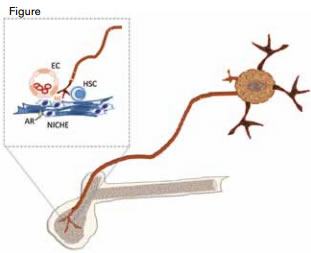
Sympathetic Nervous System and Hematopoiesis. Sympathetic nerves innervate the bone marrow and release neurotransmitters such as norepinephrine (NE) that is taken up by adrenergic receptors (AR) of the “niche” cells that make up the microenvironment of the hematopoietic stem cell (HSC). Niche cells include nestin and CXCL12 positive cells and endothelial cells (EC).
Sympathetic Nervous System and Hematopoiesis. Sympathetic nerves innervate the bone marrow and release neurotransmitters such as norepinephrine (NE) that is taken up by adrenergic receptors (AR) of the “niche” cells that make up the microenvironment of the hematopoietic stem cell (HSC). Niche cells include nestin and CXCL12 positive cells and endothelial cells (EC).
Over the past half-dozen or so years, the surprising contribution of the sympathetic nervous system (SNS) to control of hematopoiesis at the level of the stem cell has been illuminated (Figure).1,2 Observations from these studies have important translational implications, as it is well known by clinicians that the toxicity profile of a number of chemotherapeutic agents such as cisplatin, vinorelbine, gemcitabine, and vincristine includes both neurotoxicity and myelosuppression. Now, a report by Lucas and colleagues from the laboratory of Paul Frenette at Albert Einstein College of Medicine in New York provides new insights into the basis of the marrow insufficiency sustained after chemotherapy by demonstrating that drugs that cause sensory neuropathy also induce a defect in innervation of the marrow, thereby altering the cellular architecture of the hematopoietic stem cell niche.
They first showed that mice with cisplatin- or vincristine-induced sensory neuropathy are unable to sustain hematopoiesis after bone marrow transplantation. In contrast, treatment with carboplatin, a drug that does not cause neuropathy, permits full donor engraftment after bone marrow transplant. Next, they conducted experiments that tested bone marrow recovery after treatment with 5-fluorouracil (5-FU) in two animal models: one in which denervation was chemically induced, and the other in which genetic modification made it possible to induce apoptosis of peripheral sympathetic neurons in mice injected with diphtheria-toxin. In normal animals, 5-FU ablates proliferating cells and induces quiescent hematopoietic stems cells (HSCs) to repopulate the marrow. However, following treatment with 5-FU, the experimentally denervated mice were unable to recover hematopoiesis, supporting the hypothesis that the SNS is an essential component of marrow homeostasis. Marrow recovery is dependent on both β2 and β3 adrenergic receptors. The β2 type of receptors is widely expressed, including by human CD34+ cells where it regulates progenitor migration,3 and the β3 type is involved in Circadian release of HSCs by the marrow niche cells.4 Administration of β2 and β3 adrenergic antagonists was shown to alter the cellular architecture of the HSC niche, suggesting a mechanism for the failure of SNS-damaged mice to recover hematopoiesis following treatment with 5-FU. Lucas and colleagues also tested the hypothesis that the suboptimal HSC mobilization observed in patients treated with chemotherapy could be caused by bone marrow neuropathy. They found that cisplatin treatment of mice reduced by 50 percent both the number of progenitors mobilized by G-CSF and the competitive repopulating capacity of the mobilized cell product. Strikingly, the following two maneuvers reversed the cisplatin-induced neuropathy and restored bone marrow recovery in the mouse models: treatment with a chimeric glial-derived neurotrophic factor (GDNF)-Fc protein that rescues sympathetic neurons after adrenomedullectomy; and treatment with 4-methylcatechol (4-MC), a drug that induces endogenous nerve growth factor production and protects SNS fibers during cisplatin treatment. Not only was marrow hematopoiesis after transplant restored by these neuroprotectants, but G-CSF-induced mobilization was also reestablished.
In Brief
This pivotal paper demonstrates that the integrity of the SNS is critical for maintaining the structure and function of the HSC niche and, as such, participates in both blood cell recovery after chemotherapy and progenitor cell mobilization. As the same drugs that were used in the animal experiments, (i.e., cisplatin and vincristine), are routinely used during standard chemotherapy in patients (e.g., vincristine in CHOP) or in regimens that have dual roles in salvage/mobilization (e.g., cisplatin in ESHAP, DHAP, VD(orR)T-PACE), we should consider the potential clinical consequence of neuropathy on marrow function. With this issue in mind, neuroprotectant drugs could restore marrow function and mobilization, and analogs of these drugs might be available for use in the clinic. Alternatively, non-neurotoxic agents, such as carboplatin, that do not exhibit this deleterious effect on the SNS might be substituted for cisplatin in these regimens. We should also be alert to the possibility that other drugs with appreciable neurotoxicity might be harmful and consider that the effect of a damaged SNS on engraftment after allogeneic stem cell transplant may be underappreciated.
References
Competing Interests
Dr. Becker indicated no relevant conflicts of interest.