confocal images of nucleus DAPI and phalloidin (F-actin) merged (DAPI, blue; and F-actin, red) of an unstretched flat membrane; an unstretched nanopatterned membrane, and a flat membrane stretched for 24 hours at 10% strain and at 1 Hz (left). Objective, original magnification ×40; scale bar, 20 μm. The lines point at the direction of the nanogrooves for the nanopatterned membranes and the arrow at the direction of the stretch for the flat membranes. Z-stack sum projection. (Right) Cell pose segmentation. (B) Frequency distribution of individual cell projected area, aspect ratio and orientation for unstretched flat (light blue), unstretched with nanopatterns (red), and stretched 24 hours, 10% (orange). n = 2586 cells; N = 3 independent experiments.
Cell alignment. Cells align orthogonally to the stretch direction. (A) Confocal images of nucleus DAPI and phalloidin (F-actin) merged (DAPI, blue; and F-actin, red) of an unstretched flat membrane; an unstretched nanopatterned membrane, and a flat membrane stretched for 24 hours at 10% strain and at 1 Hz (left). Objective, original magnification ×40; scale bar, 20 μm. The lines point at the direction of the nanogrooves for the nanopatterned membranes and the arrow at the direction of the stretch for the flat membranes. Z-stack sum projection. (Right) Cell pose segmentation. (B) Frequency distribution of individual cell projected area, aspect ratio and orientation for unstretched flat (light blue), unstretched with nanopatterns (red), and stretched 24 hours, 10% (orange). n = 2586 cells; N = 3 independent experiments.
Continuous uniaxial cyclic stretching increases TM expression
When subjected to uniaxial cyclic elongations at ϵmax = 10%, HUVECs on flat substrates exhibited an increase of TM expression (Figure 3). Immunofluorescence quantification reveals mechanical stimulation leads to a 75% increase in the average TM expression within just 6 hours of stretching, compared with static cells. Continuous stretch results in greater variability in TM expression, as indicated by wider distributions observed at both 6 and 24 hours. This suggests that stretching not only elevates TM levels but also increases the variability in expression. The interquartile range plot further supports this observation, showing a twofold increase in variance at 6 hours. Finally, we test the case of interrupted stretching. The experiments reveal a slight decrease on the average TM expression compared with continuous stretching. Although the average level of TM is higher than in the static condition, the interruption of the stretch does not induce a significant change. These results are also confirmed by western blot (WB; supplemental Figure 8). Collectively, our observations support the hypothesis that expression of TM is enhanced under continuous short- and long-term cyclic stretch.
Time study of TM. Continuous uniaxial cyclic strain increases the antithrombotic TM. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10%, stretched for 24 hours at 10%, and interrupted stretch at 10%, all membranes used were flat. DAPI in blue, F-actin in red, and TM in magenta. Objective, original magnification ×40; scale bar 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative TM protein expression fold change (bar graph) and interquartile range (dots graph). Mean ± standard deviation (SD) values are shown for the following conditions: unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 4 independent experiments; stretched for 24 hours at 10%, N = 5 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 13 200 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment. AU, arbitrary unit.
Time study of TM. Continuous uniaxial cyclic strain increases the antithrombotic TM. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10%, stretched for 24 hours at 10%, and interrupted stretch at 10%, all membranes used were flat. DAPI in blue, F-actin in red, and TM in magenta. Objective, original magnification ×40; scale bar 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative TM protein expression fold change (bar graph) and interquartile range (dots graph). Mean ± standard deviation (SD) values are shown for the following conditions: unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 4 independent experiments; stretched for 24 hours at 10%, N = 5 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 13 200 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment. AU, arbitrary unit.
Interrupted cyclic stretching after 24 hours induces an increase in VWF expression
We performed a similar analysis for VWF. The results show that VWF expression is not influenced by continuous stretching at 10% for either short or long periods. However, interrupted stretching led to a 118% increase in VWF expression compared with static conditions, as shown in Figure 4. This trend is confirmed by WB (supplemental Figure 8). WB results show a 50% decrease of VWF when the cells undergo continuous stretching for 24 hours, and 1.3-fold increase upon interruption of the stretch.
Time study of VWF. Interrupted cyclic strain triggers thrombotic events reflected in the increase of VWF. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10% on a flat membrane, stretched for 24 hours at 10%, and interrupted stretch at 10%. DAPI in blue, F-actin in red, and VWF in green; all membranes used were flat. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative VWF protein expression fold change (bar graph) and interquartile range (dot graph). Mean ± SD values of unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 3 independent experiments; stretched for 24 hours at 10%, N = 3 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 9000 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.
Time study of VWF. Interrupted cyclic strain triggers thrombotic events reflected in the increase of VWF. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10% on a flat membrane, stretched for 24 hours at 10%, and interrupted stretch at 10%. DAPI in blue, F-actin in red, and VWF in green; all membranes used were flat. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative VWF protein expression fold change (bar graph) and interquartile range (dot graph). Mean ± SD values of unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 3 independent experiments; stretched for 24 hours at 10%, N = 3 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 9000 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.
TM increase is only triggered beyond a stretch threshold
Stretch at different magnitudes for a long duration was explored. Static, stretch for 24 hours at 10% vs 5% is presented (Figure 5A). Lower magnitudes of stretch do not allow the establishment of a clear change in TM expression.
Study of magnitude of stretch at 5%. TM expression on HUVECs is dependent on the magnitude of stretch. (A) Confocal images of HUVECs stretched for 24 hours at 5% on a flat membrane: DAPI in blue, F-actin in red, TM in magenta, and VWF in green on the same sample. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM and VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Unstretched, light blue; stretched for 24 hours at 10%, orange; and stretched for 24 hours at 5%, green. Insets: relative protein expression fold change (bar graph), mean ± SD values of unstretched and stretched for 24 hours at 10% vs stretched for 24 hours at 5%, N = 2 independent experiments, respectively (medium and multiwell Curibio cytostretcher membranes were used to facilitate averaging across duplicates). ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.
Study of magnitude of stretch at 5%. TM expression on HUVECs is dependent on the magnitude of stretch. (A) Confocal images of HUVECs stretched for 24 hours at 5% on a flat membrane: DAPI in blue, F-actin in red, TM in magenta, and VWF in green on the same sample. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM and VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Unstretched, light blue; stretched for 24 hours at 10%, orange; and stretched for 24 hours at 5%, green. Insets: relative protein expression fold change (bar graph), mean ± SD values of unstretched and stretched for 24 hours at 10% vs stretched for 24 hours at 5%, N = 2 independent experiments, respectively (medium and multiwell Curibio cytostretcher membranes were used to facilitate averaging across duplicates). ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.
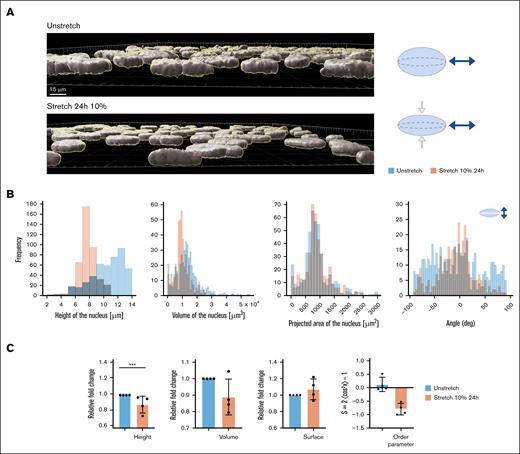
Nuclei respond to sustained stretching by flattening and reorienting
During stretching, nuclei are affected in their morphology. Figure 6A shows the 3-dimensional reconstruction of cell nuclei under static conditions compared with a 10% stretch for 24 hours. Height measurements show a significant decrease, whereas the projected areas remain constant (Figure 6B). Another effect of stretching is nuclei reorientation. As with the cell body, nuclei align orthogonal to the direction of stretching (Figure 6C).
Nuclear height study. Twenty-four hours of 10% stretch induces nuclear deformation. (A) 3-Dimensional (3D) reconstruction of confocal images of HUVEC nuclei in static vs stretched at 10% over a 24-hour period, with Imaris software (left) and model of flattening of the nucleus due to the uniaxial mechanical stretch (right). (B) Frequency distribution of quantification measured by 3D Imaris software, height of the nucleus, volume, projected area of the nucleus, and orientation of the single cell nucleus of the same experiment, N = 1 representative experiment. (C) The bar graphs show the mean and SD of the height and the volume of the cell nucleus for unstretched and stretched conditions 24 hours of 10%. N = 4 independent experiments; n = 4000 cells; ∗∗∗P < .001. deg, degree.
Nuclear height study. Twenty-four hours of 10% stretch induces nuclear deformation. (A) 3-Dimensional (3D) reconstruction of confocal images of HUVEC nuclei in static vs stretched at 10% over a 24-hour period, with Imaris software (left) and model of flattening of the nucleus due to the uniaxial mechanical stretch (right). (B) Frequency distribution of quantification measured by 3D Imaris software, height of the nucleus, volume, projected area of the nucleus, and orientation of the single cell nucleus of the same experiment, N = 1 representative experiment. (C) The bar graphs show the mean and SD of the height and the volume of the cell nucleus for unstretched and stretched conditions 24 hours of 10%. N = 4 independent experiments; n = 4000 cells; ∗∗∗P < .001. deg, degree.
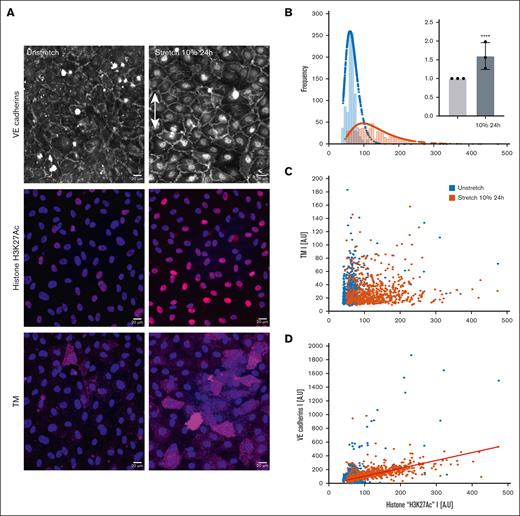
Uniaxial cyclic stretching induces an increase in histone mark acetylation, correlated with VE-cadherin expression in the nucleus
We then sought to identify a link between nuclear deformation and TM expression. We examined the epigenetic acetylation of histone H3K27ac before and after 10% stretch for 24 hours. A 1.6-fold increase was observed (Figure 7). However, the scatter in the plot points indicates no clear correlation between the 2 moieties per cell, suggesting that although the tissue protein expression changes on average after stretching, the local behavior is far more complex. Surprisingly, H3K27ac is strongly correlated with the VE-cadherin signal in the nuclei undergoing stretch, with a Pearson r coefficient of 0.560.
Histone mark acetylation study. Continuous uniaxial cyclic strain for 24 hours induces histone mark acetylation increases. (A) Confocal images of HUVECs: nuclei (DAPI), H3K27ac histone in red, merged colors. Objective, oil original magnification ×40; scale bar, 20 μm. (B) Frequency distribution of static conditions in blue and stretching 10% 24 hours in orange; n = 1 representative experiment. Inset: relative fold change of H3K27ac histone stretched for 24 hours at 10% vs static unstretched state. P < .0001 and mean ± SD values of N = 3 independent experiments; n = 4000 cells. (C) Scatterplot of TM and histone mark intensity signal. (D) Linear relationship between VE cadherins in the nucleus of the cells and histone mark intensity. N = 1 independent experiment; n = 1582 cells. The slope of the linear fit: m = 1.1453; Pearson r coefficient = 0.560.
Histone mark acetylation study. Continuous uniaxial cyclic strain for 24 hours induces histone mark acetylation increases. (A) Confocal images of HUVECs: nuclei (DAPI), H3K27ac histone in red, merged colors. Objective, oil original magnification ×40; scale bar, 20 μm. (B) Frequency distribution of static conditions in blue and stretching 10% 24 hours in orange; n = 1 representative experiment. Inset: relative fold change of H3K27ac histone stretched for 24 hours at 10% vs static unstretched state. P < .0001 and mean ± SD values of N = 3 independent experiments; n = 4000 cells. (C) Scatterplot of TM and histone mark intensity signal. (D) Linear relationship between VE cadherins in the nucleus of the cells and histone mark intensity. N = 1 independent experiment; n = 1582 cells. The slope of the linear fit: m = 1.1453; Pearson r coefficient = 0.560.
Discussion
In this work we show that uniaxial cyclic stretch induces ECs alignment. The reorientation of ECs in response to hemodynamic forces is one of the earliest signs of mechanotransduction within the endothelium.28 In vivo, ECs in areas of steady flow become elongated and align parallel to the flow,29 whereas nonaligned cells are typically found in regions with low mean shear stress, such as at stagnation points in complex flow patterns.30,31 In vitro and in static conditions, cells do not have a preferential angle and are randomly oriented. Substrate topography influences cytoskeleton orientation through focal adhesion complexes, with cells aligning in the direction of the grooved nanopatterns.32-36 The cells retain a cobblestone morphology as in nonstretched static conditions; however, the substrate induces a guidance to the tissue. Many studies have shown that subconfluent ECs subjected to stretch undergo changes in size and morphology, a phenomenon referred to as cell mechanoadaptation.37,38 This adaptation involves geometric modifications such as an increase in cell surface area and aspect ratio39,40 but also cell orientation and intense cytoskeletal remodeling.41 Actin stress fibers undergo disassembly, resulting in cytoskeletal alignment at a specific angle in the 0-strain direction,42,43 represented here by the orthogonal direction to uniaxial stretch of the substrate. Alignment has been observed for different cell types such as smooth muscle cells,44 bovine aortic ECs, fibroblasts, and osteoblasts.45 Our observations on HUVECs in confluent conditions show an analogous behavior both for shape and cytoskeleton organization, although cell density most likely influences both their orientation rate and their mechanoresponse. The confluency of our cell cultures is guaranteed by the formation of mature homotypic VE-cadherin junctions (supplemental Figure 1). VE cadherins mediate cell-cell contact by forming tight junctions between ECs,46 which are crucial for establishing a functional vascular barrier, influencing nuclear functions and gene expression.47,48 Reorientation perpendicular to the strain direction has been correlated to the stretch amplitude with a threshold of ∼3% of strain for subconfluent cells, as proposed by Dartsch et al,44 which is close to the 5% value in our experiments for which we do not observe strong changes (Figure 5; supplemental Figure 6). Faust et al42 hypothesized that these cells may tolerate a certain elongation without inducing a mechanoresponse. Moreover, the dynamics of alignment itself has been observed reaching a steady state after 3 hours49 for ϵmax = 10%. Our observations are also consistent with these findings, however, in confluent conditions of culture. We observed that TM expression is upregulated by uniaxial stretching. When exposed to 10% uniaxial cyclic elongation, TM expression increases. Similar results have been obtained by Peghaire et al24 in response to flow. Our data also show that the duration of the stretch period has a pronounced effect on TM expression dynamics. In the short-term response, cells strongly express TM, with 75% increase, although the cells are not yet aligned (supplemental Figure 2). After 24 hours, when the cells have adapted their shape to the cyclic mechanical cue, they still retain their antithrombotic expression. TM signal is stronger than in static conditions but weaker than the expression induced shortly after exposure (6 hours). This observation leads to the hypothesis that cells adapt to cyclic stretching not only morphologically but also in terms of protein regulation, reaching a steady state. This is consistent with the work of Hollosi et al that observed rapid response of BRAF protein in HUVECs after 15 and 30 minutes of stretching, although with 30% biaxial cyclic stretching.50 In our results, interrupted stretch demonstrated minimal impact on ECs TM expression. The cells quickly relieve stress, and after 6 hours without mechanical constraints, their behavior closely resembles that of the nonstretched condition. The dispersion of the TM signal from the mean value highlights the greatly increased variance of the TM expression after only 6 hours of stretch. The most elongated cells, although expressing less TM, appear to be more oriented, revealing an inverse correlation between TM expression and elongation. This hypothesis gains support from TM values obtained after 6 hours of stretching at ϵmax = 10%, during which cells are not yet aligned orthogonally to the direction of stretching but show high levels of the antithrombotic protein. When using nanopatterned membranes, we do not see a significant influence on TM expression (supplemental Figure 3B), indicating that signaling coming from substrate topography is not sufficient by itself to alter the expression of these proteins. The duration and magnitude of strain exert distinct effects on TM dynamics; both short and long stretching at 10% induce high thrombotic resistance, whereas at ϵmax = 5%, the response appears comparable with the static nonstretched condition. This, physiologically, could suggest that a minimal tension threshold is required for protein regulation, because insufficient tension (eg, 5% in our model) does not elicit a significant mechanosensing response. Relative percentage of stretch might differ from cell to cell, depending on position in the monolayer, density, and cell phase cycle, which may be the result from epigenetic regulation, meaning that in the same sample only cells that are stretched 10% are expressing more TM and the cells that do not reach this cutoff do not activate. Our study primarily addresses cellular-level mechanisms related to mechanical stretch, an area that has not been established well in the context of DVT, in which only low shear stresses have been investigated. TM expression is used here as a proxy; further investigation, including the regulation of TM and protein C,51-53 is necessary to directly confirm the impact of mechanical stretch on the antithrombotic phenotype of the endothelial monolayer, which is complex and involves many important factors. Concomitantly, VWF expression in interrupted stretching conditions lead to a significant increase of the protein at the cell surface whereas it was not influenced by surface topography nor duration of stretching at ϵmax = 10%. This finding supports the hypothesis that when cells experience the interruption of physiological stretching, they transition to a pathological state characterized by high VWF expression. Giblin et al54 studied the mechanisms of VWF release from HUVECs. Their findings indicated that in nonstimulated HUVECs, ∼80% of VWF release is due to periodic exocytosis, which is the process by which cells release the contents from vesicles into the extracellular space.54 During exocytosis, the vesicle membrane fuses with the cell membrane, allowing the content to be released outside the cell even in the absence of external stimuli that would normally trigger secretion (such as inflammation or vascular injury). Turner et al21 noted that stretching may affect exocytosis by altering membrane tension of the cells. We decided to explore nuclear deformation and H3K27ac after stretching. When cells experience mechanical stress, such as shear, stretch, or compression, they undergo various structural and functional changes especially at the level of their cytoskeleton, which is physically connected to the extracellular matrix via integrin receptors, and to the nuclear lamina by the linker of nucleoskeleton and cytoskeleton complex that spans both nuclear membranes.55 This is crucial for correct mechanotransduction, its disruption perturbs cell processes such as differentiation.56,57 The remodeling of the stress fibers is therefore acting mechanically on the nucleus of each cell. As a result of stretching, we indeed observe a flattening of the nuclei through their height reduction (Figure 6). The resulting deformation of the chromatin can directly influence gene expression. The height of the nucleus is influenced by the rigidity of the substrate and by cell density, and the high cell density used in these experiments might restrain cell spreading, affecting nuclear dimensions. Nava et al58 observed the alignment of mesenchymal cells under cyclic uniaxial stretch at a frequency of 0.1 Hz. The stretch induced a reorientation of the nuclei and increased nuclear aspect ratio, whereas the nuclear volume remained virtually unaltered.58 This is consistent with our results. As mentioned earlier, flattening and stretching can lead to changes in chromatin organization and accessibility, potentially facilitating the addition of acetyl groups to histones, such as the epigenetic mark H3K27ac. For example, ECs subjected to shear stress, increases H3K27ac, correlating with the expression of stress-responsive genes as shown by Peghaire et al.24 After 24 hours of stretching at 10%, H3K27ac, as well as TM, increased. At the local scale, correlation between H3K27ac and TM was not conclusive. However, nuclear VE-cadherin and H3K27ac levels are highly correlated in stretched ECs, when the cellular state is highly dynamic. This suggests that a quiescent state is less likely to maintain strong antithrombotic conditions when cells express higher levels of TM and H3K27ac. Transcription factor ETS-related gene regulates VE-cadherin expression and is required for the control of their junctional integrity. Deletion of TM from ECs confers an inflammatory state. This is based on the loss of the basal barrier permeability regulated by VE cadherins.59 Thus, VE cadherins and regulation of transcription in ECs are intimately interlinked. Combined effect of tumor necrosis factor α and neutrophils elevated serum TM levels, which can be associated with pathological conditions that lead to reduced TM expression on the endothelial surface.60,61 Tumor necrosis factor α enhances VWF expression, promoting procoagulant and inflammatory response.62-64 Our findings, shown in supplemental Figure 5, align with these observations, suggesting that an inflammatory chemical stimulus increases VWF by fourfold on the cell surface compared with the control, and decreases TM on HUVEC surfaces. Ultimately, we decided to explore the stress state of a valve pocket. Despite extensive research on venous valves, key details about internal structure and cell organization in deep veins remain unclear.65-67 Movement promotes valve dynamics, oscillatory flow, and high stretch. In contrast, immobilization or horizontal resting positions result in open valves and minimal sinus pocket tension, which are not enough to keep a thromboresistant state. To our knowledge, no direct tension measurements exist for the venous valve sinus pocket. We imaged a valve in the great saphenous vein using magnetic resonance imaging and micro–computed tomography, shown in supplemental Figure 4. Our analysis revealed that the sinus pocket experiences tension of 10%, with the leaflet showing unexpected extension. This suggests that ECs in the valve may be influenced by stretching.
Conclusions
This study highlights the critical role of stretching in maintaining the thromboresistant state that opposes the onset of DVT. We demonstrate that 10% stretching induces high TM expression in HUVECs, followed by morphological changes of the cells as well as nuclear flattening, and alignment correlated to an increase in histone H3K27ac, linking mechanical stretch to antithrombotic gene regulation. We also show that interrupted stretch causes a dramatic change in VWF expression, linking stasis and prothrombotic regulations. Our findings outline the importance of integrating uniaxial cyclic stretch in DVT research, offering new insights into the mechanobiology of venous valve pockets and vascular health.
Acknowledgments
The authors thank Cheng-Hsiang Kuo (National Cheng Kung University, Tainan, Taiwan) for the plasmid encoding thrombomodulin–green fluorescent protein. They also thank R. Jelinek for his technical help with the cytostretcher.
This work was publicly funded through Agence Nationale de la Recherche (the French National Research Agency) under the Programme Investissements d’avenir with the reference ANR-16-IDEX-0006.
Authorship
Contribution: A.G., C.B., and M.A. designed the research; A.G. and C.B. performed the stretching experiments and data analysis; A.P-.M., J.-C.G., and E.F. performed the vein extraction and participated in discussions; A.G., M.C., and C.G.-B. performed the computed tomography scan on the vein sample; A.G., E.G., C.B., and M.A. wrote the manuscript; and all authors discussed the results and the analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manouk Abkarian, Centre de Biologie Structurale, Centre National de la Recherche Scientifique, INSERM, Université de Montpellier, 105429 rue de Navacelles, 34090 Montpellier Cedex 5, France; email: manouk.abkarian@umontpellier.fr.
References
Author notes
Data are available from the corresponding author, Manouk Abkarian (manouk.abkarian@umontpellier.fr), on request.
The full-text version of this article contains a data supplement.


![Time study of TM. Continuous uniaxial cyclic strain increases the antithrombotic TM. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10%, stretched for 24 hours at 10%, and interrupted stretch at 10%, all membranes used were flat. DAPI in blue, F-actin in red, and TM in magenta. Objective, original magnification ×40; scale bar 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative TM protein expression fold change (bar graph) and interquartile range (dots graph). Mean ± standard deviation (SD) values are shown for the following conditions: unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 4 independent experiments; stretched for 24 hours at 10%, N = 5 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 13 200 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment. AU, arbitrary unit.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/4/10.1016_j.bvth.2025.100103/1/m_bvth_vth-2024-000288r3-gr3.jpeg?Expires=1764787049&Signature=wfVgODUMOkKRYFPZkUZPYkEJXuFaTJyP4mvkzNj42VkpbyDR7tqsM~S55d6T7oBsjZ7lrwVseclOncAWchGGYeBLmPSNT68yPpqS6eRxXzhGjs7xNbupT~MHhPvPj-7Ti1qf0rBs902YSJmZ9QdXBrLWr~XsJR0oz7JYCqKCquIAnXQhN6KwsQdzLIOc-U4xGcGS~W1lA8K8hAbHvJ~750OA3mBRhbdWq9nXAARyJ6mlyOQygkkSefw71gCEuFMtpVlhhbc9AddXbZ3wQt97hjTDL9NTEXgROSEA6Avq8LR4KD9bJ-fqSKrCYUn4Q-7lIILS9NLUSM2Ba9FElfGqtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Time study of VWF. Interrupted cyclic strain triggers thrombotic events reflected in the increase of VWF. (A) Confocal images of HUVECs unstretched, stretched for 6 hours at 10% on a flat membrane, stretched for 24 hours at 10%, and interrupted stretch at 10%. DAPI in blue, F-actin in red, and VWF in green; all membranes used were flat. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Frequency distribution at the top: unstretched (light blue), stretched for 6 hours at 10% (magenta), and stretched for 24 hours at 10% (orange). Frequency distribution at the bottom: unstretched (light blue), stretched for 24 hours at 10% (orange), and interrupted stretch at 10% (yellow). (C) Relative VWF protein expression fold change (bar graph) and interquartile range (dot graph). Mean ± SD values of unstretched, N = 4 independent experiments; stretched for 6 hours at 10%, N = 3 independent experiments; stretched for 24 hours at 10%, N = 3 independent experiments; and interrupted 10% stretch, N = 4 independent experiments. n = 9000 cells; ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/4/10.1016_j.bvth.2025.100103/1/m_bvth_vth-2024-000288r3-gr4.jpeg?Expires=1764787049&Signature=uLYX5-gmp3GxDnNdvFndubBD3aObf2SUbdV~wlqL67Y023MV6JWJhplzhoLXsZH0CObzCNwEwshT8Koz~Fdg5EGdPEspzlgG5Dcj4iob4YDIIQ-c-W8WGBmYapYc-ooCmxfKWsj-GEkf7fe7bf4XNWroJahGF4jYGLHKZ3PIWH9o2TbZTXeXNxIpvBdO6JxKJNUOQt5arfAvPeqd09idJC~Hz-EDvca08Jk0zeSBuMKa~0DA4-NphjtOMGxj69kTZ9LeLDAMOkKNSgVVaJbROntbrMnxx2yzgYS-SfFk5Z09ZQ3dmrK6AmbPZyPqA5Exbefp~rLuEqSeP8QVttzOhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Study of magnitude of stretch at 5%. TM expression on HUVECs is dependent on the magnitude of stretch. (A) Confocal images of HUVECs stretched for 24 hours at 5% on a flat membrane: DAPI in blue, F-actin in red, TM in magenta, and VWF in green on the same sample. Objective, original magnification ×40; scale bar, 20 μm. The arrow represents the direction of the uniaxial cyclic stretch. Z-stack sum projection. (B) Frequency distribution of TM and VWF expression (intensity of fluorescence I [AU]) for 1 representative experiment. Unstretched, light blue; stretched for 24 hours at 10%, orange; and stretched for 24 hours at 5%, green. Insets: relative protein expression fold change (bar graph), mean ± SD values of unstretched and stretched for 24 hours at 10% vs stretched for 24 hours at 5%, N = 2 independent experiments, respectively (medium and multiwell Curibio cytostretcher membranes were used to facilitate averaging across duplicates). ∗∗∗∗P < .0001. Every black dot represents 1 independent experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodvth/2/4/10.1016_j.bvth.2025.100103/1/m_bvth_vth-2024-000288r3-gr5.jpeg?Expires=1764787049&Signature=b0qJPvC1-nxqzuGUVcL4Uv4Qk6UnfCgXZzghpImyBa9evjvrMvOXZu4Sy6SY4mqILubZvCTFvECUJz9xegonljhp~y4gVdHPgRIi7lnywiAT09smH8I8SlNMOnlBFleGYEN0DKAj9f~Kr-KV56XltMuFlDhUl~sV1Fb1Qrd-GWebOvUgLvpHycbpmNdIDtC3DltLC9Gbvw61hr-gUfH2aaXQpRZxwEcPV3u4tz0bSStO-gBU3NpdwZ8a-QOfFUX8w3NJZ~Subc~JxD4jA89nPzS50Hwoklx2-67lmQpWZ812U5quYTrvcRLRpn~uehu-zHiLHgncVTFiWdjnrhU3Eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)