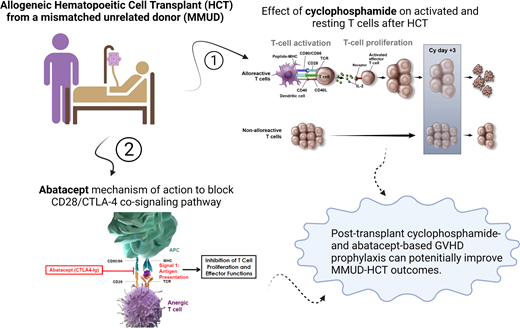
Abstract
With increasing numbers of patients with hematologic malignancies requiring allogeneic hematopoietic cell transplant (HCT), including minority racial and ethnic groups, the limited availability of matched related donors and matched unrelated donors remains a significant obstacle. Hence, the use of alternative donors such as haploidentical and mismatched unrelated donors (MMUDs) is on the rise. Herein, we present case studies to outline a rational and stepwise approach with a focus on the use of MMUD for HCT in patients with hematologic malignancies. We also review novel approaches used to reduce the incidence of severe graft-versus-host disease and improve HCT outcomes in patients undergoing MMUD HCT.
Learning Objectives
Describe the need for mismatched unrelated donors (MMUDs) for hematopoietic cell transplant (HCT) when matched donors are not available for patients with hematologic malignancies
Illustrate the impact of different human leukocyte antigen locus mismatch on HCT outcomes
Define novel strategies used to improve HCT outcomes in the MMUD setting
Introduction
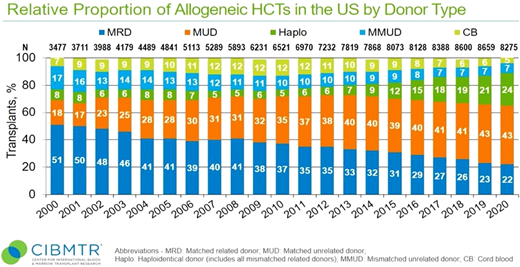
Allogeneic hematopoietic cell transplantation (HCT) offers a potentially curative treatment for patients with most hematologic malignancies.1 Historically, HCT from a human leukocyte antigen (HLA) matched sibling donor (MSD) is considered the gold standard with the lowest nonrelapse mortality (NRM) and graft-vs-host disease (GVHD)–related mortality and morbidity.2 Comparable transplant outcomes have been reported with matched unrelated donors (MUDs), which became a widely acceptable alternative for patients with no MSD.3,4 The likelihood of identifying an 8/8 MUD varies with ethnicity and race; for example, this could be as high as 70% for Caucasians but falls to below 20% for African American and other ethnic minorities and even more challenging for mixed-race individuals.5 Other factors affecting donor availability include typing issues, medical deferral, and donor or donor center availability.5 With the recent advancement and improvement in outcomes, alternative donors have been increasingly used in close to 36% of cases where match donors are not available (Figure 1). These include mismatched unrelated donors (MMUDs), haploidentical donors (Haplo), and umbilical cord blood.
CIBMTR-reported relative proportion of allogeneic HCTs in the United States by donor type.
CIBMTR-reported relative proportion of allogeneic HCTs in the United States by donor type.
MMUDs
Matching at HLA loci plays an integral role in selecting donors for HCT. When a matched donor (MSD or MUD) is not available, alternative donors are sought such as haploidentical donors, MMUDs, and cord blood units.
Outcomes using standard (conventional) GVHD prophylaxis for MMUD transplants
Large single-center and registry retrospective comparative studies have shown worse outcome with higher degree of mismatch in the setting of conventional GVHD prophylaxis, regardless of disease type or risk, HCT conditioning intensity, or stem cell source (Table 1). Conventional GVHD prophylaxis usually includes a calcineurin inhibitor (ie, tacrolimus or cyclosporine) combined with mycophenolate mofetil, methotrexate, or sirolimus. In 1 study, mostly in patients undergoing myeloablative conditioning (MAC) for bone marrow (BM) HCT,6 difference in survival is less when disease risk is higher, but this was not confirmed in other studies when expanded to other conditioning intensities and stem cell source. For example, patients with poor-risk acute myeloid leukemia (AML) in complete remission 1 who underwent HCT from MSD (n = 226), 8/8 MUD (n = 254), and MMUD (n = 104) had similar leukemia-free survival (LFS) and overall survival (OS) to MSD and 8/8 MUD transplants, but LFS and OS were worse for MMUD HCT.7 Also, in patients with other diseases, such as myelodysplastic syndrome, similar results were reported with worse outcomes in MMUD HCT.8
Studies using conventional GVHD prophylaxis for MMUD HCTs
| Study . | Population . | Design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Gupta et al (2010)7 | Poor-risk AML in CR1 underwent HCT from MSD (n = 226), 8/8 MUD (n = 254), and MMUD (n = 104) | CIBMTR—retrospective | NA | 1. Similar LFS and OS for MSD and 8/8 MUD transplants but LFS and OS were worse for MMUD HCT |
| Al Malki et al (2020)9 | 482 patients with hematologic malignancies underwent MUD/MMUD HCT with tacrolimus and sirolimus combination for GVHD prophylaxis | Single-institution retrospective study | NA | 1. 5-year OS: 39% for MMUD vs 50.7% for MUD, P = .022 2. NRM: 33.5% for MMUD vs 21.7% for MUD, P = .038 3. Severe GVHD: 20.8% for MMUD vs 12.8% for MUD, P = .22 4. Infection 33.0% for MMUD vs 18.1% for MUD, P < .01 5. No improvement in rates of relapse with MMUD HCT |
| Kornblit et al (2020)10 | 77 patients who underwent RIC PBSC HCT from MMUD for hematologic malignancies (76 evaluable patients) | Multicenter phase 2 trial | Historical | 1. Day 100 CI of grade II to IV aGVHD was 36% 2. OS at 4 years = 62% 3. NRM = 18% 4. Relapse/progression = 30% |
| Study . | Population . | Design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Gupta et al (2010)7 | Poor-risk AML in CR1 underwent HCT from MSD (n = 226), 8/8 MUD (n = 254), and MMUD (n = 104) | CIBMTR—retrospective | NA | 1. Similar LFS and OS for MSD and 8/8 MUD transplants but LFS and OS were worse for MMUD HCT |
| Al Malki et al (2020)9 | 482 patients with hematologic malignancies underwent MUD/MMUD HCT with tacrolimus and sirolimus combination for GVHD prophylaxis | Single-institution retrospective study | NA | 1. 5-year OS: 39% for MMUD vs 50.7% for MUD, P = .022 2. NRM: 33.5% for MMUD vs 21.7% for MUD, P = .038 3. Severe GVHD: 20.8% for MMUD vs 12.8% for MUD, P = .22 4. Infection 33.0% for MMUD vs 18.1% for MUD, P < .01 5. No improvement in rates of relapse with MMUD HCT |
| Kornblit et al (2020)10 | 77 patients who underwent RIC PBSC HCT from MMUD for hematologic malignancies (76 evaluable patients) | Multicenter phase 2 trial | Historical | 1. Day 100 CI of grade II to IV aGVHD was 36% 2. OS at 4 years = 62% 3. NRM = 18% 4. Relapse/progression = 30% |
CR1, complete remission 1; NA, not available.
A large single-center retrospective study compared outcomes of MUD and MMUD with tacrolimus and sirolimus as GVHD prophylaxis in 482 peripheral blood stem cell (PBSC) HCTs. With a long-term follow-up (median = 6.2 years), 5-year OS was significantly worse in patients undergoing MMUD HCT (39% vs 50.7%, P = .022), mostly due to higher risk of NRM (33.5% vs 21.7%, P = .038), with more severe GVHD (20.8% vs 12.8%, P = .22) and infection (33.0% vs 18.1%, P < .01). There was no improvement in rates of relapse with MMUD HCT.9 A multicenter phase 2 trial used a triplet of cyclosporine, mycophenolate mofetil, and sirolimus as GVHD prophylaxis for reduced intensity conditioning (RIC) transplant using MMUD for hematologic malignancies.10 A combination of fludarabine and total body irradiation (2-3 Gy) was used as conditioning therapy. The study included 76 evaluable patients with a median age of 63 years. With a median follow-up of 47 months (4-94 months), the cumulative incidence of day 100 grade II to IV acute GVHD (aGVHD) was 36%. The study reported a cumulative incidence of NRM (18%), relapse/progression (30%), and OS (62%) after 4 years.
Mismatch type, number, and location
Multiple studies have attempted to correlate HCT outcomes with mismatch at certain HLA loci (Table 2). In 1 study, outcomes of 1874 donor-recipient pairs undergoing myeloablative BM HCT were analyzed with respect to high-resolution typing at HLA-A, -B, -C, -DRB1, -DQ, and -DP.11 Survival was worse with higher number of mismatched loci regardless of the mismatched locus location (A, B, C, or DR) or level of testing (antigen vs allele).11 Although mismatches at loci HLA-A, -B, -C, or -DRB1 all showed more frequent aGVHD, the incidence of grade III/IV GVHD was much higher with mismatch at HLA-A (in this particular study) compared with mismatch at HLA-B, -C, or -DRB1. Mismatches at HLA-DQ and -DP showed little effect on HCT outcomes. Another study showed that for the selection of an appropriate MMUD, preference should always be to match at high resolution with HLA-A, -B, -C, and -DR, allowing for HLA-DQ and/or -DP mismatch.11
Landmark studies for MMUD transplant (HCT)
| Study . | Population . | Study design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Flomenberg et al (2004)11 | 1874 patients in NMDP who underwent HCT for HMs and nonmalignant disorders | Retrospective | 1. Mismatches at loci HLA-A (n = 374), -B (n = 477), -C (n = 749), or -DRB1 (n = 311), -DQ (n = 415), and -DP (n = 1648) 2. Study included 108 patients with 8/8 match | 1. Reduced OS after MMUD HCT with mismatches at loci HLA-A, -B, -C, or -DRB1 2. Survival was worse with increasing number of mismatches. 3. Mismatches at loci HLA-A, -B, -C, or -DRB1 all showed more frequent aGVHD 4. The incidence of grade III/IV aGVHD was much higher with mismatch at HLA-A as compared to mismatch at HLA-B, -C, or -DRB1. 5. Mismatches at HLA-DQ and -DP had little effect on HCT outcomes. 6. Class I HLA mismatches with or without HLA-DQ mismatch had similar outcomes. |
| Lee et al (2007)6 | 3857 patients with HMs (early stage, intermediate stage, and advanced/higher stage) who underwent MAC HCT • 94% received BMSCs • 78% received T-cell replete grafts • GVHD prophylaxis included calcineurin inhibitor | Retrospective (CIBMTR) | 1. 8/8 MUD (n = 1840) 2. Single (n = 985) or multiple (n = 1032) MMUD | 1. Higher-risk disease at the time of HCT had a higher association with poor survival as compared to increased number of HLA mismatches. 2. No statistically significant difference in survival outcomes between single-antigen mismatch vs allele mismatch on the same locus. No statistically significant difference between 8/8 and 7/8 with respect to engraftment, relapse, and chronic GVHD. 3. A single mismatch at HLA-A, -C, or -DRB1 was associated with significantly reduced OS compared to 8/8 matches. 4. Multiple mismatches were associated with poorer survival outcomes. 5. Single mismatch at HLA-DQ was not associated with any effect on survival. 6. HLA-DP mismatch did not affect survival but was associated with increased risk of aGVHD. |
| Saber et al (2012)4 | 2223 adult patients with AML who underwent alloHCT between 2002 and 2006 | Retrospective (CIBMTR) | 1. MSD (n = 624) 2. 8/8 MUD (n = 1193) 3. 7/8 MUD (MMUD) (n = 406) | 1. Significantly lower 100-day cumulative incidence of grade II to IV aGVHD in MSD HCT recipients than in 8/8 MUD and 7/8 MUD HCT recipients (33%, 51%, and 53%, respectively; P < .001). 2. 8/8 MUD HCT recipients had a similar survival rate compared with MSD HCT recipients (RR, 1.03; P = .62). 3. 7/8 MUD HCT recipients had higher early mortality than MSD HCT recipients (RR, 1.40; P < .001), but beyond 6 months after HCT, their survival rates were similar (RR, 0.88; P = .30). |
| Pidala et al (2014)12 | 8003 patients with HMs (early stage, intermediate stage, and advanced/higher stage) who underwent MUD HCT with MAC between 1999 and 2011 | Retrospective (CIBMTR) | 1. 8/8 MUD (n = 5449) 2. 7/8 MMUD (n = 2071) 3. 6/8 MMUD (n = 483) | 1. 6/8-7/8 led to significantly increased risk of grade II to IV and III to IV aGVHD, chronic GVHD, TRM, and reduced OS as compared to 8/8 matched pairs. 2. For 8/8 HCTs, mismatch at both HLA-DQB1 and HLA-DPB1 loci led to an increased incidence of aGVHD and mismatch at HLA-DPB1 resulted in reduced relapse. Both PR and no-PR HLA-DPB1 allele mismatches led to significantly increased incidence of grade II to IV and grade III to IV aGVHD and reduced risk of relapse. 3. Significantly increased risk of TRM in no-PR pairs as compared to matched pairs or pairs with PR DPB1 allele mismatches. 4. Adjusted OS for 8/8 matched HCT was best among the HLA-DPB1 allele mismatched pairs followed by fully matched HLA-DPB1, with worst adjusted OS for nonpermissive mismatches (P = .015). 5. No difference in outcomes for 1 or double allele mismatches at HLA-DPB1 for permissive or nonpermissive mismatches. |
| Malki et al (2020)14 | 310 patients RIC HCT | Retrospective | 1. 12/12 MUD 2. 11/12 MUD (DPB1-mismatched) 3. 10/12 MUD (DPB1-mismatched) | 1. Better OS and relapse in 11/12 when compared to 12/12 and better OS as compared to 10/12. 2. Among the 11/12 pairs, when compared with permissive DP mismatch (PR), nonpermissive DP mismatch (no-PR) is associated with increased risk of grade II to IV aGVHD and NRM. 3. When DP expression was considered, using high DP expressors as donors for low-expressor recipients was associated with increased risk of mortality. DP PR 11/12 mismatch was associated with higher OS when both donor and patient were DP low expressors when compared with other combinations. |
| Study . | Population . | Study design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Flomenberg et al (2004)11 | 1874 patients in NMDP who underwent HCT for HMs and nonmalignant disorders | Retrospective | 1. Mismatches at loci HLA-A (n = 374), -B (n = 477), -C (n = 749), or -DRB1 (n = 311), -DQ (n = 415), and -DP (n = 1648) 2. Study included 108 patients with 8/8 match | 1. Reduced OS after MMUD HCT with mismatches at loci HLA-A, -B, -C, or -DRB1 2. Survival was worse with increasing number of mismatches. 3. Mismatches at loci HLA-A, -B, -C, or -DRB1 all showed more frequent aGVHD 4. The incidence of grade III/IV aGVHD was much higher with mismatch at HLA-A as compared to mismatch at HLA-B, -C, or -DRB1. 5. Mismatches at HLA-DQ and -DP had little effect on HCT outcomes. 6. Class I HLA mismatches with or without HLA-DQ mismatch had similar outcomes. |
| Lee et al (2007)6 | 3857 patients with HMs (early stage, intermediate stage, and advanced/higher stage) who underwent MAC HCT • 94% received BMSCs • 78% received T-cell replete grafts • GVHD prophylaxis included calcineurin inhibitor | Retrospective (CIBMTR) | 1. 8/8 MUD (n = 1840) 2. Single (n = 985) or multiple (n = 1032) MMUD | 1. Higher-risk disease at the time of HCT had a higher association with poor survival as compared to increased number of HLA mismatches. 2. No statistically significant difference in survival outcomes between single-antigen mismatch vs allele mismatch on the same locus. No statistically significant difference between 8/8 and 7/8 with respect to engraftment, relapse, and chronic GVHD. 3. A single mismatch at HLA-A, -C, or -DRB1 was associated with significantly reduced OS compared to 8/8 matches. 4. Multiple mismatches were associated with poorer survival outcomes. 5. Single mismatch at HLA-DQ was not associated with any effect on survival. 6. HLA-DP mismatch did not affect survival but was associated with increased risk of aGVHD. |
| Saber et al (2012)4 | 2223 adult patients with AML who underwent alloHCT between 2002 and 2006 | Retrospective (CIBMTR) | 1. MSD (n = 624) 2. 8/8 MUD (n = 1193) 3. 7/8 MUD (MMUD) (n = 406) | 1. Significantly lower 100-day cumulative incidence of grade II to IV aGVHD in MSD HCT recipients than in 8/8 MUD and 7/8 MUD HCT recipients (33%, 51%, and 53%, respectively; P < .001). 2. 8/8 MUD HCT recipients had a similar survival rate compared with MSD HCT recipients (RR, 1.03; P = .62). 3. 7/8 MUD HCT recipients had higher early mortality than MSD HCT recipients (RR, 1.40; P < .001), but beyond 6 months after HCT, their survival rates were similar (RR, 0.88; P = .30). |
| Pidala et al (2014)12 | 8003 patients with HMs (early stage, intermediate stage, and advanced/higher stage) who underwent MUD HCT with MAC between 1999 and 2011 | Retrospective (CIBMTR) | 1. 8/8 MUD (n = 5449) 2. 7/8 MMUD (n = 2071) 3. 6/8 MMUD (n = 483) | 1. 6/8-7/8 led to significantly increased risk of grade II to IV and III to IV aGVHD, chronic GVHD, TRM, and reduced OS as compared to 8/8 matched pairs. 2. For 8/8 HCTs, mismatch at both HLA-DQB1 and HLA-DPB1 loci led to an increased incidence of aGVHD and mismatch at HLA-DPB1 resulted in reduced relapse. Both PR and no-PR HLA-DPB1 allele mismatches led to significantly increased incidence of grade II to IV and grade III to IV aGVHD and reduced risk of relapse. 3. Significantly increased risk of TRM in no-PR pairs as compared to matched pairs or pairs with PR DPB1 allele mismatches. 4. Adjusted OS for 8/8 matched HCT was best among the HLA-DPB1 allele mismatched pairs followed by fully matched HLA-DPB1, with worst adjusted OS for nonpermissive mismatches (P = .015). 5. No difference in outcomes for 1 or double allele mismatches at HLA-DPB1 for permissive or nonpermissive mismatches. |
| Malki et al (2020)14 | 310 patients RIC HCT | Retrospective | 1. 12/12 MUD 2. 11/12 MUD (DPB1-mismatched) 3. 10/12 MUD (DPB1-mismatched) | 1. Better OS and relapse in 11/12 when compared to 12/12 and better OS as compared to 10/12. 2. Among the 11/12 pairs, when compared with permissive DP mismatch (PR), nonpermissive DP mismatch (no-PR) is associated with increased risk of grade II to IV aGVHD and NRM. 3. When DP expression was considered, using high DP expressors as donors for low-expressor recipients was associated with increased risk of mortality. DP PR 11/12 mismatch was associated with higher OS when both donor and patient were DP low expressors when compared with other combinations. |
BMSC, bone marrow stem cell; HM, hematological malignancies; NMDP, National Marrow Donor Program; PR, permissive match; no-PR, nonpermissive match; TRM, transplant-related mortality.
CLINICAL CASE 1
A 65-year-old woman in complete remission 1 after induction therapy for FLT3+ acute myeloid leukemia, with no suitable sibling donors, presented in consultation. A MUD search revealed four 8/8 or 10/10 HLA matched donors. Patient's high-resolution HLA typing showed that HLA-DPB1 was homozygous at 04:01, which are low expresser HLA-DPB1 alleles. Review of the HLA typing of the 3 potential donors revealed one 30-year-old donor with 11/12 match with permissive HLA-DPB1 at 02:01, which is a low expressor allele that was activated. Patient underwent HCT with conditioning therapy consisting of fludarabine and melphalan, as well as GVHD prophylaxis of tacrolimus and sirolimus. Patient is at 2.5 months posttransplant, in continued complete remission (CR) and no evidence of GVHD.
Role of HLA-DPB1 mismatch
HLA-DPB1 mismatch was studied in multiple different large studies. In a study by Pidala et al,12 8003 donor-recipient pairs underwent MUD HCT with MAC between 1999 and 2011. Mismatch at A, B, C, or DRB1 led to significantly increased risk of grade II to IV and III to IV aGVHD, chronic GVHD, transplant-related mortality, and reduced OS compared with 8/8 matched pairs, confirming prior results. GVHD was higher with no impact on OS/NRM in patients with HLA-DQB1 and/or HLA-DPB1 mismatch. However, mismatch at HLA-DPB1 resulted in reduced rate of relapse. Based on T-cell epitope matching algorithm, risk of NRM was higher in pairs with nonpermissive HLA-DPB1 mismatch.13 Adjusted OS for 8/8 matched HCT was best among patients with permissive HLA-DPB1 allele mismatched pairs followed by fully matched HLA-DPB1 with worst adjusted OS for nonpermissive mismatches (P = .015). Same results were obtained in a smaller cohort with RIC undergoing PBSC MUD HCT.14
In addition, since risk of GVHD associated with HLA-DPB1 mismatching is influenced by the HLA-DPB1 rs9277534 expression marker, patients with higher HLA DPB1 expression allele have a high risk for GVHD when receiving transplants from donors with the low-expression allele, regardless of the conditioning intensity.15
HLA-B leader match in HLA-B mismatch
HLA-B has high polymorphism at the peptide binding site. Mismatch at HLA-B leads to increased risk of GVHD and lower disease-free survival.16 Leader peptides have methionine (M) or threonine (T) at position 2 containing sequence dimorphisms in exon 1 of HLA-B.16 These leader peptides are bound by HLA-E, which serves as a ligand for the natural killer receptors. The 2 HLA-B allotypes determine the leader genotype in a person. T- and M-leaders can affect the T-cell and natural killer–cell responses by changing the expression of HLA-E.16-19 These changes could affect alloimmune responses. A large retrospective study reported outcomes of 33 982 patients who underwent alloHCT from a MUD/MMUD between 1988 and 2016. The study included 1457 transplants with mismatch at HLA-B and evaluated outcomes based on B-leader match/mismatch. Presence of M-leader in recipients or donors increases the risk of grade III to IV aGVHD in patients who underwent HCT from an HLA-B mismatched donor. GVHD risk is also increased with the presence of different leaders at the mismatched HLA-B allotype.16
In this setting, if available donors are mismatched at HLA-B, the selection of the most suitable donor is based on the recipient's leader genotype. For single-locus HLA-B mismatched hematopoietic cell transplants, 94.6% of the recipients had the MT or TT leader genotype. Patients with the MT leader genotype had 2 leader-matched donor possibilities: a donor matched at the M-leader allotype and mismatched for the T-leader allotype (TTM) or a donor mismatched at the M-leader allotype and matched for the T-leader allotype (MMT). Authors reported that TTM transplants had a higher risk of severe aGVHD compared with MMT (odds ratio, 1.99; 95% confidence interval [CI], 1.10-3.59; P = .022), preferring MMT over TTM to improve HCT outcomes.16 The donor choices for recipients with the TT leader genotype include leader matched (TTT) and leader mismatched (TMT), and TTT pairing is preferred over TMT for improved GVHD outcomes.16
HLA-C mismatch and role of permissive C mismatch
For HCTs involving mismatch at HLA-C, the immunogenicity is affected by the level of expression of mismatched HLA-C in the patient.20 A large retrospective study compared outcomes of 1933 unrelated donor-recipient pairs who underwent PBSC alloHCT for hematologic malignancies. This study included 189 pairs mismatched at the HLA-C antigen. HLA-C mismatch at high resolution was associated with significantly lower leukemia-free survival (relative risk [RR], 1.36; 95% CI, 1.13-1.64; P = .0010) and significantly increased risk for mortality (RR, 1.41; 95% CI, 1.16-1.70; P = .0005), transplant-related mortality (RR, 1.61; 95% CI, 1.25-2.08; P = .0002), and grade III to IV aGVHD (RR, 1.98; 95% CI, 1.50-2.62; P < .0001).21 Single-allele mismatch at HLA-C, C*03:03/C*03:04, was very frequent in a registry study of patients with Caucasian ancestry (68.7%).22 When compared with 8/8 matched HCTs, 7/8 allele level match with C*03:03/C*03:04 mismatch was associated with comparable outcomes such as OS, NRM, disease-free survival, and risk of aGVHD.22 Due to this finding, C*03:03/C*03:04 mismatch is referred to as a permissive mismatch and is preferred at least in the setting of myeloablative BM HCT.
CLINICAL CASE 2
A 69-year-old Mediterranean woman in complete remission 2 after induction therapy for NPM1-positive AML with hypomethylating agents with venetoclax was seen in consultation. HLA typing for patient and 2 siblings did not reveal MSD. No MUD was found in the registry. She had multiple high titers (Mean Fluorescent Intensity >5000-10 000) and donor-specific antibodies (DSAs) against HLA-A, -B, and -DRB1 loci in her children. Unrelated donor search revealed a young 7/8 donor with HLA-C mismatch that was permissive, for which the patient had no DSAs. Patient underwent PBSC MMUD HCT with HLA-C mismatched donor using GVHD prophylaxis of tacrolimus and sirolimus. The patient developed grade II skin GVHD, which responded to steroid. Day +100 BM biopsy specimen showed minimal residual disease CR and full donor chimerism. Patient is at >6 months post-HCT in continued remission and no evidence of GVHD.
Donor selection for patients with high-risk HLA antibodies
Antibodies to HLA can be detected at elevated frequencies in patients with prior sensitizing events such as pregnancy or transfusion,23 but HLA antibodies are also sometimes present in otherwise healthy individuals in the absence of known sensitizing events. Although the impact of humoral sensitization in HCT outcomes remains poorly understood compared with the significance of DSAs developing in solid organ transplantation,24 recent studies of HLA antibodies pre- and early post-HCT have been shown to exert a negative impact on engraftment and graft function.25,26 To avoid graft failure or graft dysfunction, screening patients for HLA antibodies when undergoing workup prior to HLA mismatched (related or unrelated) HCT has become a standard practice.23 Patients with clinically significant DSAs may undergo DSA desensitization to mitigate risk and facilitate engraftment in most cases but not all.27 With recent advances in GVHD prophylaxis and improvement in outcome of MMUD,27-29 avoiding the mismatched loci with DSAs and mismatching to a non-DSA locus has become one of the advantages of this donor type.28
Novel approaches to improve outcomes in MMUD HCT
The role of posttransplant cyclophosphamide (PTCy) in haploidentical transplant is well established.30 Alloreactive T cells are sensitive to the killing effect of cyclophosphamide, while memory and regulatory T cells, with expression of aldehyde dehydrogenase, are resistant, making PTCy a highly effective therapy to prevent GVHD while preserving T-cell regulatory and memory function.31-34 A phase 2 prospective multicenter trial was conducted to study outcomes of MMUD HCT using PTCy.35 All patients (n = 80) received fresh BM grafts on day 0, PTCy on days 3 and 4 after HCT, and sirolimus with mycophenolate starting on day 5. The study included 48% of the patients who were racial or ethnic minorities, and 39% of the patients received a BM graft with more than 1 HLA allele mismatch (4-6/8). One-year OS for the whole group was 76%, as well as 72% and 79% for MAC and RIC, respectively. Degree of HLA mismatch did not affect the OS (75% for 7/8 and 77% for 4-6/8) in this small cohort, which was later confirmed in a longer follow-up presentation. The incidence of day 100 grade II to IV aGVHD, III to IV aGVHD, and 1-year chronic GVHD was 43%, 18%, and 36% for patients receiving MAC and 33%, 0%, and 18% for patients who received RIC, respectively.35 These results were comparable to the outcome of a historical control undergoing Haplo HCT and reported to the Center for International Blood, and Marrow Transplant Research (CIBMTR) around the same time.35 Similar retrospective but timely comparison was also published by the European Society for Blood and Marrow Transplantation36 in patients with AML in CR with patients undergoing MMUD HCT (n = 155) compared with Haplo BM (n = 647) and Haplo PB (n = 949) in the setting of PTCy-based GVHD prophylaxis. Haplo BM and Haplo PB had a higher NRM compared with MMUD (hazard ratio, 2.28; 95% CI, 1.23-4.24; P < .01 and hazard ratio, 2.65; 95% CI, 1.46-4.81; P < .01, respectively), with lower LFS and OS. All raised the need for a randomized study comparing the 2 graft sources in a prospective and systemic manner.
A concurrently conducted, single-center, prospective study of 38 patients who underwent PBSC MMUD HCT (Figure 2) with a similar design showed similar outcomes.29 Median number of HLA mismatches was 2 (range, 1-4) of 12. Racial and ethnic minorities made up 61% of the study population. The 1-year OS and GVHD-free/relapse-free survival was 87% and 68%, respectively. The incidences of grade II to IV and III to IV aGVHD at day +100 and chronic GVHD at 1 year were 50%, 18%, and 48%, respectively.29 Combined together, these studies showed that MMUD HCT with PTCy is feasible and well tolerated with promising results using either BM or PBSCs. A large National Marrow Donor Program-sponsored study (ACCESS) is ongoing to confirm these results using PBSC in adults and BM in children (NCT04904588).
Study schema.
(A) RIC regimen. Fludarabine was administered at the daily dose of 25 mg/m2 from days −7 to −3 before HCT. Melphalan was given on day −2 at 140 mg/m2 or 100 mg/m2 for patients ≥60 years old. (B) MAC regimen consisted of daily fludarabine at 30 mg/m2, from days −7 to −5 before HCT. Total body irradiation was administered in 8 fractions of 150 cGy, 2 times a day, from days −4 to −1, for a total of 1200 cGy. Graft source was PBSCs for both strata. ANC, absolute neutrophils count; G-CSF, granulocyte colony stimulating factor; TBI, total body irradiation.
Study schema.
(A) RIC regimen. Fludarabine was administered at the daily dose of 25 mg/m2 from days −7 to −3 before HCT. Melphalan was given on day −2 at 140 mg/m2 or 100 mg/m2 for patients ≥60 years old. (B) MAC regimen consisted of daily fludarabine at 30 mg/m2, from days −7 to −5 before HCT. Total body irradiation was administered in 8 fractions of 150 cGy, 2 times a day, from days −4 to −1, for a total of 1200 cGy. Graft source was PBSCs for both strata. ANC, absolute neutrophils count; G-CSF, granulocyte colony stimulating factor; TBI, total body irradiation.
The European Society for Blood and Marrow Transplantation conducted a retrospective analysis on 272 patients with AML who underwent 9/10 HLA matched HCT with GVHD prophylaxis consisting of PTCy-based (n = 93) or antithymocyte globulin–based (n = 179) regimens. HLA mismatch involved class I in 74% and class II in 26%; half of the patients received MAC and other half received RIC. Use of PTCy was associated with a lower incidence of severe aGVHD and higher LFS and GVHD-free/relapse-free survival.37
Abatacept (ABA) is a CTLA-4-IgG1 fusion protein that binds to CD80/CD86 on the surface of antigen presenting cells and blocks the CD28-mediated costimulation.38 A single-arm feasibility study of ABA as prophylaxis of GVHD was conducted enrolling 10 patients with hematologic malignancies using unrelated donors.39 Four patients underwent HCT from 8/8 HLA matched donors, and 6 patients received 7/8 HLA matched donor grafts. Two patients received a BM graft, and 8 patients received PBSCs. Preparative regimen consisted of MAC in 7 and RIC in 3 patients. GVHD prophylaxis was abatacept, cyclosporine, and methotrexate.39 This helped establish proof of concept and prepared for a larger phase 2 study evaluating the use of ABA in combination with calcineurin inhibitor (CNI) and methotrexate in HLA matched unrelated donors for hematologic malignancies.40 Thirty-eight patients in this study reported to have received MMUD, mostly MAC, with 48% receiving PBSCs. This was reported in comparison to a historical cohort from CIBMTR with or without antithymocyte globulin. Abatacept in combination with CNI and methotrexate (MTX) and in comparison to a historical cohort was shown to be effective in decreasing day 100 grade III to IV and day 180 severe aGVHD-free survival with a minimal effect on the incidence and severity of chronic GVHD.40 A large multicenter prospective study is ongoing to confirm these results and to test if extended exposure to ABA would affect incidence and severity of chronic GVHD (NCT04380740).
A recent abstract, presented at an American Society of Hematology meeting in 2021, compared the real-world outcome of 7/8 HLA MMUD HCTs for hematologic malignancies reported to CIBMTR between 2011 and 2018 using either CNI + MTX with (n = 54) or without ABA (n = 162).41 The OS at day +180 for the cohort receiving ABA was 98% compared with 75% for CNI + MTX alone (P = .0028). In an exploratory analysis focused on short-term end point (OS at 180 days), outcome of patients undergoing MMUD HCT with ABA with CNI + MTX was comparable to patients undergoing PTCy.41 Further long-term analysis will be needed to define the effect of this novel GVHD prophylaxis regimen on chronic GVHD and ultimately on OS and the efficacy of this regimen in a higher degree of mismatch (<7/8).
Other novel GVHD prophylaxis approaches in the MMUD HCT setting, used recently, with a short-term follow-up include graft manipulation, such as CD34+ cells with add back of memory CD45RA+ T cells, α/β T-cell receptor depletion, and CD34+ cell depletion with add back of a conventional T cells and regulatory T cells approach. Those promising approaches are still under investigation (Table 3).
Studies using PTCy or abatacept for GVHD prophylaxis for MMUD HCT
| Study . | Population . | Design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Shaw et al (2021)35 | 80 patients who underwent RIC/MAC BMSC MMUD HCT with PTCY/MMF/sirolimus-based GVHD prophylaxis | Multicenter, prospective, phase 2 trial | NA | 1. The 1-year OS was 76% (72% for MAC and 79% for RIC) 2. Degree of HLA mismatch did not affect the OS (75% for 7/8 and 77% for 4-6/8). 3. The day +100 incidence of grade II to IV and grade III to IV aGVHD was 43% and 18% for MAC and 33% and 0% for RIC. 4. The 1-year incidence of chronic GVHD MAC and RIC was 36% and 18%, respectively. |
| Al Malki et al (2021)29 | 38 patients who underwent PBSC MMUD HCT | Single-center, prospective study | 1. The 1-year OS was 87%. 2. The 1-year GRFS 68%. 3. The day +100 incidence of grade II to IV and III to IV aGVHD was 50% and 18%, respectively. 4. The 1-year incidence of chronic GVHD was 48%. | |
| Battipaglia et al (2022)36 | Patients with AML in CR undergoing MMUD HCT (n = 155) compared to Haplo BM (n = 647) and Haplo PB (n = 949) with PTCy-based GVHD prophylaxis | Retrospective study | Haplo HCT | 1. Haplo BMSC and Haplo PBSC had a higher NRM compared to MMUD (HR, 2.28; 95% CI, 1.23-4.24; P < .01 and HR, 2.65; 95% CI, 1.46-4.81; P < .01, respectively) with lower LFS and OS. |
| Battipaglia et al (2019)37 | 272 patients with AML underwent 9/10 HLA matched HCT with GVHD prophylaxis consisting of PTCy-based (n = 93) or ATG-based (n = 179) regimens. HLA mismatch involved class I in 74% and class II in 26%. Half of the patients received MAC, and the other half received RIC. | Retrospective study | 1. Use of PTCy was associated with lower incidence of severe aGVHD and higher LFS and GRFS. | |
| Watkins et al (2021)40 | 38 received MMUD, mostly MAC, with 48% receiving PBSCs and 52% received BMSCs. GVHD prophylaxis with abatacept in combination with CNI and MTX | Phase 2 | Historical cohort from CIBMTR with or without ATG | 1. The incidence of grade II to IV aGVHD was 2.3% (CNI/MTX plus abatacept, intention-to-treat population), which compared favorably with a nonrandomized matched cohort of CNI/MTX (30.2%, P < .001), and the SGFS was better (97.7% vs 58.7%, P < .001). |
| Kean et al (2021)41 | 7/8 HLA MMUD HCTs for hematologic malignancies between 2011 and 2018 using either CNI + MTX with (n = 54) or without ABA (n = 162) | CIBMTR retrospective | 1. The OS at day +180 for the cohort receiving ABA was 98% compared to 75% for CNI + MTX alone (P = .0028). 2. In an exploratory analysis focused on short-term end point (OS at 180 days), outcome of patients undergoing MMUD HCT with ABA with CNI + MTX was comparable to patients undergoing PTCy. |
| Study . | Population . | Design . | Comparison . | Key findings . |
|---|---|---|---|---|
| Shaw et al (2021)35 | 80 patients who underwent RIC/MAC BMSC MMUD HCT with PTCY/MMF/sirolimus-based GVHD prophylaxis | Multicenter, prospective, phase 2 trial | NA | 1. The 1-year OS was 76% (72% for MAC and 79% for RIC) 2. Degree of HLA mismatch did not affect the OS (75% for 7/8 and 77% for 4-6/8). 3. The day +100 incidence of grade II to IV and grade III to IV aGVHD was 43% and 18% for MAC and 33% and 0% for RIC. 4. The 1-year incidence of chronic GVHD MAC and RIC was 36% and 18%, respectively. |
| Al Malki et al (2021)29 | 38 patients who underwent PBSC MMUD HCT | Single-center, prospective study | 1. The 1-year OS was 87%. 2. The 1-year GRFS 68%. 3. The day +100 incidence of grade II to IV and III to IV aGVHD was 50% and 18%, respectively. 4. The 1-year incidence of chronic GVHD was 48%. | |
| Battipaglia et al (2022)36 | Patients with AML in CR undergoing MMUD HCT (n = 155) compared to Haplo BM (n = 647) and Haplo PB (n = 949) with PTCy-based GVHD prophylaxis | Retrospective study | Haplo HCT | 1. Haplo BMSC and Haplo PBSC had a higher NRM compared to MMUD (HR, 2.28; 95% CI, 1.23-4.24; P < .01 and HR, 2.65; 95% CI, 1.46-4.81; P < .01, respectively) with lower LFS and OS. |
| Battipaglia et al (2019)37 | 272 patients with AML underwent 9/10 HLA matched HCT with GVHD prophylaxis consisting of PTCy-based (n = 93) or ATG-based (n = 179) regimens. HLA mismatch involved class I in 74% and class II in 26%. Half of the patients received MAC, and the other half received RIC. | Retrospective study | 1. Use of PTCy was associated with lower incidence of severe aGVHD and higher LFS and GRFS. | |
| Watkins et al (2021)40 | 38 received MMUD, mostly MAC, with 48% receiving PBSCs and 52% received BMSCs. GVHD prophylaxis with abatacept in combination with CNI and MTX | Phase 2 | Historical cohort from CIBMTR with or without ATG | 1. The incidence of grade II to IV aGVHD was 2.3% (CNI/MTX plus abatacept, intention-to-treat population), which compared favorably with a nonrandomized matched cohort of CNI/MTX (30.2%, P < .001), and the SGFS was better (97.7% vs 58.7%, P < .001). |
| Kean et al (2021)41 | 7/8 HLA MMUD HCTs for hematologic malignancies between 2011 and 2018 using either CNI + MTX with (n = 54) or without ABA (n = 162) | CIBMTR retrospective | 1. The OS at day +180 for the cohort receiving ABA was 98% compared to 75% for CNI + MTX alone (P = .0028). 2. In an exploratory analysis focused on short-term end point (OS at 180 days), outcome of patients undergoing MMUD HCT with ABA with CNI + MTX was comparable to patients undergoing PTCy. |
ATG, antithymocyte globulin; GRFS, GVHD free relapse-free survival; HR, hazard ratio; MMF, mycophenolate mofetil; SGFS, severe aGVHD-free survival.
Conclusion
Recent advances in GVHD prophylaxis and donor selection have reignited interest in MMUD as a feasible and suitable option for patients without a matched donor for life-saving HCT. Careful selection of optimal MMUDs involving high-resolution HLA typing and HLA antibody measurement to detect DSAs helps guide in the selection of HLA mismatches to improve outcomes. Novel GVHD prophylaxis approaches such as PTCy, abatacept, and graft manipulation seem to improve GVHD outcomes, although prospective studies are under way. There is constant need for novel therapies to improve the outcomes using MMUDs.
Acknowledgments
The authors thank Steven Devine, MD, for discussion, feedback, and critical reading of the manuscript and Sally Mokhtari, PhD, for reviewing the manuscript.
Conflict-of-interest disclosure
Shukaib Arslan: no competing financial interests to declare.
Monzr M. Al Malki: no competing financial interests to declare.
Off-label drug use
Shukaib Arslan: cyclophosphamide is discussed.
Monzr M. Al Malki: cyclophosphamide is discussed.