The bone marrow microenvironment is known to play a role in the regulation of normal hematopoietic stem cells (HSCs). In recent years, many laboratories have contributed mechanistic data identifying different cellular constituents and signals that regulate not only the most primitive HSCs, but also more differentiated progenitors. From this work, we know that the bone marrow microenvironment is heterogeneous, with multiple niches likely supporting heterogeneous stem and progenitor pools. Concurrently, targeting the cancer microenvironment has revolutionized the treatment strategy for patients with solid organ tumors. However, with the notable exception of multiple myeloma, in which the role of the microenvironment has long been acknowledged and targeted, in other cancers of the hematopoietic system, current therapeutic strategies focus primarily on the diseased clone. In this Mini Review, we discuss the current understanding of the role of the bone marrow microenvironment in myelodysplastic syndromes (MDS), disorders with often dismal prognosis and few approved and effective treatment modalities, with the aim of advocating for novel therapeutic opportunities targeting the microenvironment.
Conceptual and Experimental Arguments for the Role of the Microenvironment in MDS Pathogenesis
Recent work has highlighted the mutational heterogeneity of MDS. Data show that most mutations are represented in both low- and high-risk MDS, and that multiple mutations are often present in the same individual.1 While efforts should certainly be aimed at targeting the clonal mutations, focusing on the bone marrow microenvironment may provide a strategy based on stereotyped interactions of clonal cells with mesenchymal and immune cell populations2,3 in the marrow that may cooperate with individual mutations in determining disease pathology and outcomes. Such approaches could complement clone-targeted treatments. Conceptually, certain characteristics of MDS suggest a role of the microenvironment in contributing to disease pathogenesis. Most investigators support the concept that the cell of origin for MDS is within the stem cell pool — a population typically thought to be more resistant to mutations given its quiescence. Yet in MDS there is clonal expansion in the setting of mutations that ordinarily would not provide an intrinsic advantage at least in vitro, suggesting that non-cell autonomous forces may drive clonal selection in the bone marrow. Additionally, deep-sequencing studies have shown the presence of residual normal hematopoiesis in the setting of clonal disease. Based on the potential of normal HSCs to reconstitute the entire hematopoietic system, the development of marrow failure is therefore conceptually surprising and implies the inhibition of normal hematopoiesis by environmental signals. Given the complex homeostatic system known to maintain the synthetic function of the hematopoietic marrow, more functional mutational clones or nonmutated stem cells would be expected to contribute preferentially to hematopoiesis. Therefore, clonal emergence of severely dysfunctional MDS clones remains poorly unexplained without invoking contribution from the bone marrow microenvironment.
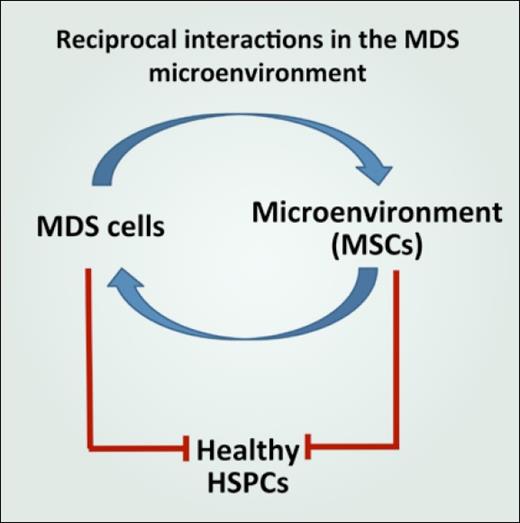
In addition to these conceptual arguments, in MDS there is abundant experimental evidence to support disruption of the normal environment and its hijacking to improve support of the malignant clone. Multiple studies have reported data showing that mesenchymal stromal cells from patients with MDS exhibit functional deficits in vitro.4 These cell populations were found to have impaired growth capacity, accelerated senescence, impaired osteogenic differentiation, and diminished capacity to support HSCs.5 Additionally, in follow up to the observation that human MDS cells engraft poorly in xenotransplantation models, studies showed that cotransplantation of MDS-derived mesenchymal stromal cells improves human MDS cell engraftment.6 Together these data suggest a model of pathogenic reciprocal interactions in the MDS microenvironment where MDS-derived signals disrupt normal HSCs and progenitor cells directly as well as through induction of a dysfunctional bone marrow microenvironment that insufficiently supports normal hematopoiesis but instead facilitates MDS clonal expansion (Figure).
Signals From MDS That Induce Microenvironmental Disruption
Not only is experimental evidence building to show disruption of the bone marrow microenvironment in the setting of MDS, but in fact, data are being used to identify dependence of bone marrow microenvironmental abnormalities on the presence of MDS stem and progenitor cells. For example, there are data showing that exposure of normal mesenchymal cells to MDS marrow could induce these cells to become supportive of MDS.6 Additionally, a large body of work is showing the importance of inflammation in MDS.7 For example, aberrancies found in MDS (5q–) and also in clonal hematopoiesis of indeterminate potential (Tet2 mutations) are capable of initiating an inflammatory program that can be acquired by normal bone marrow mesenchymal cells.6,8-11 To date, however, strategies to target these inflammatory signals have not been extensively tested in therapeutic settings.
Impact of Microenvironmental Disruption on Signals That Support HSCs
Studies of the bone marrow microenvironment have revealed a dominant role of mesenchymal-osteolineage cells in the production of critical HSC maintenance factors such as CXCL12, KIT ligand (stem cell factor), and angiopoietin-1.12-16 These signals contribute to regulation of HSC retention, proliferation, and differentiation that are necessary for maintenance of hematopoietic homeostasis. With MDS-induced dysfunction of this supportive mesenchymal cell population, decreased HSC function would be expected to follow.17 Indeed, genetically altered murine models have demonstrated that defects in mesenchymal and osteoprogenitor populations are sufficient to drive the emergence of not only MDS but also acute myeloid leukemia and myeloproliferative neoplasms.18-20 In the setting of myeloproliferative neoplasms or MDS, in murine models and human samples, the microenvironment demonstrates decreased HSC-supportive signals, including decreased CXCL12, increased stromal cell senescence, abnormal remodeling of blood vessels, osteoblastic defects, and marrow neural damage. It is also the source of disruptive inflammatory signals such as transforming growth factor β (TGFβ), the alarmins S100A9 and S100A8, interleukin-1β (IL1β). and the chemokine CCL3.19,21-29 Data have also shown that some of these inflammatory signals may be generated by the MDS clone.26,27 Therefore, numerous signals from the microenvironment have already come to light that could cooperate with clonal mutations and could represent potential therapeutic MDS targets.
Microenvironmental Targeting Strategies
Identification of the signals that govern reciprocal interactions of MDS and the bone marrow microenvironment is ongoing, and some of the recently identified targets are already being studied, often by repurposing therapies previously approved for other disorders. For example, identification of activation of the inflammasome (NLRP3) in 5q– MDS provides rationale for examining the impact of TLR/TRAF6 inhibitors, NLRP3 inhibitors and IL1 signaling inhibitors. Similarly, inhibitors are available for TGFβ and chemokines such as CCL3, making therapeutic targeting of the microenvironment feasible.
It is our opinion that several factors limit the impact of this work in MDS. First, the genetic heterogeneity of MDS and the frequent co-occurrence of mutations make MDS difficult to model in vivo. Whether different mutations or their combinations induce unique microenvironmental responses remains unknown and should continue to be an active area of research. Second, one of the key populations responsible for HSC support (mesenchymal stromal cells) are extremely rare and remain poorly defined in the human marrow, with lack of consensus as to isolation and definition of these populations. Therefore, studies identifying these populations are often difficult to compare. However, given the availability of agents to potentially target MDS–microenvironment reciprocal signals, the potential for novel therapies that could finally change MDS prognosis by rationally targeting both the MDS clone and its microenvironment should continue to drive discovery and clinical investigation.
Acknowledgements
The authors thank their laboratory members and Dr. Michael W. Becker for helpful discussion. Supported by funds from the Department of Defense, the Mangurian Foundation, the Taub Foundation, and funds from the James P. Wilmot Cancer Institute.
References
Competing Interests
Dr. Calvi and Dr. Liesveld indicated no relevant conflicts of interest.