Ten years ago, multiple groups published ground-breaking work describing the somatic activating JAK2V617F mutation and its central role in the pathogenesis of myeloproliferative neoplasms (MPN).1-4 Further studies identified JAK-STAT pathway mutations in the thrombopoietin receptor (MPL) and the calreticulin gene (CALR) in JAK2-nonmutated MPN.5-7 The pivotal role of aberrant JAK-STAT signaling in MPN has provided the rationale for the development of JAK kinase inhibitors. The JAK1/JAK2 inhibitor ruxolitinib was recently approved for patients with hydroxyurea-resistant or intolerant polycythemia vera (PV), which follows its initial approval for myelofibrosis (MF) in 2011. In both MF and PV, core benefits include improvement of systemic symptoms and splenomegaly. It has been observed that MPN patients have markedly elevated levels of proinflammatory cytokines, and that ruxolitinib (and well as other JAK inhibitors) is associated with a decrease of cytokine levels.8 However, the exact mechanisms by which JAK inhibitors impact the production of cytokine levels have been largely speculative. In a collaborative study originating from the Memorial Sloan Kettering Cancer Center, Dr. Maria Kleppe and colleagues use single-cell cytokine profiling of mouse models and patient samples to show that the attenuating effects of JAK inhibitors on cytokine secretion is only efficacious if it occurs in bothnonmalignant and malignant cell populations.
Using two mouse models of MF (MPLW515L bone marrow [BM] transplanted mutant mice and JAK2V617F knock-in mice), the authors show that proinflammatory cytokines are elevated in the serum and/or BM supernatant of these mice, and that short-term ruxolitinib treatment could normalize serum cytokine levels. Next, the authors performed single-cell cytokine profiling experiments that demonstrated aberrant cytokine secretion profiles of MF murine and human bone marrow cells compared to control BM cells. These findings included: 1) an increased fraction of cytokine-secreting cells, 2) increased per-cell cytokine secretion, and 3) co-secretion of multiple cytokines. The authors showed that sorted mature myeloid cells and megakaryocyte/erythroid progenitor (MEP) cells both had increased fractions of cytokine-secreting cells and increased per-cell cytokine secretion in MF compared to controls. The two cell types, however, had discrete cytokine secretion profiles: mature myeloid cells primarily secreted TNF-α and CCL3, whereas MEP were the predominant source of IL-6 and IL-10. Similar techniques performed on circulating granulocytes from patients with MF confirmed these results. These studies support the idea of functional heterogeneity regarding cell-specific cytokine secretion in MF.
The authors next turned their attention to examining the signaling pathways that promote cytokine production in MF. Experiments demonstrated constitutive STAT3 activation in cell lines, MPLW515L mutant mice, and in the BM of patients with MF. STAT3 phosphorylation was reduced by JAK1/2 inhibition in western blot analysis from MPLW515L mouse splenocytes. In murine MPLW515L-positive mice transplanted with BM from either control or SAT3-knockout mice, the authors demonstrated that STAT3 deletion reduced cytokine production, and ameliorated MF disease features in vivo, including lower white blood cell counts, reduced spleen weights and reticulin fibrosis, and prolonged survival. In fact, complete hematopoietic STAT3 deletion mimicked the effects of JAK1/2 inhibition by ruxolitinib.
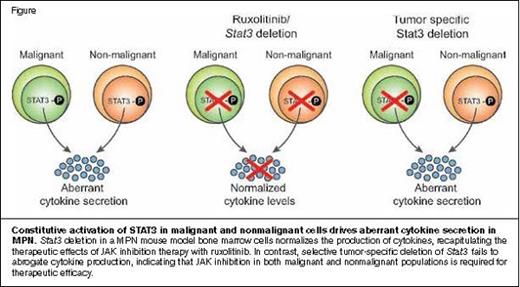
Constitutive Activation of STAT3 in Malignant and Nonmalignant Cells Drives Aberrant Cytokine Secretion in MPN.Stat3 deletion in a MPN mouse model bone marrow cells normalizes the production of cytokines, recapitulating the therapeutic effects of JAK inhibition therapy with ruxolitinib. In contrast, selective tumor-specific deletion of Stat3 fails to abrogate cytokine production, indicating that JAK inhibition in both malignant and nonmalignant populations is required for therapeutic efficacy.Reprinted from Cancer Discovery, 2015, 5/3, 316-331, M. Kleppe et al, JAK-STAT Pathway Activation in Malignant and Nonmalignant Cells Contributes to MPN Pathogenesis and Therapeutic Response, with permission from AACR.
Constitutive Activation of STAT3 in Malignant and Nonmalignant Cells Drives Aberrant Cytokine Secretion in MPN.Stat3 deletion in a MPN mouse model bone marrow cells normalizes the production of cytokines, recapitulating the therapeutic effects of JAK inhibition therapy with ruxolitinib. In contrast, selective tumor-specific deletion of Stat3 fails to abrogate cytokine production, indicating that JAK inhibition in both malignant and nonmalignant populations is required for therapeutic efficacy.Reprinted from Cancer Discovery, 2015, 5/3, 316-331, M. Kleppe et al, JAK-STAT Pathway Activation in Malignant and Nonmalignant Cells Contributes to MPN Pathogenesis and Therapeutic Response, with permission from AACR.
The authors sought to determine whether JAK inhibitors exert their effects on the malignant MPN cell population alone, or also on nonmalignant cells in the tumor microenvironment. They examined this question by transplanting lethally irradiated recipient mice with MPLW515L, STAT3-deleted BM with STAT3 wild-type BM. In contrast to the effects observed with pan-hematopoietic STAT3 deletion, tumor-specific STAT3 deletion did not reduce disease severity or attenuate inflammatory cytokine production. These data indicate that aberrant STAT3 signaling and cytokine production from both the malignant clone and nonmalignant hematopoietic cells in the BM microenvironment contribute to the MPN phenotype (Figure).9 The authors extended these observations by analyzing cytokine mRNA expression using NanoString technology in sorted MPLW515L-mutant (GFP+) and wild-type (GFP−) BM cells. They found that MPLW515L-positive myelofibrosis cells express high levels of several inflammatory cytokines, including IL-6; cytokines such as CCL2 and TNF-α were derived from both GFP-positive and GFP-negative populations; other cytokines such as IL-12 and CXCL9, were derived predominantly from nonmutant cells. These findings of increased and heterogeneous cytokine secretion from mutant and nonmutant cells were corroborated in a murine model of JAK2V617Fpositive disease and a patient with JAK2V617Fpositive primary myelofibrosis.
In Brief
This work by Dr. Kleppe and colleagues sheds light on the complex interactions between malignant cells and supporting cells in the BM microenvironment of MPN. These findings may also extend to other cancers since inflammation arising from pathologic cytokine elaboration is a cardinal feature of many tumors. Numerous questions are generated from this work. For example, how do differences in cell-specific cytokine secretion profiles influence the phenotype and severity of MPNs? What role do host genetic factors versus diseases-specific variables (e.g., JAK2 mutant allele burden) play in initiating and perpetuating the aberrant cytokine storm? Given the common tendency for disease persistence to develop over time with JAK inhibition, it will be important to interrogate whether these pathways can be therapeutically exploited to improve outcomes for patients.
References
Competing Interests
Dr. George indicated no relevant conflicts of interest.