Hematologists commonly care for patients with iron-related problems, including iron-deficiency anemia (IDA) or insufficient supply of marrow iron (anemia of chronic inflammation [ACI] or malignancy). Other patients are phlebotomized to remove iron from the body and/or restrict iron supply to the marrow in polycythemia vera, abnormal accumulation of dietary iron (hemochromatosis), transfusion-related hemosiderosis, or porphyria cutanea tarda. Some patients with significant anemia combined with iron overload and/or transfusion requirements, such as in thalassemia or sickle cell disease, may require iron chelation therapy. Among the most important discoveries for hematologists in 2014 is the report of Dr. Leon Kautz and colleagues,1 from the laboratory of Dr. Tomas Ganz, that identified erythroferrone (ERFE) as a hormone produced by erythroblasts that suppresses hepcidin transcription in the liver. As predicted from studies in patients with thalassemia and hemolytic anemia, ERFE appears to be the regulator of iron absorption and plasma iron mobilization that, “…originates in immature erythroblasts in need of more iron.”2 The liver hormone hepcidin, the transcription of which is suppressed by ERFE, modulates cellular export of iron by binding and down-regulating surface expression of the cellular iron exporter, ferroportin.3
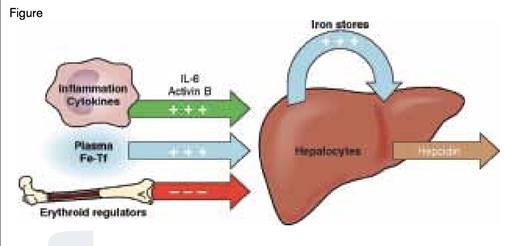
Regulation of Hepcidin Production by the Liver. Three major processes that influence hepcidin transcription are 1) induction by infection/inflammation (green), 2) induction by iron status (blue), or 3) suppression by expanded erythroid precursors (red). Modified slightly from Ganz T, Systemic Iron Homeostasis, Physiol Rev. 2013;93:1721-1741.
Regulation of Hepcidin Production by the Liver. Three major processes that influence hepcidin transcription are 1) induction by infection/inflammation (green), 2) induction by iron status (blue), or 3) suppression by expanded erythroid precursors (red). Modified slightly from Ganz T, Systemic Iron Homeostasis, Physiol Rev. 2013;93:1721-1741.
In terms of providing transferrin-bound iron required for cellular iron uptake, including the very large amounts required by erythroid precursors, the regulation of ferroportin by hepcidin is most important for macrophages that recycle iron from phagocytosed senescent erythrocytes, hepatocytes that are a major iron storage site, and duodenal enterocytes that are the site of dietary iron absorption. At least three major processes acting in concert regulate hepcidin gene transcription (Figure): 1) increased iron stores and transferrin iron saturation,4,5 which induce transcription by the BMP6-SMAD pathway via a complex receptor mechanism involving BMP6 receptors and other components, including transferrin receptor 2, hemojuvelin, and HFE (each associated with hereditary hemochromatosis when mutated); 2) inflammation/infection, which induces transcription via IL-6 signaling through the STAT3 pathway4 and activin B signaling through the BMP/Smad1/5/8 pathway6 ; and 3) erythropoietin-induced erythroblast expansion, which suppresses transcription by ERFE signaling through an unknown mechanism.1 Using disease models and ERFE-deficient mice, Dr. Kautz and colleagues demonstrated that during stress erythropoiesis, such as following blood loss or hemolysis, prompt induction of ERFE provides erythroblasts with greater amounts of iron, permitting an optimal recovery of circulating erythrocyte numbers.1 During ineffective erythropoiesis with expanded erythroid precursors but reduced erythrocyte production, such as in β-thalassemia, increased ERFE causes iron overload,1 a major cause of morbidity and mortality in the more severe thalassemias. In a murine ACI model, ERFE deficiency augments the increases in serum hepcidin caused by inflammation, worsens iron restriction and anemia, and retards recovery from inflammation-induced anemia.7
These ERFE studies may lead to potential therapies based on regulation of hepcidin activity for disorders of iron absorption, storage, and mobilization that will be more specific than current modalities such as iron supplementation, chelation, and phlebotomy. Hepcidin mimetics and anti-hepcidin demonstrated therapeutic potential in animal models of hemochromatosis8 and ACI,9 respectively. Potential therapies for iron overload in hemochromatosis or iron restriction in ACI involving modulation of hepcidin transcription rates have been based on altering the signaling mechanisms of iron stores/transferrin iron saturation or inflammatory cytokines.10 Therapies based on inhibiting ERFE activity may help patients with β-thalassemia and disorders associated with inappropriate iron accumulation during ineffective erythropoiesis, whereas therapies based on increasing ERFE activity may ameliorate ACI, especially in those patients in which treatment of the underlying inflammatory condition is limited.
Among other noteworthy 2014 publications in the area of erythroid cell biology is another report about hepcidin. Dr. Xing-gang Wu and colleagues11 demonstrated that HFE, the mutated forms of which are associated with the large majority of hereditary hemochromatosis cases, interacts with the ALK3 component of the BMP6 receptor in the pathway that induces hepcidin transcription in response to increased iron (Figure). The more severe phenotype of C282Y mutation appears to inhibit surface expression of ALK3, while the less severe H63D mutation appears to increase proteasomal degradation of ALK3.
Finally, for those with an interest in erythrocytes and thrombosis, Dr. Douglas Cines and colleagues12 showed that the viscoelasticity of erythrocytes permits a change from discs to polyhedrocytes within clots following contraction mediated by platelets and fibirin located mainly on the outer surfaces of the clots. This previously unrecognized property of erythrocytes helps explain impermeability, density, and stiffness of clots and has clinical implications for bleeding and wound healing.