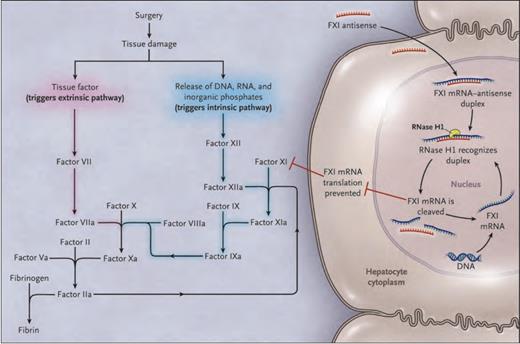
Animal studies suggest that inhibition of factor XI (FXI) prevents thrombosis without disrupting hemostasis.1,2 Epidemiologic data indicate that patients with congenital FXI deficiency are protected from venous thromboembolism (VTE), even though their bleeding phenotype is variable and often quite mild.3 On the basis of these observations, Dr. Harry Büller and colleagues hypothesized that targeting FXI may provide effective antithrombotic therapy without the bleeding risk associated with conventional anticoagulants. They used a FXI antisense oligonucleotide (FXI-ASO) to test this hypothesis. FXI-ASO binds FXI messenger RNA in hepatocytes (the site of FXI synthesis) with high specificity, targeting the messenger RNA (mRNA) for degradation before it can be translated to protein. FXI synthesis is thus curtailed, resulting in reduced plasma FXI levels (Figure).
Effect of FXI-ASO on the Coagulation System. FXI-ASO (ISIS 416858) Is a Factor XI (FXI)–targeted Second-generation Antisense Oligonucleotide. Tissue damage after surgery exposes tissue factor and results in the release of DNA, RNA, and inorganic polyphosphate from damaged cells and from activated platelets and neutrophils. Tissue factor binds factor VIIa and initiates the extrinsic pathway of coagulation, whereas DNA, RNA, and polyphosphate activate factor XII and initiate the intrinsic pathway of coagulation. FXI–targeted antisense oligonucleotide attenuates the intrinsic pathway by binding to factor XI messenger RNA (mRNA) in the liver, which results in ribonuclease H1 (RNase H1) –mediated degradation of FXI messenger RNA, thereby preventing protein synthesis and reducing circulating FXI levels.From The New England Journal of Medicine, Harry R. Büller, Claudette Bethune, Sanjay Bhanot, et al, Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis, Volume 372, Page 233 Copyright © 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Effect of FXI-ASO on the Coagulation System. FXI-ASO (ISIS 416858) Is a Factor XI (FXI)–targeted Second-generation Antisense Oligonucleotide. Tissue damage after surgery exposes tissue factor and results in the release of DNA, RNA, and inorganic polyphosphate from damaged cells and from activated platelets and neutrophils. Tissue factor binds factor VIIa and initiates the extrinsic pathway of coagulation, whereas DNA, RNA, and polyphosphate activate factor XII and initiate the intrinsic pathway of coagulation. FXI–targeted antisense oligonucleotide attenuates the intrinsic pathway by binding to factor XI messenger RNA (mRNA) in the liver, which results in ribonuclease H1 (RNase H1) –mediated degradation of FXI messenger RNA, thereby preventing protein synthesis and reducing circulating FXI levels.From The New England Journal of Medicine, Harry R. Büller, Claudette Bethune, Sanjay Bhanot, et al, Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis, Volume 372, Page 233 Copyright © 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
In a randomized, open-label, phase II trial, the authors assigned 300 adults scheduled for elective unilateral total knee arthroplasty to daily subcutaneous enoxaparin 40 mg (n = 75) or subcutaneous FXI-ASO at a dose of 200 mg (n = 147) or 300 mg (n = 78). Because of its slow onset of action, FXI-ASO was initiated five weeks before surgery and given again at 33, 31, 28, 21, 14, and seven days before surgery, at six hours after surgery, and at three days after surgery. Enoxaparin was initiated either the evening before surgery or six to eight hours after surgery and continued through at least postoperative day 8. The primary efficacy outcome was VTE, defined as a composite of symptomatic VTE or silent deep vein thrombosis (DVT), identified by mandatory bilateral lower extremity venography between postoperative days 8 and 12. The principal safety outcome was major and clinically relevant non-major bleeding. An independent committee blinded to treatment assignment adjudicated all outcomes.
The treatment groups were well-balanced at study entry. Mean perioperative FXI levels were normal (0.93 U/mL) in the enoxaparin group and were reduced in a dose-dependent fashion in the 200-mg (0.38 U/mL) and 300-mg (0.20 U/mL) groups. The primary efficacy outcome occurred in 30 percent, 27 percent, and 4 percent of the treatment groups, respectively. The FXI-ASO 200-mg regimen was non-inferior to, and the 300-mg regimen was superior to enoxaparin for prevention of VTE. Most VTE events were silent below-the-knee DVTs; only three events (one in the enoxaparin group and two in the FXI-ASO 200-mg group) were symptomatic. The incidence of the principal safety outcome was not significantly different between the treatment groups (8% in the enoxaparin group, 3% in the FXI-ASO 200-mg group, and 3% in the FXI-ASO 300-mg group). There was only one major bleeding event (in the FXI-ASO 300-mg group). Injection site reactions occurred in 26 percent of patients in the FXI-ASO groups, but were generally mild and did not result in discontinuation of study drug.
In Brief
Dr. Büller and colleagues provide valuable proof of concept that targeting FXI is a potentially effective and safe antithrombotic strategy. The clinical potential of FXI-ASO itself may be limited by relatively slow onset and offset of antithrombotic effect, need for subcutaneous administration, and frequent injection site reactions. Nevertheless, its promising performance in the present study engenders enthusiasm for development of alternative approaches to FXI inhibition, including small molecule and antibody inhibitors.
The study results also challenge two widely held “common sense” dogmas in coagulation. First, activation of the extrinsic pathway by tissue factor at the site of surgery has long been viewed as the predominant mechanism of postoperative VTE. The antithrombotic efficacy of FXI-ASO suggests that the intrinsic pathway has an important and previously underappreciated role in the pathophysiology of this disorder, perhaps through activation by DNA, RNA, and inorganic polyphosphates released from damaged cells (Figure). Second, antithrombotic therapy has traditionally been viewed as a double-edged sword – an inevitable trade-off between antithrombotic efficacy and bleeding risk. In keeping with preclinical data,1,2 FXI-ASO reduced thrombosis without increasing bleeding, although the study was not powered to detect differences in bleeding rates between treatment groups. Larger studies with patient-important outcomes will be needed to confirm whether targeting FXI can prevent clinically meaningful thrombosis while sparing hemostasis.
References
Competing Interests
Dr. Adam Cuker indicated no relevant conflicts of interest.