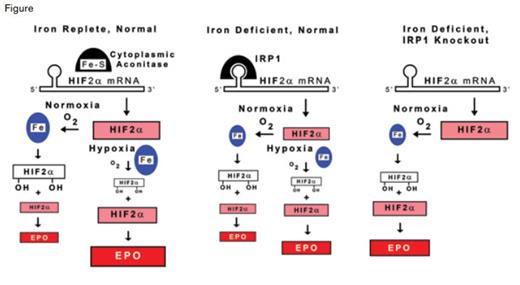
IRP1 Regulation of Erythropoietin (EPO) Production. The production of EPO in renal interstitial fibroblasts is shown for normal individuals under iron-replete conditions, and for normal and IRP1 knockout individuals under iron-deficient conditions. Under iron-replete conditions (left panel), IRP1 contains an iron-sulfur cluster and has cytoplasmic aconitase activity, but it does not bind the iron responsive element (IRE) of HIF2α. Under conditions of iron deficiency, IRP1 lacks the iron-sulfur cluster, and in this state, binds the IRE of HIF2α mRNA (center panel). HIF2α is a component of the transcription factor complex that controls EPO production. Relative amounts of HIF2α (pink), hydroxylated HIF2α (white), and EPO (red) are indicated by the size of rectangles. In iron-deficient individuals (center panel), IRP1 binding to the 5’ IRE of HIF2α mRNA inhibits translation, thereby reducing the availability of HIF2α, ultimately restricting EPO production by renal interstitial cells and thereby contributing to the anemia of iron deficiency. When oxygen delivery to the kidneys is normal, HIF2α is rapidly hydroxylated by iron-containing prolyl-hydroxyase (blue ovals) and undergoes proteasomal degradation so that renal HIF2α is low and only a few cells produce EPO under either iron-replete (left panel) or iron-deficient (middle panel) conditions. Under conditions of renal hypoxia, as occurs with anemia, less oxygen is available to support the prolyl-hydroxylation reaction. Consequently, degradation of HIF2α is limited, resulting in increased renal HIF2α and greater production of EPO compared with renal normoxia. This process contributes to the increase in EPO production that is observed in response to anemia. In normal individuals with intact IRP1 function, iron deficiency reduces the amount of HIF2α formed and, therefore, restricts the amount of EPO produced, even under hypoxic conditions (middle panel). Therefore, renal HIF2α and EPO production are decreased in iron deficiency as compared with iron-replete conditions. In the IRP1 knockout condition (right panel), basal HIF2α and EPO production are increased under normal conditions, and iron deficiency exacerbates these already elevated levels, presumably by decreasing the activity of iron-containing prolyl-hydroxyase (smaller blue oval), even when the kidney is normoxic.
IRP1 Regulation of Erythropoietin (EPO) Production. The production of EPO in renal interstitial fibroblasts is shown for normal individuals under iron-replete conditions, and for normal and IRP1 knockout individuals under iron-deficient conditions. Under iron-replete conditions (left panel), IRP1 contains an iron-sulfur cluster and has cytoplasmic aconitase activity, but it does not bind the iron responsive element (IRE) of HIF2α. Under conditions of iron deficiency, IRP1 lacks the iron-sulfur cluster, and in this state, binds the IRE of HIF2α mRNA (center panel). HIF2α is a component of the transcription factor complex that controls EPO production. Relative amounts of HIF2α (pink), hydroxylated HIF2α (white), and EPO (red) are indicated by the size of rectangles. In iron-deficient individuals (center panel), IRP1 binding to the 5’ IRE of HIF2α mRNA inhibits translation, thereby reducing the availability of HIF2α, ultimately restricting EPO production by renal interstitial cells and thereby contributing to the anemia of iron deficiency. When oxygen delivery to the kidneys is normal, HIF2α is rapidly hydroxylated by iron-containing prolyl-hydroxyase (blue ovals) and undergoes proteasomal degradation so that renal HIF2α is low and only a few cells produce EPO under either iron-replete (left panel) or iron-deficient (middle panel) conditions. Under conditions of renal hypoxia, as occurs with anemia, less oxygen is available to support the prolyl-hydroxylation reaction. Consequently, degradation of HIF2α is limited, resulting in increased renal HIF2α and greater production of EPO compared with renal normoxia. This process contributes to the increase in EPO production that is observed in response to anemia. In normal individuals with intact IRP1 function, iron deficiency reduces the amount of HIF2α formed and, therefore, restricts the amount of EPO produced, even under hypoxic conditions (middle panel). Therefore, renal HIF2α and EPO production are decreased in iron deficiency as compared with iron-replete conditions. In the IRP1 knockout condition (right panel), basal HIF2α and EPO production are increased under normal conditions, and iron deficiency exacerbates these already elevated levels, presumably by decreasing the activity of iron-containing prolyl-hydroxyase (smaller blue oval), even when the kidney is normoxic.
Intracellular iron content is post-transcriptionally controlled by two iron regulatory proteins (IRP1 and IRP2) that bind to iron-responsive elements (IREs), which are specific RNA nucleotide sequences forming stem-loop structures in 5’- and 3’-untranslated regions (UTRs) of mRNAs encoding proteins involved in cellular iron import, export, and storage. In iron-replete cells, IRP1 contains an iron-sulfur cluster at its active site and has cytosolic aconitase activity. In iron-deficient cells, IRP1 loses its iron-sulfur cluster and assumes the configuration that binds IREs. IRP2 is stable and binds IREs during iron deficiency but undergoes rapid degradation under iron-replete conditions. Examples of IREs can be found in the 5’-UTR of mRNAs encoding ferritin and ferroportin where IRP binding inhibits translation, thereby decreasing iron storage and exportation, respectively, and in the 3’-UTR of transferrin receptor mRNAs where IRP binding stabilizes and enhances mRNA translation, thereby increasing transferrin receptors and iron importation.
IREs are also found in mRNAs that encode proteins related to iron utilization, including heme synthesis, energy metabolism, and response to hypoxia. Among these mRNAs are those encoding HIF2α, the hypoxia-responsive component of the transcription factor complex that binds the hypoxia-responsive elements of many genes, including erythropoietin(EPO) (Figure). Under normoxic conditions, two prolines of HIF2α are rapidly hydroxylated by prolyl-hydroxylases that contain iron in their active site and use molecular oxygen, thereby targeting HIF2α for polyubiquitination by the von Hippel–Lindau protein and subsequent proteasomal degradation. This oxygen-dependent HIF2α hydroxylation process maintains low EPO production under normoxic conditions. Under hypoxic conditions,such as anemia, decreased oxygen availability limits HIF2α hydroxylation, resulting in greater HIF2α stability and activity, with the end result being rapid transcription, translation, and secretion of EPO by renal interstitial fibroblasts, the major source of EPO.
Tight feedback control of EPO production by this oxygen-regulated system is consistent with the absence of polycythemic overshoot in association with periods of increased erythropoiesis, such as following the period of rapid growth in post-natal mice or following acute blood loss. However, Ghosh et al., and the authors of two other recent publications,1,2 report that following the post-natal growth period, IRP1 knockout (Irp1-/Irp1-) mice develop increased hematocrit/hemoglobin (Hct/Hgb), serum EPO, and HIF2α in renal interstitial EPO-producing cells. These findings suggest an oxygen-independent mechanism in which HIF2α activity is controlled by IPR1 (Figure). In addition to renal interstitial cells, Ghosh et al. demonstrated that IPR1 is the prevalent IRP in pulmonary endothelium, and that Irp1-/Irp1- mice have pulmonary hypertension associated with increased lung HIF2α and endothelin-1, the product of another HIF2α regulated gene. Thus, Irp1-/Irp1- mice provide a model for diseases with polycythemia and/or pulmonary hypertension due to increased HIF2α, such as Chuvash polycythemia, von Hippel–Lindau disease, and activating HIF2α mutations.
In Brief
When fed a low-iron diet, Irp1-/Irp1- mice have decreased splenic iron, serum iron, and transferrin saturation compared with Irp1-/Irp1- mice fed a normal diet. Compared with Irp1-/Irp1- mice fed a normal diet or wild-type mice fed either type of diet, iron-starved Irp1-/Irp1- mice have increased renal HIF2α, serum EPO, Hct/Hgb, and extramedullary erythropoiesis, but they have lower MCV and MCH. Many iron-starved Irp1-/Irp1- mice die suddenly as a result of intra-abdominal hemorrhage. Thus, iron-starved Irp1-/Irp1- mice have a phenotype like some polycythemia vera patients (i.e., elevated Hct/Hgb with hypochromic, microcytic indices, and increased vascular accidents.) In polycythemia vera, erythropoiesis increases despite limited iron availability because mutant JAK2 kinase associated with EPO receptors increases erythropoietic responses when EPO levels are low. In Irp1-/Irp1- mice, elevated HIF2α, EPO, and erythropoiesis as a consequence of derepression of HIF2α mRNA translation are increased further with iron starvation. In this situation, iron starvation most likely inhibits HIF2α hydroxylation by iron-containing prolyl-hydroxylases (Figure). Thus, Ghosh et al. demonstrate that IRP1 suppression of HIF2α mRNA translation during iron deficiency inhibits EPO production, thereby restricting erythropoiesis. This IRP1-induced inhibition of erythropoiesis is crucial for development of anemia during iron deficiency and treatment of polycythemia vera by phlebotomy-induced, iron-restricted erythropoiesis.
References
Competing Interests
Dr. Koury indicated no relevant conflicts of interest.