Update/Commentary
Several new developments have emerged in the field of systemic mastocytosis (SM) since the publication of the “Ask The Hematologist” article on this subject in 2013.
Classification
In the revised 2016 World Health Organization Classification of hematolymphoid neoplasms, “mastocytosis” was removed as one of the subtypes under the major category of “myeloproliferative neoplasms” and is now classified as its own major category.1 Smoldering systemic mastocytosis is now a full variant of SM, and no longer a provisional subvariant under indolent SM. Additionally, “systemic mastocytosis with an associated hematologic non-mast cell lineage disease” (SM-AHNMD) has been given a “name makeover”; the designation “SM with an associated hematologic neoplasm” (SM-AHN), a simpler designation, can now be used in place of, or interchangeably with, SM-AHNMD.1
More attention is being focused on how to better capture the clinical heterogeneity within SM subtypes. For example, for patients with aggressive systemic mastocytosis (ASM) and increased numbers of neoplastic mast cells on a bone marrow aspirate (e.g., 5-19%), the term ASM-t (ASM in transformation) has been proposed to reflect the worse prognosis of these patients and higher risk of transformation to mast cell leukemia (MCL) compared to individuals with ASM patients with <5 percent mast cells on a marrow aspirate.2 Although the life expectancy of MCL is often less than six months, such patients may also exhibit variable clinical outcomes. The terms “acute MCL” (one or more signs of organ damage) and “chronic MCL” (no evidence of organ damage) have been proposed as variants that respectively denote relatively worse and better outcomes within the spectrum of MCL.3 However, retrospective and prospective data are needed to confirm whether the absence of presence of organ damage adequately discriminates prognosis between these two MCL subgroups.
Biology
Although the KITD816V mutation is identified in approximately 90 percent of SM patients, SM-AHNMD is usually characterized by multiple myeloid mutations, the most common being TET2, SRSF2, ASXL1, CBL, and RUNX1.4 Patients with a mutation in one or more of these genes (and especially SRSF2, ASXL1, or RUNX1) exhibit a worse prognosis compared to patients who only exhibit the KIT D816V mutation.5 Sequencing of granulocyte-macrophage colony-forming progenitor cells (CFU-GM) indicates that such mutations often precede the acquisition of KIT D816V.6 This parallels the finding that TET2 mutations often arise before JAK2 V617F in Philadelphia chromosome-negative MPNs.7 Notably, CFU-GM from ISM or SSM patients only exhibited the KIT D816V mutation, indicating that indolent SM may be primarily driven by this mutation alone.6
Treatment
In the fully accrued global trial of the multikinase/KIT inhibitor midostaurin in 89 evaluable patients with advanced SM (ASM, SM-AHNMD, and MCL), the overall response rate was 60 percent; 45 percent of patients achieved a major response, defined by complete resolution of ≥1 type of organ damage.U8 The median duration of response was 24.1 months. Median overall survival was 28.7 months and progression-free survival was 14.1 months. Response rates were similar regardless of advanced SM subtype, KIT mutation status, or prior therapy. The serum tryptase level and bone marrow mast cell burden decreased by >50 percent in the majority of patients. In addition to reducing splenomegaly, midostaurin elicited significant improvement of patient-reported symptoms and quality of life that may be related to the combined inhibitory effects of midostaurin on neoplastic mast cell proliferation and mediator release. In the MCL patients, where median survival of <6 months is typical, the median overall survival was 9.4 months and not reached in the eight of 16 MCL patients who responded. Further studies are warranted to evaluate the role of combining midostaurin with other drugs with activity in advanced SM (e.g. 2-chlorodeoxadenosine; 2-CdA) and in the pre- and post-transplant setting. New KIT D816V-specific inhibitors (e.g. BLU-285) have recently commenced clinical trial evaluation of patients with advanced SM.
Recent data from Dr. Celalettin Ustun and colleagues have provided more clarity on the role of hematopoietic stem cell transplantation (HSCT) in advanced SM which heretofore has been primarily based on case reports and small series.9 In a large, multicenter retrospective analysis published in 2014, Dr. Ustun and colleagues evaluated the outcomes of 57 SM patients (SM-AHNMD, n=38; ASM, n=7; and MCL, n=12) who underwent allogeneic HSCT. responses were observed in 70 percent of patients, including a 16 percent complete remission (CR) rate. The remaining 30 percent of responses were split between stable disease (21%) and primary refractory disease (9%). All 38 patients with SM-AHNMD achieved CR of the AHNMD component, but 10 subsequently relapsed with AHNMD, and half of these patients died. The median overall survival for all patients at three years was 57 percent, for all patients, consisting of 74 percent for patients with SM-AHNMD, and 43 and 17 percent for ASM and MCL patients, respectively. The strongest risk factor for worse overall survival was a diagnosis of MCL. Additionally, lower survival was observed in patients undergoing reduced intensity vs. myeloablative conditioning. These data suggest that transplantation can provide extended survival in selected patients, particularly for patients with SM-AHNMD.
Updated References
The Question
What is your approach to the diagnosis and management of mastocytosis?
Defining Mastocytosis
Mastocytosis is characterized by the accumulation and proliferation of neoplastic mast cells in one or more organs (e.g., skin, bone marrow, spleen, lymph nodes, liver, and gastrointestinal tract). Flushing, diarrhea, anaphylaxis, and neuropsychiatric symptoms resulting from mast cell release of bioactive molecules such as histamine, leukotrienes, and various inflammatory cytokines can impose a significant burden on a patient’s quality of life. Such mediator symptoms may be triggered by physical stimuli, exercise, alcohol, NSAIDs, opioids, insect stings, or certain foods. In advanced disease, mast cell infiltration of tissues can lead to organ damage and shortened survival.
Table. World Health Organization Diagnostic Criteria for Systemic Mastocytosis*
| Major criterion | Multifocal dense infiltrates of mast cells (>15 mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organ(s) |
| Minor criteria |
|
| Major criterion | Multifocal dense infiltrates of mast cells (>15 mast cells in aggregates) detected in sections of bone marrow and/or other extracutaneous organ(s) |
| Minor criteria |
|
*Requires 1 major + 1 minor criterion or 3 minor criteria
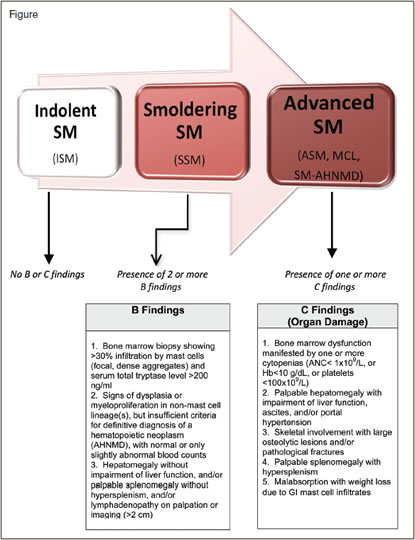
The World Health Organization (WHO) includes mastocytosis within the category of myeloproliferative neoplasms (MPN) and divides these disorders into cutaneous and systemic forms.1 The diagnosis of systemic mastocytosis (SM) is based on consensus diagnostic criteria (Table) and requires one major plus one minor criterion or three minor criteria. Clinicopathologic criteria are used to further classify SM into subtypes that generally reflect clinical severity and prognosis. In indolent SM (ISM), where bone marrow mast cell burden is relatively low, survival is similar to age-matched populations, and clinical issues are usually related to mediator symptoms.2 Smoldering SM (SSM) is a subtype of ISM that reflects SM in transition toward more advanced disease and is characterized by two or more so-called “B-findings” (Figure), including organomegaly, and indicators of a relatively higher mast cell burden (e.g., > 30% bone marrow mast cells and serum tryptase level > 200 ng/ml). Advanced SM collectively refers to SM subtypes in which organ damage (referred to as “C” findings) is present. This includes the WHO-defined subtypes aggressive SM (ASM), mast cell leukemia (MCL), and SM with an associated hematologic non-mast cell lineage disease (SM-AHNMD). Approximately 90 percent of AHNMDs represent a myeloid neoplasm such as myelodysplastic syndrome (MDS), MPN, MDS/MPN (e.g., chronic myelomonocytic leukemia), eosinophilic disorders, or acute myeloid leukemia (AML). Rarely, a lymphoid neoplasm may be concurrently diagnosed with SM.
Mastocytosis is a challenging disease for many reasons. In the normal state, mast cells are not identified on the peripheral blood differential and represent a small fraction of the myeloid lineage on visual inspection of the marrow aspirate. The idiom “out of sight, out of mind” is apt in the case of SM and, together with its rare incidence, tests the physician to think about this neoplasm in the first place. If present, prototypic mediator symptoms such as flushing, diarrhea, and anaphylaxis may make this task easier. In advanced disease, organ damage such as cytopenias, liver dysfunction, ascites, lytic bone lesions, and hypoalbuminemia with weight loss due to mast cell infiltration of the gastrointestinal tract are sufficiently protean to conceal a unifying diagnosis. Because SM often partners with a myeloid disease, immunohistochemical (IHC) stains (e.g., tryptase, CD117 [KIT], and CD25) are necessary to identify and quantify neoplastic mast cells in trephine biopsies and can additionally help unmask SM when it lives in the shadow of other neoplasms. Clinical and biologic heterogeneity, even within specific SM disease subtypes, can make it difficult to gauge disease trajectory and prognosis. In patients with an AHNMD, prognosis is usually related to the associated myeloid neoplasm, highlighting the need to fully characterize and stage the concomitant disease.
Diagnosis
In addition to a high index of suspicion, obtaining the expertise of a cadré of subspecialists (hematologists, dermatologists, and allergists/immunologists) as well as hematopathologists versed with the nuances of mast cell disease is important in establishing the correct diagnosis. A combination of clinical, morphologic, immunophenotypic, and molecular studies is required to establish the diagnosis of SM and its subtype. Bone marrow mast cell burden is best quantified by morphologic analysis, and use of the aforementioned IHC stains on the core biopsy is essential. The major criterion for SM requires demonstration of multifocal mast cell aggregates in the bone marrow (or other extracutaneous organ), of which > 25 percent are atypical, often spindle-shaped mast cells. In MCL, mast cells account for > 20 percent of nucleated cells on the BM aspirate and form a diffuse infiltrate on the core biopsy with or without circulating mast cells. Multi-parameter flow cytometry of the BM aspirate can also be used to quantify mast cells in the marrow and complements morphologic evaluation. The overwhelming majority of SM patients (> 80%) carry the activating KIT D816V mutation, which should be obtained from the bone marrow aspirate or from a core biopsy that is preserved in formalin to avoid degradation of DNA for PCR analysis. Determination of KIT D816V mutation status is critical to the diagnostic evaluation of SM, but it also guides treatment decisions. Although KIT D816V is an imatinib-resistant mutation, this tyrosine kinase inhibitor has been reflexively misapplied and overused in the treatment of SM. However, a small minority of patients (< 5%) may exhibit juxtamembrane KIT mutations, which exhibit sensitivity to imatinib in vitro as well as in clinical practice. Such cases illustrate that sequencing the remainder of the KIT gene in SM patients who are negative for codon 816 mutations may prove fruitful. Total serum tryptase levels generally reflect the increased burden of mast cells in patients with SM. A serum tryptase level > 20 ng/ml is an additional minor criterion for the diagnosis of SM. Although not always concordant, it is the most useful blood marker to assess changes in mast cell burden in response to cytoreductive therapy. In advanced disease, staging studies include CT of the abdomen to assess hepato/splenomegaly, lymphadenopathy, and ascites; metastatic skeletal survey to evaluate osteolyses and/or pathologic fractures; and DEXA scans to follow osteoporosis, which is common in SM. Some patients with mediator symptoms who do not fulfill criteria for SM (e.g., only one or two minor criteria and/or exhibit a low burden of atypical mast cells without aggregates on biopsy) have been given the provisional diagnosis of mast cell activation syndrome (MCAS).2 The diagnostic criteria for this mast cell disorder are still evolving and its natural history is not well-defined.
Treatment
Individuals with symptomatic skin-only disease or SM with mediator symptoms are educated to avoid known triggers and are encouraged to carry an Epipen®, particularly those with a history of anaphylaxis or anaphylactoid symptoms. Antihistamines (H1- and H2-blockers) serve as the foundation for symptom palliation; leukotriene antagonists (e.g., monteleukast) and mast cell stabilizers (e.g., cromolyn sodium) are typically used for refractory mediator symptoms. Osteoporosis is treated with conventional approaches, and radiotherapy and/or IV bisphosphonates may be used to treat osteolyses or pathologic fractures. Patients with advanced SM exhibit shortened survival, and cytoreductive therapy is used in an attempt to reverse organ damage. Interferon-α with or without corticosteroids and 2-chloro-deoxyadenosine demonstrate overall response rates in the range of 30 to 60 percent, including major responses denoting normalization of organ dysfunction.3 Intensive chemotherapy has been used in MCL with modest benefit, and there is a paucity of published data regarding the utility of stem cell transplantation for advanced SM. The generally short-lived responses and tolerability of these approaches have reinforced the need to enroll patients in clinical trials evaluating novel agents. The tyrosine kinase inhibitors dasatinib and midostaurin exhibit in vitro activity against D816V-mutated KIT, with the latter demonstrating encouraging activity in an ongoing international, multi-center clinical trial.4 In patients with SM-AHNMD, the clinical approach for such patients has been to treat the SM component as if the myeloid neoplasm were not present and to treat the myeloid neoplasm as if SM were not present. However, in clinical practice, priority should be given to treating the disease component that is contributing to the most urgent clinical concerns. Because KIT D816V or other pathogenetic abnormalities may reside in both the abnormal mast cell and associated myeloid clonal cell populations, the future availability of agents that inhibit shared therapeutic targets may make the distinction between the two disease compartments less relevant. Next-generation sequencing approaches of sorted cell populations should inform this approach. The recent identification of CD30 expression on neoplastic mast cells5 also provides an opportunity for testing anti-CD30 antibody approaches (e.g., brentuximab vedotin) in advanced SM.
References
Author notes
The update/commentary section was added in 2016 when this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium.
Competing Interests
Dr. Gotlib is Chairman of the Study Steering Committee for the global trial of idostaurin and receives reimbursement for travel expenses from Novartis; Dr. Gotlib receives research funding for administration of the BLU-285 trial.