Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma that is incurable with standard chemoimmunotherapy. The recent approval of the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib represents a major therapeutic breakthrough in MCL, as the overall response rate is nearly 70 percent in the relapsed/refractory setting. The majority of patients treated with ibrutinib, however, do not achieve complete remission, and the median progression-free survival is approximately 14 months. Thus, effective new treatment options are needed both for non-responders and for those patients who experience disease progression while taking ibrutinib.
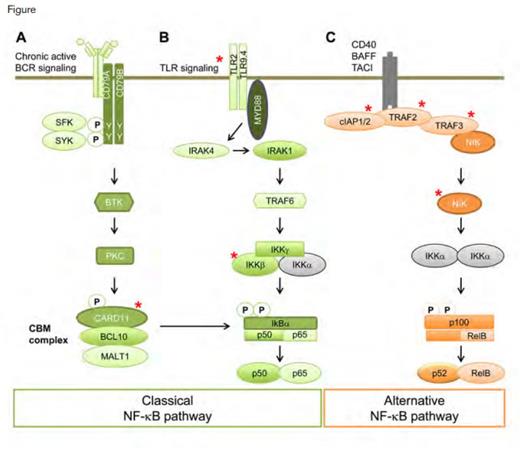
Somatic Mutation and Activation of the NF-κB Pathway in MCL. Activation of the classical and alternative NF-κB pathway in MCL may occur by chronic active BCR signaling and other mechanisms. Genomic sequencing studies have identified mutations in several elements of this regulatory pathway (denoted by red asterisks). Chronic active BCR (A) or Toll-like receptor (TLR) (B) signaling activate the classical NF-κB pathway. MCL that is dependent on BCR activation (A) may be blocked by BCR signaling inhibitors. Somatic mutations in the inhibitors of the alternative pathways (C) cIAP1 and cIAP2 (gene products of BIRC2 and BIRC3, respectively) and TRAF2/3 activate the alternative NF-κB pathway and confer resistance to inhibitors of the BCR signaling pathway. Somatic mutations in other elements of these pathways (CARD11, IKKβ, encoded by IKBKB, TLR2, and NIK, encoded by MAP3K14) also have been found in MCL by whole-exome or genome sequencing.Reprinted from Cancer Cell, Volume 25, Issue 1, Colomer D and Campo E, Unlocking New Therapeutic Targets and Resistance Mechanisms in Mantle Cell Lymphoma, Pages 7-9, Copyright 2014, with permission from Elsevier.
Somatic Mutation and Activation of the NF-κB Pathway in MCL. Activation of the classical and alternative NF-κB pathway in MCL may occur by chronic active BCR signaling and other mechanisms. Genomic sequencing studies have identified mutations in several elements of this regulatory pathway (denoted by red asterisks). Chronic active BCR (A) or Toll-like receptor (TLR) (B) signaling activate the classical NF-κB pathway. MCL that is dependent on BCR activation (A) may be blocked by BCR signaling inhibitors. Somatic mutations in the inhibitors of the alternative pathways (C) cIAP1 and cIAP2 (gene products of BIRC2 and BIRC3, respectively) and TRAF2/3 activate the alternative NF-κB pathway and confer resistance to inhibitors of the BCR signaling pathway. Somatic mutations in other elements of these pathways (CARD11, IKKβ, encoded by IKBKB, TLR2, and NIK, encoded by MAP3K14) also have been found in MCL by whole-exome or genome sequencing.Reprinted from Cancer Cell, Volume 25, Issue 1, Colomer D and Campo E, Unlocking New Therapeutic Targets and Resistance Mechanisms in Mantle Cell Lymphoma, Pages 7-9, Copyright 2014, with permission from Elsevier.
Using pharmacologic and genomic profiling, Dr. Rami Rahal and colleagues at the Novartis Institutes for Biomedical Research in Cambridge, Massachusetts, recently reported the results of experiments that demonstrate the importance of the NF-κB pathway in the pathogenesis of MCL (Figure). After testing 119 leukemia and lymphoma cell lines with a variety of therapeutic agents that are active against known targets involved in the pathophysiology of hematologic malignancies, the authors chose for further study 10 MCL cells lines, four that were sensitive and six that were resistant to ibrutinib and sotrastaurin (STN), a pan inhibitor of protein kinase C (PKC).
In vitro experiments demonstrated that the cell lines that are responsive to ibrutinib and STN are dependent on signaling through the NF-κB pathway. PKC-β, the primary isoform of PKC in MCL, is downstream of BTK and activates the CARD11-BCL10-MALT1 (CBM) complex, leading to cell proliferation through the classical NF-κB pathway (Figure). Cell lines that were sensitive to ibrutinib and STN had detectable cleavage of Rel-B, a marker of activity of the CBM complex. Following the administration of STN, RelB cleavage decreased, as did phosphorylation of IκΒα, both results indicating suppression of the NF-κB pathway. In confirmatory experiments, short hairpin (sh) RNA-mediated inhibition of PKC-β resulted in selective loss of growth in both ibrutinib and STN responsive cell lines. Subsequent gene set-enrichment analysis confirmed the up-regulation of BCR pathway components in sensitive cell lines and demonstrated the down-regulation of NF-κB pathway target genes in response to treatment with ibrutinib and STN. Further, the authors reported that a constitutively active allele of CARD11 restored the expression of NF-κB target genes in the presence of STN.
Upon further analysis of NF-κB signaling, both pharmacologic and shRNA-mediated inhibition of IKK-β was shown to suppress the growth of all MCL cell lines irrespective of response to ibrutinib and STN. To elucidate the mechanisms by which NF-κB signaling was important in the resistant cell lines, the authors subjected samples to RNA sequencing and identified a nonsense mutation in TRAF2 and biallellic deletion of TRAF3. In the alternative NF-κB pathway, TRAF2 and TRAF3 are negative regulators of NIK (also called mitogen-activated protein 3 kinase or MAP3K14), a kinase that phosphorylates p100, generating its active isoform, p52 (Figure). Levels of p52 were low in ibrutinib- and STN-sensitive cell lines and elevated in resistant cell lines. ShRNA inhibition of NIK resulted in depleted p52 levels and prevented cell growth. In a zenograft mouse model using a resistant MCL cell line, knockdown of NIK resulted in decreased tumor growth and reduced expression of NF-κB target genes. Sequencing of key genes in the NF-κB alternative pathway in 165 patient-derived MCL samples revealed mutations in TRAF2 and its downstream protein, BIRC3, in 6 percent and 10 percent of samples, respectively. Subsequent analysis showed that mutant BIRC3 had dominant negative properties that resulted in the loss of inhibition of NIK–NF-κB signaling (Figure).
In Brief
The elegant work by Dr. Rahal and colleagues identifies the critical role of NF-κB signaling in MCL and provides key insights into the pathogenic mechanisms in ibrutinib-sensitive and -resistant MCL. Understanding these pathways may allow for the prediction of patient response to ibrutinib and lead to development of rational treatment combinations. In addition, the authors have identified NIK as a novel therapeutic target in patients who are resistant to ibrutinib.
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.