T-cell acute lymphoblastic leukemia (T-ALL) represents 10 to 15 percent of newly diagnosed cases of acute leukemia and is notable for its distinctive biological features and aggressive clinical course. While outcomes were once inferior, they now approach those observed in B-lymphoblastic disease (B-ALL). However, intensive treatment regimens are required to achieve these outcomes, and recurrent disease is very difficult to salvage. Moreover, in contrast to B-ALL where targeted therapeutic options have emerged for disease subtypes, targeted therapies have not yet been routinely adopted for T-lineage leukemia. Several oncogenic transcription factors have been implicated in the pathogenesis of the disease, but have proved difficult to target. An alternative strategy is the therapeutic targeting of chromatin-modifying enzymes, which affect the expression of oncogenic gene targets.
The chromatin complex is composed of DNA in association with histone proteins and other molecules, and post-translational modifications of histone tails regulate diverse biological processes, including gene transcription. Well-characterized histone modifications are lysine acetylation and methylation. Some modifications are associated with active transcription while others lead to repression. The histone 3 lysine 27 (H3K27) methylation mark is associated with chromatin condensation and transcriptional repression.1
Based on prior studies demonstrating the importance of NOTCH1-driven epigenetic changes in the pathogenesis of T-ALL,2 Dr. Panagiotis Ntziachristos in the Department of Pathology at New York University and colleagues hypothesized that removal of methyl groups from H3K27 mediates T-ALL progression. They investigated the role of two H3K27 demethylases in T-ALL initiation and maintenance: Jumongi D3 (JMJD3) and ubiquitously transcribed tetratricopeptide repeat X-linked protein (UTX). The authors first demonstrated that JMJD3 protein and transcript levels, but not UTX1, were highly expressed in T-ALL relative to physiological T-cell subsets as well as other leukemic subtypes. They further showed that JMJD3 regulates the expression of oncogenic NOTCH1 target genes (HEY1, NRARP, and HES1).
To further clarify the roles of JMJD3 and UTX in the maintenance of T-ALL, the authors performed genomic knockdown of JMJD3 in human T-ALL cells using short hairpin RNAs (shRNAs) and showed that knockdown of JMJD3, but not UTX, selectively decreased T-ALL cell viability and reduced the expression of NOTCH1 targets. This was accompanied by loss of JMJD3 and gain of H3K27me3 on target promoters. Curiously, JMJD3 expression was significantly upregulated upon UTX silencing, suggesting opposing roles for these two demethylases in T-ALL.
The authors next provided additional lines of evidence suggesting that UTX might in fact act as a tumor suppressor in T-ALL, in contrast to the role of JMJD3 as a transcriptional activator.
Whereas xenograft models of T-ALL cell lines treated with shRNAs against JMJD3 showed a significant growth disadvantage, silencing of UTX led to cell proliferation. In bone marrow transplant mouse model experiments, UTX germline knock-out mice demonstrated more rapid disease progression. Blasts from UTX knockout mice further demonstrated a decrease in the expression of other tumor suppressors as well as upregulation of genes promoting cell proliferation, including JMJD3.
A panel of primary pediatric T-ALL samples was screened for genetic alterations of the UTX locus, and eight patients were identified with somatic focal deletions or inactivating mutations, seven of which occurred in male patients. UTX is an X-linked gene, and this gender pattern is consistent with another recent report by Dr. Joni Van der Meulen and colleagues showing that UTX is recurrently mutated in males and escapes X-inactivation in females.3 These observations are consistent with the 3:1 male-to-female predominance of T-ALL that is observed clinically. Finally, overexpression of UTX in T-ALL cell lines led to suppression of tumor growth. Together, these studies strongly suggest that UTX acts as a tumor suppressor in human T-ALL.
In contradistinction to the biologic function of UTX, the authors provided evidence supporting an oncogenic role for JMJD3. Genetic ablation of JMJD3 in a murine model of T-ALL resulted in reduced disease burden and improved survival rates, which prompted the authors to explore therapeutic targeting of JMJD3 activity in T-ALL with the small molecule GSKJ4. GSKJ4, which was shown to affect the demethylase activity of JMJD3, significantly impacted the growth of human T-ALL cell lines and primary human T-ALL cells, leading to cell cycle arrest and increased apoptosis. These effects were observed exclusively in T-ALL and not in myeloid leukemia or in nonmalignant hematopoietic cells. Following treatment, changes in gene expression were coupled with an increase in H3K27me3 levels at repressed genes. Notably, genes that were upregulated with UTX knockdown overlapped with the GSKJ4 down-regulated gene signature, further supporting the opposing roles of the demethylases in T-ALL.
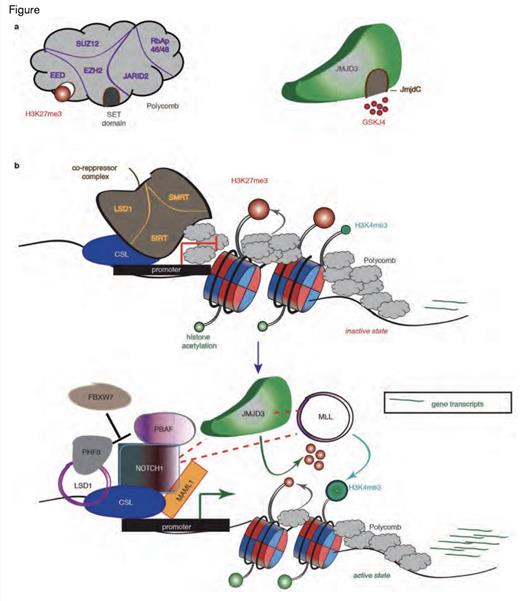
JMJD3 as a Pivotal Factor in NOTCH1-Mediated Oncogenic Activation in T-cell Leukemia. A) Schematic representation of the H3K27me3 writer (the polycomb complex, left) and eraser (JMJD3, right). EZH2 contains the catalytic subunit of the complex through its SET domain, whereas the EED subunit recognizes the H3K27me3 mark and aids in polycomb binding. JmjC domain activity is inhibited by the small molecule inhibitor GSKJ4. B) The main idea about the key role of JMJD3 in the NOTCH1transcriptional complex. Before activation of the NOTCH1 signaling pathway, the promoters of classical NOTCH1 target genes are bound by RBP-Jk, together with components of the co-repressor complexes and PRC2, leading to low gene expression. After the binding of NOTCH1 and its co-activator MAML1, the genes are activated through the recruitment of JMJD3 and the MLL complex, with simultaneous eviction of PRC2, which leads to the demethylation of H3K27me3 and the methylation of H3K4me3.Reprinted by permission from Macmillan Publishers Ltd: Nature. Volume 514: pg. 513-517, copyright 2014.
JMJD3 as a Pivotal Factor in NOTCH1-Mediated Oncogenic Activation in T-cell Leukemia. A) Schematic representation of the H3K27me3 writer (the polycomb complex, left) and eraser (JMJD3, right). EZH2 contains the catalytic subunit of the complex through its SET domain, whereas the EED subunit recognizes the H3K27me3 mark and aids in polycomb binding. JmjC domain activity is inhibited by the small molecule inhibitor GSKJ4. B) The main idea about the key role of JMJD3 in the NOTCH1transcriptional complex. Before activation of the NOTCH1 signaling pathway, the promoters of classical NOTCH1 target genes are bound by RBP-Jk, together with components of the co-repressor complexes and PRC2, leading to low gene expression. After the binding of NOTCH1 and its co-activator MAML1, the genes are activated through the recruitment of JMJD3 and the MLL complex, with simultaneous eviction of PRC2, which leads to the demethylation of H3K27me3 and the methylation of H3K4me3.Reprinted by permission from Macmillan Publishers Ltd: Nature. Volume 514: pg. 513-517, copyright 2014.
In conclusion, the authors proposed targeting JMJD3 as a novel treatment option for T-ALL and formulated a model where JMJD3 plays a key role in NOTCH1-driven oncogenic target gene activation. Recent studies have shown that the polycomb repressive complex 2 (PRC2) mediates H3K27 trimethylation and plays a role as a tumor suppressor in T-ALL by repressing gene transcription and antagonizing NOTCH1.2 The authors also proposed that JMJD3 could reverse the effect in a model where NOTCH1 recruitment leads to PRC2 eviction and recruitment of JMJD3 to target promoters with a resulting demethylation of H3K27me3 and activation of target gene transcription (Figure).
In Brief
This work by Dr. Ntziachristos and colleagues elegantly demonstrates the opposing roles of H3K27 demethylases in T-ALL, with UTX1 functioning as a tumor suppressor and JMJD3 as a key enzyme mediating NOTCH1-induced leukemogenesis. The study also suggests a novel therapeutic approach for the treatment of T-ALL. While outcomes for this disease have improved in recent years, therapy is intensive and associated with acute and late toxicities, and there are few successful treatment options for recurrent disease. Oncogenic transcription factors have proven difficult to target, and this study offers promise for a novel epigenetic approach inhibiting JMJD3 and potentially other chromatin modifying agents for UTX-mutant disease.
References
Competing Interests
Dr. Elizabeth Raetz indicated no relevant conflicts of interest.