The Use of Genotyping in Transfusion Medicine
Required testing for red blood cell (RBC) transfusion includes serologic ABO and RhD typing, as well as screening for alloantibodies to “minor” (non-ABO and non-RhD) RBC antigens (Figure 1). In general, blood banks and transfusion services no longer rely on human-source antibodies for these tests, as ABO and RhD typing can now be accomplished quickly and accurately using monoclonal antibody reagents. To confirm the ABO type, all donors and recipients are tested for the presence of isoagglutinins directed against A and B antigens, as plasma from all immunologically normal individuals without an A or B antigen should contain such antibodies. For example if a recipient’s RBCs type as A, the plasma from that recipient will have anti-B antibodies. Anti-D is neither expected nor naturally occurring in D-negative individuals. In addition, a sensitive screen for alloantibodies to other blood group antigens is done to avoid the mostly delayed hemolytic transfusion reactions that can be caused by such alloantibodies. While small laboratories still use traditional tube agglutination methodologies for these initial tests, blood donor centers and larger hospital-based laboratories now use high-throughput automated testing instruments to ascertain ABO and Rh type, as well as to perform antibody screens designed to detect alloantibodies.
Clinical Need
One to two percent of all patients who receive transfusions develop antibodies to RBC antigens. In patients receiving chronic transfusion (e.g., for sickle cell disease [SCD]), the frequency of alloimmunization is higher, affecting ≈20 percent to 30 percent of recipients,1 ,2 though most patients who develop alloantibodies do so before the 15th transfusion.3 For patients with hematologic malignancies, the incidence of RBC alloimmunization is estimated at nine percent to 15 percent,3 ,4 despite the immunosuppressive effects of the chemotherapy most patients receive. Thus, alloimmunization to RBC antigens in hematology patients is relatively common.
Once alloimmunization occurs, the likelihood of additional antibody responses is also relatively high. In surgical, pregnant, and non–hematologic malignancy patients, once RBC antibodies have been induced, 20 percent to 25 percent of patients form additional antibodies after subsequent transfusions and thus become multiply alloimmunized. In a retrospective study from the Netherlands that covered a 24-year period, 25 of 115 immunized patients (21.7%) formed 30 additional antibodies after receiving a median of seven additional RBC transfusions. The median interval between primary and additional antibody detection was four months. Interestingly, diagnosis or treatment intensity did not significantly influence development of additional antibodies.5
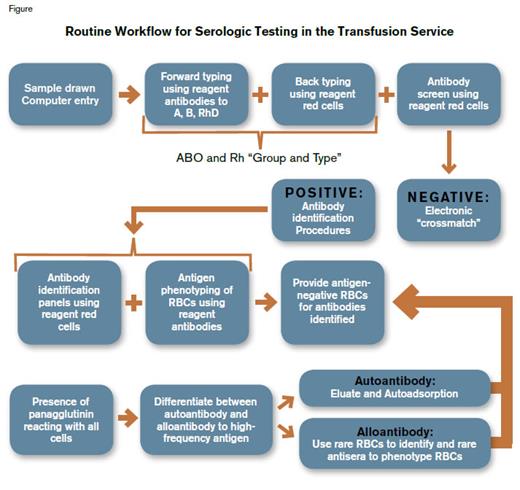
Routine Workflow for Serologic Testing in the Transfusion Service. Once the patient’s red blood cells (RBCs) are phenotyped for ABO and RhD antigens, providing appropriate ABO- and RhD-matched RBCs for transfusion is straightforward if no alloantibodies or autoantibodies are detected. However, if the patient’s serum or plasma reacts with reagent RBCs, the specificity of each antibody present must be identified. The patient’s own RBCs are phenotyped to confirm the validity of antibody identification. In the presence of autoantibody or an antibody that reacts with a very high frequency antigen, the process of antibody identification and phenotyping of the patient’s RBCs becomes technically difficult, often requires the availability of rare reagent cells and sera, and is highly time-consuming.
Routine Workflow for Serologic Testing in the Transfusion Service. Once the patient’s red blood cells (RBCs) are phenotyped for ABO and RhD antigens, providing appropriate ABO- and RhD-matched RBCs for transfusion is straightforward if no alloantibodies or autoantibodies are detected. However, if the patient’s serum or plasma reacts with reagent RBCs, the specificity of each antibody present must be identified. The patient’s own RBCs are phenotyped to confirm the validity of antibody identification. In the presence of autoantibody or an antibody that reacts with a very high frequency antigen, the process of antibody identification and phenotyping of the patient’s RBCs becomes technically difficult, often requires the availability of rare reagent cells and sera, and is highly time-consuming.
There are currently a variety of methods used to detect alloantibodies to minor blood group antigens; however, most laboratories still rely on the indirect Coombs’ method in which patient antibodies bound to erythrocytes of the test panel are detected by agglutination mediated by Coombs’ serum (rabbit antiserum against human IgG and complement C3). Alternatively, an isolated, specific antibody against human IgG (usually a murine monoclonal antibody) can be used rather than Coomb’s serum to mediate the RBC crosslinking. While this method is technically straightforward, successful identification of the target of the alloantibody is reagent dependent. Using this technique, identification of the target antigen of a single alloantibody is relatively uncomplicated, but when multiple alloantibodies are present, a transfusion laboratory needs a supply of often rare reagent RBCs with unusual phenotypes to be able to characterize fully and accurately the spectrum of alloantibodies present (Figure 1).
Additionally, once the presence or history of alloimmunization has been documented, the availability of RBC units for transfusion may be severely restricted by compatibility issues, and, depending upon the rarity of target-antigen negative RBCs, the time necessary to identify compatible units may be prolonged. In general, the majority of banked blood has only been tested for the presence of ABO and RhD antigens. Therefore, when specific antigen-negative units are needed, either the blood supplier or the transfusion service must test donor units for the presence of the relevant antigens that must be avoided. This testing is accomplished through either the traditional agglutination methodology described earlier in this article, or, when it is necessary to screen large numbers of donor units, through automated systems. In either case, however, such screening is a time-consuming and expensive procedure that is also dependent on the availability of human sera directed against specific blood group antigens.
Therefore, large transfusion services, as well as some blood donor centers, have begun to genotype selected samples. At Duke Hospital, patients with hemoglobinopathies are genotyped prospectively at the time of their initial evaluation. Other patients are genotyped if they are multiply alloimmunized, alloimmunized and expected to need recurrent transfusions, or alloimmunized and have a co-existing autoantibody. The single most common context outside hemoglobinopathies in which patients are genotyped is hematologic malignancies, with or without a history of hematopoietic stem cell transplantation. At Duke Hospital, nonhemoglobinopathy diagnoses associated with samples referred for genotyping were 30.9 percent hematologic malignancies, 11 percent solid tumors, 16.9 percent surgery, 11.8 percent autoimmune hemolytic anemia, and 29.4 percent other medical problems, including obstetric issues and hemolytic disease of the newborn (HDN).
Molecular Basis of Blood Group Antigens and Genotyping Methodology
The molecular basis of erythrocyte blood group antigens began to be defined in the 1980s and 1990s. Before then, relatively few antigens were even characterized biochemically. Most blood group antigens were elucidated at the molecular level during the 1990s and the first decade of the 21st century. Today, 26 erythrocyte blood group antigen systems have been characterized at the molecular level. Most minor blood group antigens reside on proteins whose polymorphisms are due to exchange of one amino acid, arising from a single nucleotide substitution in the encoding gene. The notable exception to this is the MN polymorphism, which involves exchange of two nonadjacent amino acids. For carbohydrate antigens, such as ABO and Lewis, the genetic mechanism of polymorphism resides in the alteration of genes encoding glycosyltransferases involved in synthesis of the antigenic oligosaccharides. However, some variant phenotypes are caused by more complex genetic changes, including intra- and intergenic exchanges, inversions, insertions, and deletions.6 The RH system alone now has 51 characterized antigens. In addition, the two RH genes (RHD and RHCE) each have a large number of alleles, with more than 200 alleles recognized in RHD alone.7
Although initial research into the molecular bases of blood group antigens often used simple PCR-based Sanger sequencing methodologies, advances have now been made in the development of high-throughput methods by which each sample can be simultaneously typed at multiple loci. The most commonly used genotyping system in the United States was developed by Bioarray Solutions Ltd. (Warren, NJ) and is now supplied by Immucor Inc.; it has recently been approved by the U.S. Food and Drug Administration as a diagnostic test. This system currently predicts the phenotype for antigens in the RHCE, KEL, FY, DO, LW, CO, SC, LU, DI, JK, and MNS systems, as well as for HbS. It does not include ABO or RHD genotyping. Additional platforms are available specifically for detecting more than 100 RHD and RHCE variants, though these are not widely used. Unlike assays that rely on agglutination or interactions of antibodies with antigens, this system is not affected by immunoglobulin coating of the RBCs, the presence of recently transfused RBCs (since DNA is derived from leukocytes), or by any form of polyagglutination. The human erythrocyte antigen genotyping platform (BioArray™ molecular BeadChip™) uses functionalized color-coded beads with attached oligonucleotide probes. Areas containing SNPs corresponding to blood group antigen polymorphisms are amplified by PCR, denatured and then annealed to derivatized beads. An extension reaction adds a labeled nucleotide to the 3’ end of the probe, and the labeled beads are then placed on a beadchip array. After obtaining an automated readout, the genotype is determined and the phenotype predicted.
Potential Benefits of Genotype-Matched RBC Transfusion
SCD
Dr. Daniel Ambruso and colleagues have found in two prospective studies that the incidence of developing antibodies per unit transfused was diminished 10-fold when selected donors were used for transfusion of patients with SCD.8 ,9 Dr. Oswaldo Castro and colleagues calculated that if all transfusions had been selected by limited phenotype matching (C/c, E/e, K, ABO, and D), alloantibody development would have been prevented for more than half (53.3%) of the 137 alloimmunized SCD patients studied.10 Recently, Dr. Sule Mine Bakanay and colleagues showed that previous transfusion led to errors in traditional phenotyping in 51 percent of such patients.11 Moreover, the RHD and RHCE genes are highly polymorphic, so that D-positive and e-positive individuals, especially, may make alloantibodies to these antigens despite typing positive for them by traditional serologic methods. Genotypic analysis of such individuals invariably identifies the molecular alterations responsible for such apparent discrepancies.12 ,13 Thus, for SCD patients, who frequently undergo transfusion and are frequently alloimmunized, genotypic data can clarify their alloimmune status and facilitate provision of compatible RBC units, which are sometimes critical for survival of SCD complications such as stroke and acute chest syndrome.
Autoimmune hemolytic anemia
Transfusing patients with autoimmune hemolytic anemia is challenging because the autoantibody coats the patient’s own RBCs and typically reacts with all cell tested (i.e., the antibody is a panagglutinin). Autoantibodies therefore interfere with patient blood group antigen phenotyping, identification of alloantibodies, and crossmatching. Technical procedures can be used to remove autoantibody from serum or plasma, either in order to identify alloantibodies or to perform crossmatching, but these are labor-intensive and time-consuming procedures. In addition, accurate serologic phenotyping of RBCs coated with autoantibody is challenging because the IgG already coating the cells obscures the differences between negative and positive reactions obtained by using Coombs’ serum (anti-IgG) –induced agglutination. Serological phenotyping in such situations requires that the autoantibody first be chemically stripped off the patient’s own cells before they can be phenotyped. Thus, genotyping of the patient and donors has the potential to allow the transfusion of antigen-matched blood to patients with autoimmune hemolytic anemia, saving both time and money. Moreover, methods such as absorption, which are used to remove reactivity due to autoantibodies, run the risk of obscuring the presence of alloantibodies, especially if they are of low titer. Thus, transfusion of genotypically matched blood would also be a safer practice than the use of traditional serological methods in patients with an IgG autoantibody.
Recently transfused patients
In the setting of suspected immune destruction of transfused cells, the transfusion service needs to determine the native phenotype of the recipient in order to correlate that information with any detected RBC alloantibodies. However, after transfusion of several units of blood, much of the circulating RBCs are of donor origin. Given the short survival of transfused leukocytes and the fact that an increasing proportion of transfused RBCs are now leukoreduced, genotyping in the setting of recent transfusion can accurately determine the recipient’s blood group antigen genotype without concerns about contamination from donor-derived DNA.
Rare phenotypes
Alloantibodies to common antigens are only rarely made because individuals with the RBC phenotype that puts them at risk for developing such alloantibodies are by definition rare. Therefore, because many antigen phenotypes are still defined serologically, the capacity to identify rare antigen-negative phenotypes resides only in a few reference laboratories that have the scarce (often expensive) test reagents available. Again, genotyping relies neither on rare reagents nor on reagents of human origin. Thus blood donors can be more readily genotyped than phenotyped for several common antigens, to identify those rare donors who are negative for the common antigen.
Prenatal diagnosis and risk of HDN
Routine serologic methods for the prediction of risk for HDN consist of performing a serologic screen for unexpected alloantibodies in the mother’s serum, and then titering any antibodies found. However, it is well recognized that titer and severity of HDN do not necessarily correlate, especially for some common culprits such as anti-K. A second method for determining risk is to phenotype the father’s cells serologically to determine if the father is likely to confer the gene for a particular antigen to the fetus. Fetal genotyping, however, can predict risk much more accurately by defining which antigens the fetus is actually capable of expressing. Amniocytes, whether obtained directly from amniotic fluid or cultured, can be genotyped as easily as blood cells. Such testing is especially valuable when the father is not available, his phenotype is indeterminate serologically, or he is heterozygous for an antigen of interest. Definitive determination of the fetus’ genotype allows alloimmunized mothers whose fetuses cannot express the target antigen to receive routine prenatal management, without concern for HDN. In addition, genotyping of a father can define zygosity (e.g., of RhD), which can also determine the likelihood that the fetus has inherited a particular allele.
Are We Ever Going to be Able to Avoid Transfusion of Units Likely to Engender Alloimmunization?
Although delayed hemolytic transfusion reactions rarely cause more than transient clinical problems, there are particular populations in which this is not true. Patients at particular risk are those with frequent transfusion requirements, limited capacity to produce RBCs, or chronic hemolysis. High-throughput genotyping methods can be applied to both patients and blood donors, resulting for the first time in the possibility of providing genotype-matched, rather than just ABO/RhD-matched or crossmatch-compatible, RBCs. This approach has the great advantage of potentially avoiding almost all alloimmunization, along with the consequent medical complications of such alloimmunization.
It is also likely that even genotype-matching of RBCs will neither be foolproof nor become the sole methodology used in blood centers and transfusion services.14 DNA-based methods thus far are designed to detect known gene variations that affect protein sequence or, in rare cases, protein expression (such as the GATA site mutation in the FY allele). Rare alleles that interfere with gene expression (such as ones affecting mRNA splicing, for example) are likely to lead to results predicting positivity for an antigen that is actually unexpressed, and occasional variants may exist but evade detection for technical reasons. Some complex genetic changes, such as hybrid alleles common in the RH and MNS systems, may lead to false-negative and false-positive results. And the number of known alleles for some blood group systems (especially RH and ABO) are too large to be practicably addressed by currently available technology. Finally, some systems remain to be characterized on the molecular level. Nevertheless, it is likely that technologies such as next-generation sequencing will ultimately supplant current methods and lead to the capacity to genotype more completely even the complex RH and ABO systems, as well as to identify the molecular basis of yet uncharacterized antigens.
Figure Legends
Figure 1. Routine Workflow for Serologic Testing in the Transfusion Service.
Once the patient’s red blood cells (RBCs) are phenotyped for ABO and RhD antigens, providing appropriate ABO- and RhD-matched RBCs for transfusion is straightforward if no alloantibodies or autoantibodies are detected. However, if the patient’s serum or plasma reacts with reagent RBCs, the specificity of each antibody present must be identified. The patient’s own RBCs are phenotyped to confirm the validity of antibody identification. In the presence of autoantibody or an antibody that reacts with a very high frequency antigen, the process of antibody identification and phenotyping of the patient’s RBCs becomes technically difficult, often requires the availability of rare reagent cells and sera, and is highly time-consuming.
References
Competing Interests
Dr. Telen indicates no relevant conflicts of interest.