The Question:
What is your approach to the treatment of elderly patients with acute myeloid leukemia?
My Response:
Acute myeloid leukemia (AML) is not a significant public health hazard, accounting for less than 2 percent of all cancers diagnosed yearly in the United States. The American Cancer Society estimates that 18,860 new cases of acute myeloid leukemia (AML) were diagnosed in 2014.1 Nonetheless, AML is second only to chronic lymphocytic leukemia as the most common subtype of leukemia in adults. The median age at diagnosis is 67 years, and more than 60 percent of newly diagnosed patients are older than 60 years.2 The management of elderly patients with AML poses unique therapeutic challenges. These individuals disproportionally account for greater than 75 percent of AML deaths yearly. In addition, recent data from the Surveillance, Epidemiology, and End Results (SEER) Program demonstrate that 50 to 60 percent of newly diagnosed AML patients older than 65 years do not receive any form of antileukemia therapy.3 In this article I will discuss decision-making strategies and treatment approaches that the practicing hematologist can use in elderly patients with AML.
Definition of “Elderly” in Patients with AML
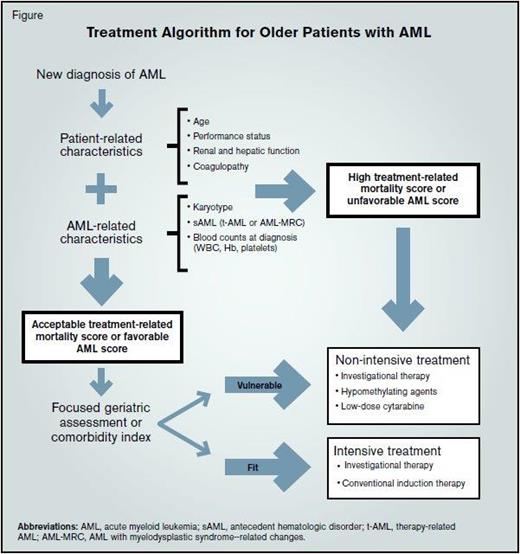
Older patients can be classified into three categories of chronological age: 1) young-old patients are 65 to 75 years of age; 2) old patients are 76 to 85 years; and 3) oldest-old patients are older than 85 years.4 In AML, clinical outcomes worsen with advancing age.2 Recognizing this relationship between older age and outcomes, contemporary AML treatment protocols have typically used an arbitrary cutoff of 55 to 65 years to distinguish between younger and older subjects. However, chronologic age by itself is not reliable in estimating life expectancy, functional reserve, or the risk of treatment complications. Comprehensive geriatric assessments (CGA) are multidisciplinary, in-depth evaluations designed to assess life expectancy and attendant morbidity and mortality risks in older patients. CGAs include tools to predict the functional age based on functional status, comorbidities, polypharmacy, nutritional status, and geriatric syndromes.5 For this reason, pre-treatment CGAs or more focused geriatric assessment tools may replace chronologic age as an identifier of “functionally older” AML patients ineligible for aggressive treatment approaches.
AML and Non-AML–Related Factors Influencing Treatment Decisions
Recent studies suggest that age may be a suboptimal sole or primary criterion for allocation to intensive AML treatment protocols, and that additional variable may improve the ability to predict toxicity and outcomes. To this effect, population-based data demonstrate that older age was only one of the several covariates (including history of antecedent hematologic disorder [sAML], higher comorbidity score, poor performance indicators, marital status, and lower household incomes) associated with lack of antileukemia therapy in newly diagnosed older patients with AML.3 A retrospective analysis showed that a pre-treatment geriatric assessment, focused on cognitive and physical function, improved the prediction of survival among older adults with AML treated with conventional induction chemotherapy.6 Two large retrospective studies have identified several AML and non-AML covariates that predict early outcomes, such as induction mortality and likelihood of complete remission, following intensive chemotherapy. In the first, investigators developed a simplified early death score with moderate discriminatory power (area under curve: 0.8-0.83) that incorporates performance status at diagnosis, age, platelet count, albumin, sAML, white blood cell count, percentage of peripheral blood blasts, and serum creatinine (cstaging.fhcrc-research.org/TRM/Default.aspx).7 Interestingly, removal of age as covariate had minimal impact on the predictive power of this model. Similarly, investigators from the Study Alliance Leukemia demonstrated that the risk of induction mortality and the chance of complete remission could be predicted in older patients with AML using a combination of pre-treatment covariates, including age, hemoglobin, platelet count, fibrinogen, type of AML, karyotype and limited molecular abnormalities at diagnosis (www.aml-score.org).8 These data suggest that age alone is a poor predictor of treatment intent and outcomes. Instead, it is likely a surrogate for other covariates associated with inferior prognosis and challenges the strategy of using age as the singular basis for assignment of intensive AML treatment.
Therapeutic Options for Elderly Patients with AML
In routine clinical practice, it remains challenging to know how to best utilize these data to inform patients and families on the optimal therapeutic approach for an individual with AML. Although these predictive models provide some guidance on the potential risks and benefits associated with conventional induction chemotherapy, treatment decisions regarding therapeutic intensity remain a highly individualized exercise. In my practice, I direct patients with low treatment-related mortality scores or favorable AML risk prediction scores to intensive standard or investigational therapy; conversely, I consider patients with high treatment-related mortality scores or unfavorable AML risk profiles for lower-intensity investigational regimens.
Moreover, results from population studies and randomized clinical trials add to the challenge of deciding optimal therapeutic intensity. Recent SEER-Medicare linked data showed that, after using a propensity score-matched survival analysis to account for all confounders, similar reductions in the risk of death were observed for elderly patients receiving either intensive chemotherapy or hypomethylating agents.3 Similar results have been reported in randomized clinical trials with similar overall survival between single agent hypomethylating agents (decitabine or azacitidine) and conventional care regimens.9,10
Targeted Therapies in AML
A molecular revolution, stimulated by the identification of novel, recurrent, and cytogenetically silent genomic abnormalities, has promoted a shift in the treatment paradigm of AML. These molecular abnormalities not only improve our understanding of the mechanisms of leukemogenesis, but also provide the genomic footprint for the development of small molecule, targeted therapies in AML. Activating mutations in signaling pathways, including FLT3, KIT, RAS, and others, have been described in approximately 60 percent of AML patients.11 Clinical trials testing the activity (as single agents or in combinations) of FLT3, KIT and MEK inhibitors are readily available in most academic centers and may reshape treatment strategies for these patients. Mutations in the DNMT3a and TET2 genes have been linked to increased sensitivity to hypomethylating agents.12,13 Identification of oncogenic alterations in the isocitrate (IDH) genes can be used to identify patients for current AML research protocols testing specific small molecule inhibitors of mutated IDH1 and IDH2.14,15
Treatment Algorithm for Older Patients with AML. Abbreviations: AML, acute myeloid leukemia; sAML, antecedent hematologic disorder; t-AML, therapy-related AML; AML-MRC, AML with myelodysplastic syndrome--related changes.
Treatment Algorithm for Older Patients with AML. Abbreviations: AML, acute myeloid leukemia; sAML, antecedent hematologic disorder; t-AML, therapy-related AML; AML-MRC, AML with myelodysplastic syndrome--related changes.
Future Directions in the Management of Elderly Patients with AML
“It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness...” The opening words to A Tale of Two Cities by Charles Dickens accurately describes the sentiment from hematologists worldwide regarding the current state of AML management in older patients. On one hand, while the understanding of the genomic alterations occurring in AML, which are ultimately responsible for leukemogenic transformation, has skyrocketed in recent years, few, if any, of these discoveries thus far have been translated into new treatment strategies for older patients with AML beyond “7+3” conventional induction chemotherapy. Fortunately, results of several recent studies suggest that significant breakthroughs may be within reach of most patients in the not-so-distant future. Examples of novel agents in clinical development include specific small-molecule inhibitors of IDH1 and IDH2, DOT1-L inhibitors for patients with MLL rearranged acute leukemia, the introduction of novel epigenetic modifiers such as the bromodomain (BRD4) inhibitors and development of second-generation anti-CD33 conjugated monoclonal antibodies. Also, the addition of multityrosine kinase (sorafenib) or aurora kinase inhibitors to conventional induction chemotherapy is associated with promising antileukemic activity and improved survival. Finally, improved patient selection (genomic profile, AML-MRD, or adverse-risk karyotype) or combination with novel agents (Pracinostat or Pevonedistat) may optimize the clinical benefit of inhibitors of DNA methyltransferase, such as azacitidine. At last, physicians caring for older patients with AML may say farewell to the age of foolishness when patients and physicians were painstakingly faced with choosing between non-specific and toxic therapeutic regimens or palliative care and welcome the age of wisdom when genomics-driven, targeted, and risk-adapted treatment strategies can be incorporated into the management of AML. There is great enthusiasm in the field, with the promise that the best of times will be upon us soon.
References
Competing Interests
Dr. Medeiros indicated no relevant conflicts of interest.