Childhood malaria accounts for more than a million deaths annually, and survivors are often left with severe neurologic and cognitive impairment as a consequence of cerebral involvement that may complicate the disease. Advances in mosquito control, new anti-malarials, and vaccines have tempered this plague. Nonetheless, malaria remains a dominant public health problem in sub-Saharan Africa, as the offending parasite, Plasmodium falciparum, has developed, through thousands of years of coevolution with humans, elaborate mechanisms for utilizing host properties to assure its survival. Vascular sequestration of infected RBCs, a process that is mediated by binding of members of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family to receptors on the endothelial lining of blood vessels, plays a central role in the pathobiology of severe malaria. Each P. falciparum parasite genome contains approximately 60 var genes that encode different PfEMP1 types, enabling the parasite to attach infected erythrocytes to a number of different receptors that are normally expressed by vascular endothelial cells. Despite a high rate of var gene recombination, many tandem domain arrangements, called domain cassettes (DCs), have been maintained through evolution and are therefore thought to be of functional importance. An example is DC2, which mediates binding of infected erythrocytes within the placenta in patients with the malaria of pregnancy. Severe malaria in children is associated with expression of a subset of PfEMP1s, DC8 and DC13, but prior to the studies of Turner and colleagues, the endothelial receptor for these proteins had remained enigmatic.
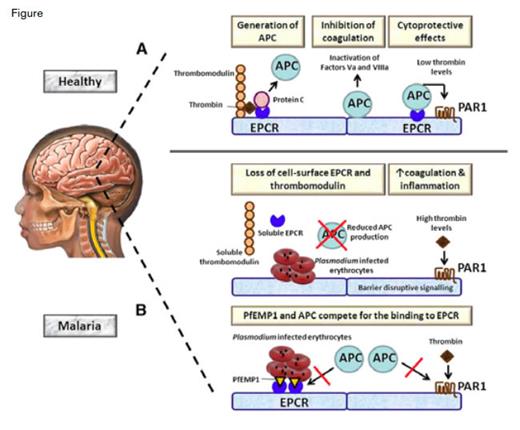
Role of EPCR in Cerebral Malaria. A) During normal homeostasis, excessive thrombin is bound by thrombomodulin expressed by endothelial cells. The thrombomodulin-thrombin complex activates protein C to APC, a process that is strongly accelerated by EPCR. APC exerts the anticoagulant effects when it becomes detached from EPCR by inactivating clotting factor Va and VIIIa. In addition, APC bound to EPCR has cytoprotective properties by activating PAR1. B) (Upper) In malaria, parasite-infected erythrocytes induce the loss of EPCR and thrombomodulin from the endothelial cell surface at least in part by shedding of these receptors. As a consequence, the capacity to produce APC is greatly impaired, resulting in enhanced coagulation. The resulting high thrombin levels can induce proinflammatory-barrier disruptive effects on blood vessels via PAR1. (Lower) Plasmodium-infected erythrocytes transport PfEMP1 to their membrane, which can bind EPCR in the same region as APC. As a result, APC is less capable of inducing cytoprotective effects via PAR1.Figure from van der Poll, Blood. 2013;122:625
Role of EPCR in Cerebral Malaria. A) During normal homeostasis, excessive thrombin is bound by thrombomodulin expressed by endothelial cells. The thrombomodulin-thrombin complex activates protein C to APC, a process that is strongly accelerated by EPCR. APC exerts the anticoagulant effects when it becomes detached from EPCR by inactivating clotting factor Va and VIIIa. In addition, APC bound to EPCR has cytoprotective properties by activating PAR1. B) (Upper) In malaria, parasite-infected erythrocytes induce the loss of EPCR and thrombomodulin from the endothelial cell surface at least in part by shedding of these receptors. As a consequence, the capacity to produce APC is greatly impaired, resulting in enhanced coagulation. The resulting high thrombin levels can induce proinflammatory-barrier disruptive effects on blood vessels via PAR1. (Lower) Plasmodium-infected erythrocytes transport PfEMP1 to their membrane, which can bind EPCR in the same region as APC. As a result, APC is less capable of inducing cytoprotective effects via PAR1.Figure from van der Poll, Blood. 2013;122:625
To identify the receptor, Turner et al. generated recombinant, full-length DC8-PfEMP1 and used it to screen an array of 2,505 full-length human plasma membrane proteins. One specific hit identified the endothelial protein C receptor (EPCR) as a potential binding partner. P. falciparum-infected RBCs bound EPCR on brain microvascular endothelial cells, and binding was inhibited by anti-EPCR antibodies. To show functional relevance to activation of protein C, the authors demonstrated that domains derived from both DC8 and DC13 competed with activated protein C (APC) for the same binding site, and an antibody that blocks APC binding to EPCR also prevented parasite binding to endothelial cells. Soluble EPCR shed from endothelial cells by treatment with the metalloproteinase TNF-α converting enzyme was shown to block parasite attachment to the vessel wall cells. Together, these studies establish the EPCR as the binding site for DC8 and DC13 and suggest that hijacking of the receptor for APC contributes to the pathobiology of severe childhood malaria (Figure).
The studies of Turner and colleagues dovetail with the recent findings of Moxon and colleagues who examined the brains of 10 children who had died of cerebral malaria (CM). They observed sequestration of infected erythrocyte (IE) within the cerebral vasculature and showed significantly more fibrin deposition and microvascular hemorrhage in those samples when compared with the brains of malaria-infected children without evidence of cerebral vascular IE sequestration who died of non-malarial causes based on autopsy findings. The rains of those with CM demonstrated endothelial loss of anticoagulant receptors EPCR and thrombomodulin (TM) that are critical components of the protein C pathway of anticoagulation (Figure). As previously reported,1 TM was not seen in control brain neuroendothelium, however, TM is expressed in the vasculature of the subcutaneous fat tissue, and examination of such tissue samples from children with CM showed evidence of IE sequestration and reduced expression of both EPCR and TM. Turner et al. also reported that soluble EPCR and TM were increased in the CSF of CM patients. Retinopathy is a sensitive marker of IE sequestration, and retinopathypositive CM patients had evidence of activation of coagulation with higher concentrations of thrombin-anti-thrombin III complexes, longer prothrombin times, and elevated systemic APC levels.
The protein C anticoagulant system also has anti-inflammatory and cytoprotective activity.2 APC binding to EPCR activates PAR1 G-protein signaling in the endothelium (Figure), decreasing vascular barrier permeability through sphingosine-1-phosphate signaling. Thus, P. falciparum-infected RBCs can selectively bind to EPCR on neuroendothelium (that are constitutively lacking in TM) causing shedding of EPCR and loss of both APC’s cytoprotective and anti-thrombotic properties, leading to vascular microthromboses and hemorrhage in CM (Figure). The latter process may be responsible for the long-term neurologic and cognitive defects experienced by survivors of CM.
In Brief
Van der Poll nicely summarizes the potential mechanisms by which P. falciparum interactions with EPCR contribute to the pathophysiology of CM (Figure).3 The data suggest that recombinant APC or TM infusions or therapies that target the PAR1/sphingosine-1-phosphate anti-inflammatory signaling pathway may limit intracranial bleeding and tissue damage and thereby reduce the morbidity and mortality of CM.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.