Treatment options for both newly diagnosed and relapsed multiple myeloma (MM) have been expanding in the past decade, resulting in improved median overall survival (OS) outcomes of more than eight to 10 years.1,2 Nevertheless, patients with high-risk features continue to have inferior outcomes, typically experiencing only short-term disease control. Immunomodulatory drugs, proteosome inhibitors (PIs), corticosteroids, and anti-CD38 monoclonal antibodies remain cornerstones of MM treatment, and these agents are usually adjunct to high-dose melphalan and autologous stem cell transplantation (ASCT) in eligible patients.2,3 Selinexor reversibly inhibits exportin 1, which results in reduction of oncoproteins, accumulation of tumor suppressor proteins in the nucleus, cell cycle arrest, and subsequent cancer cell apoptosis.4 Given its unique mechanism of action, selinexor has been explored in clinical trials for both hematologic malignancies and solid tumors. The STORM phase IIb single-arm study of selinexor and dexamethasone showed an overall response rate of 25 percent and duration of response of 4.4 months in 83 patients with triple-class refractory MM.5 The combination of selinexor 80 mg/dose twice weekly on days 1 and 3 each week (in combination with dexamethasone 20 mg twice weekly) has been approved by the U.S. Food and Drug Administration (FDA) in patients with MM who have received at least four prior MM therapies with a disease that is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
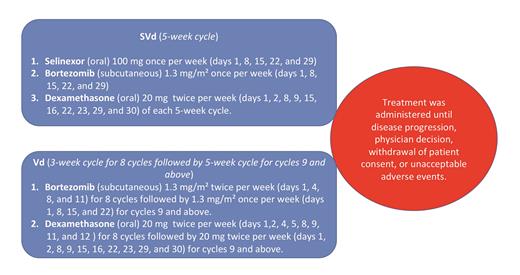
Recently, the BOSTON clinical trial reported results of selinexor, bortezomib, and dexamethasone (SVd) in patients with MM with one to three previous therapies. In this phase III clinical trial, SVd was compared with bortezomib and dexamethasone (Vd; Figure). Previous use of PIs (alone or as part of a combination treatment) was allowed. Patients were required to have had at least a partial response to previous PI-based therapy and at least a six-month interval since their last PI therapy, with no history of discontinuation of bortezomib due to grade 3 or higher toxicity. Patients were required to be refractory to the most recent regimen as part of inclusion criteria.
Treatment administration in the BOSTON clinical trial. SVd, Selinexor, bortezomib, and dexamethasone; Vd, bortezomib and dexamethasone.
Treatment administration in the BOSTON clinical trial. SVd, Selinexor, bortezomib, and dexamethasone; Vd, bortezomib and dexamethasone.
The study was open-label, which means that both patients and their physicians were aware of the trial assignment. A total of 402 patients were enrolled onto the study at 123 different sites. The SVd arm had a shorter median follow-up of 13.2 months, compared with 16.5 months for the Vd arm. Of the patients enrolled onto the trial, 36 (9%) were from 31 centers in Canada and the United States, 35 percent had previous ASCT, 49 percent received one previous line of therapy, 69 percent received prior bortezomib, 38 percent received prior lenalidomide, 4 percent received prior daratumumab, and 48 percent had high-risk cytogenetic abnormalities. Most patients (87%) had Revised International Staging System disease stage I-II.
The median progression-free survival (PFS) was 4.47 months longer in the SVd arm (13.93 vs. 9.46 months). The proportion of patients with a very good partial response or better was 12 percent higher in the SVd group (44.6% vs. 32.4%; odds ratio [OR], 1.66; 95% CI, 1.1-2.5, p=0.008). Minimal residual disease negativity was low, though similar between both groups (5% in SVd vs. 4% in Vd). Median duration of response was longer with SVd (20.3 vs. 12.9 months) though the difference was not statistically significant (hazard ratio [HR], 0.81; 95% CI, 0.56-1.17; p=0.14).
More patients on the SVd arm discontinued treatment due to adverse events (AEs) or patient withdrawal (70 vs. 41 patients). Grade 3-4 AEs were significantly higher in the SVd arm. Those included thrombocytopenia (39% vs. 17%), fatigue (13% vs. 1%), anemia (16% vs. 10%), neutropenia (9% vs. 3%), nausea (8% vs. 0%), diarrhea (6% vs. <1%), cataract (9% vs. 1%), and decreased appetite (4% vs. 0%). Grade 3-4 peripheral neuropathy was higher in the Vd arm (8.8% vs. 4.6%), though this difference was not statistically significant (OR, 0.48; 95% CI, 0.21-1.11; p=0.08). Serious AEs were higher in the SVd arm (52% vs. 38%). Of note, for patients who crossed over to the SVd arm, AEs that occurred after crossover were not included.
A prespecified subgroup analysis showed that patients with high-risk cytogenetics (n=192) responded better to SVd (OR, 0.67; 95% CI, 0.45-0.98), with patients who had isolated del(17p) (n=37) achieving the strongest benefit (OR, 0.38; 95% CI, 0.16-0.86). The subgroup analysis showed that the PFS benefit was only statistically significant in patients who received one previous line of therapy (OR, 0.63; 95% CI, 0.41-0.96), with patients who didn’t receive a previous PI deriving a larger benefit (OR, 0.26; 95% CI, 0.11-0.60).
Eighteen percent of patients who received SVd required a thrombopoietin receptor agonist to manage thrombocytopenia (while trying to limit dose interruption or drug discontinuation) compared to only 1 percent of the patients who received Vd. Despite the higher utilization of thrombopoietin agonists in the SVd arm, there were more significant bleeding events (grade 3 or higher, 2% vs. <1%) with one case of cerebral hemorrhage in the SVd arm. On December 18, 2020, the FDA approved SVd for the treatment of adult patients with MM who have received at least one prior therapy.6,7 It is being studied in other hematologic malignancies, including diffuse large B-cell lymphoma where it showed an initial overall response rate of 28 percent,8 and in some solid tumors.9
In Brief
Selinexor appears to be an active agent in the treatment of MM. The reduction in selinexor dose between the STORM and BOSTON trials along with the use of supportive care measures helped make selinexor more tolerable. One key observation was that the benefit seen in del(17p) patients seemed similar to that of standard-risk patients, which supports the preclinical rationale for this MM disease subset. What remains unknown is when to introduce selinexor in the treatment of relapsed/refractory MM, especially with significant short-term AE profiles and unknown long-term effects. In the United States, patients with MM who progress on a given drug will be switched to a different drug from the same class or to a totally new drug class. The BOSTON trial did not enroll many patients in the United States owing to the fact that it allowed patients to be re-treated with the same PI at time of progression and owing to the luxury of available standard effective therapies with longer follow-up time. Financial toxicity is a growing problem in the field of MM,10 and the cost of selinexor ($22,000/month)11 has come under critique by the U.S. oncology community since its FDA approval. While the BOSTON trial provided evidence that the addition of selinexor to Vd resulted in better PFS, the MM community aspires to use the most effective therapy with the least AEs profile at the most appropriate time for the most ideal patient outcome — for SVd, it may be the high-risk relapsed/refractory MM subset.
Competing Interests
Dr. Hadidi indicated no relevant conflicts of interest. Dr. Usmani has received research funding from Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, and Takeda; consulting fees from Amgen, BMS, Celgene, EdoPharma, GSK, Janssen, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio; and speaker fees from Amgen, BMS, Janssen, and Sanofi.