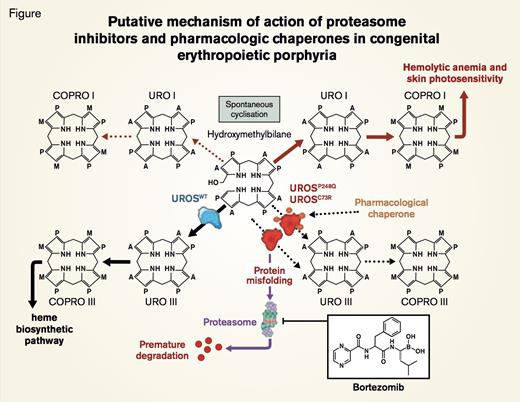
Putative Mechanism of Action of Proteasome Inhibitors and Pharmacologic Chaperones in Congenital Erythropoietic Porphyria. UROSC73R and UROSP248Q proteins are misfolded and subjected to premature degradation by the proteasome pathway, leading to deficiency of UROS enzymatic activity and accumulation of uroporphyrinogen I (URO I) and coproporphyrinogen I (COPRO I). Proteasome inhibitors such as bortezomib partially rescue UROSC73R and UROSP248Q expression and restore the metabolic heme biosynthetic pathway by preventing proteolysis. Conceivably, pharamacologic chaperone therapy could also be used to stabilize UROSP248Q and UROSC73R proteins and prevent premature degradation by the proteasome pathway.Blouin J et al. PNAS 2013;110:18238-18243 © 2013 by National Academy of Sciences
Putative Mechanism of Action of Proteasome Inhibitors and Pharmacologic Chaperones in Congenital Erythropoietic Porphyria. UROSC73R and UROSP248Q proteins are misfolded and subjected to premature degradation by the proteasome pathway, leading to deficiency of UROS enzymatic activity and accumulation of uroporphyrinogen I (URO I) and coproporphyrinogen I (COPRO I). Proteasome inhibitors such as bortezomib partially rescue UROSC73R and UROSP248Q expression and restore the metabolic heme biosynthetic pathway by preventing proteolysis. Conceivably, pharamacologic chaperone therapy could also be used to stabilize UROSP248Q and UROSC73R proteins and prevent premature degradation by the proteasome pathway.Blouin J et al. PNAS 2013;110:18238-18243 © 2013 by National Academy of Sciences
Congenital erythropoietic pophyria (CEP), also called Günther disease, is an autosomal recessive porphyria caused by deficiency of the heme biosynthetic enzyme uroporphyrinogen III synthase (UROS) that converts linear hydroxymethylbilane (HMB) to cyclic uroporphyrinogen III (Figure). Unlike amino-levulinic acid and porphobilinogen, the two intermediates that precede UROS in the heme biosynthetic pathway and that accumulate in patients with acute intermittent porphyria, HMB is unstable. Consequently, HMB is not detected in excess in the plasma or urine of patients with CEP. Rather HMB cyclizes non-enzymatically to uroporphyrinogen I and coproporphyrinogen I (Figure). These two porphyrin isomers are not substrates for downstream enzymes of the heme biosynthetic pathway (uroporphyriongen III synthase and coproporphyrin III oxidase), and, hence, they accumulate in erythroid elements, plasma, urine, feces, teeth, eyes, bones, and skin. Clinical manifestations (primarily hemolysis due to ineffective erythropoiesis and marked dermal photosensitivity) may begin in early infancy. Although phenotype varies, disease-related morbidity, including disfiguring facial scarring, hypertrichosis, and skin discoloration, can be debilitating. Standard treatment is largely supportive and includes protection from sunlight, proper dental care, and careful management of anemia. Complete resolution of biochemical, hematologic, and cutaneous abnormalities has been reported in a small number of children who have undergone allogeneic hematopoietic stem cell transplants. Gene therapy would be ideal, but remains hypothetical.
The genetic basis of CEP is heterogeneous with gene rearrangements, splicing mutations, and single-base substitutions having been reported, including missense mutations affecting any of the 10 coding exons of UROS. Missense mutations may lead to a functional decrease in enzyme activity, but Fortian et al.1 described a UROS missense mutation that altered protein folding, resulting in reduced protein stability without alterations in intrinsic enzymatic activity. In the current paper, Jean-Marc Blouin and colleagues in the laboratory of Emmanuel Richard in Bordeaux, France, demonstrate that a proteasome inhibitor (bortezomib) can retard degradation of two UROS mutants (Figure), thereby preserving cellular enzyme activity. Two UROS missense mutations, C73R and P248Q, found in CEP patients were shown to cause structural instability that resulted in rapid degradation of the mutant proteins. Boulin et al. reported that treatment with proteasome inhibitors, but not with lysosomal inhibitors, retarded degradation of UROSC73R and UROSP248Q. In a murine model of CEP (UROSP248Q/P248Q), bortezomib treatment increased enzyme expression 30- to 53-fold and significantly reduced uroporphyrin I accumulation in RBC. While bortezomib did not cure the hemolytic disease in these mice, photosensitivity was ameliorated.
In Brief
This paper emphasizes the importance of determining the mechanism by which a genetic abnormality causes disease. In this case, the missense mutations spared protein catalytic activity but diminished the total cellular content of the protein because the structurally abnormal protein undergoes rapid proteasomal degradation. Blouin and colleagues demonstrate how this observation translated into a targeted approach to therapy. Notably, proteasome inhibition has been used successfully to treat other genetic diseases, including muscular dystrophy, in which disease pathology involves rapid proteasome degradation of the mutant protein. Another approach that targets protein-folding abnormalities in genetic disease is pharmacologic chaperone therapy in which molecular chaperones assist in folding of mutated proteins, thereby preserving catalytic activity while preventing premature protein degradation (Figure). Whether bortezomib can be adapted to treat CEP requires further investigation. In the porphyric animal model, bortezomib was injected intraperitoneally every 48 hours, a schedule that likely would be tolerated poorly by human bone marrow and peripheral nervous tissue. Nonetheless, the studies of Blouin and colleagues serve as proof of principal that aberrant protein catabolism can be approached pharmacologically and suggest a novel approach to therapy for a subset of patients with CEP.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.