In the early 1900s, Dr. James Homer Wright and colleagues observed that a reduction in the “dust of the blood” (platelets) is associated with bleeding.1 Today we know that while their primary function is hemostasis, platelets also participate in antimicrobial host defense, inflammation, and tissue repair. Chronic inflammation is often associated with reactive thrombocytosis, and responses to acute infections may be accompanied by sudden thrombocytopenia, making platelets bellwethers of health and disease. A steady platelet supply is ensured by continuous platelet production and clearance of ≈1011 platelets daily. The rate of production rises sharply under conditions of platelet destruction to maintain levels of 150,000 to 400,000 platelets per microliter of blood. Platelet production and clearance therefore must be tightly regulated to avoid spontaneous bleeding or arterial occlusion and organ damage.
Platelet production (thrombopoiesis) is a complex process that requires differentiation of hematopoietic stem cells into specialized progenitors and their organized interplay with the bone marrow microenvironment and hematopoietic cytokines.2 A major milestone in understanding the molecular mechanisms of thrombopoiesis was the discovery of thrombopoietin (TPO) in 1994. TPO is the primary regulator of platelet production, supporting the survival, proliferation, and differentiation of bone marrow megakaryocytes.2 Since the discovery of TPO, many molecular mechanisms of thrombopoiesis have been identified, including the development of polyploidy and pro-platelet formation, and the final fragmentation of the megakaryocyte cytoplasm to yield blood platelets. However, a principal unanswered question regarding thrombopoiesis is how TPO production is regulated under steady-state and pathologic conditions.
Historically, TPO regulation has been explained by a two-step model: 1) hepatocytes are the major source of TPO, which is released into circulation in a constitutive manner; and 2) circulating plasma TPO binds to its high-affinity receptor Mpl, expressed on the surface of platelets and megakaryocytes, resulting in subsequent degradation of TPO. Thus, the removal and destruction of circulating TPO has been thought to be mediated solely by expression of Mpl on circulating platelets and marrow megakaryocytes.3,4 This reciprocal relationship between platelet number and circulating TPO level is clearly evident in patients undergoing bone marrow transplantation and in Mpl–/–mice. However, several human and mouse phenotypes lend credence to the assertion that platelet TPO metabolism is not the sole determinant of plasma TPO levels. For example, serum TPO levels are lower than expected in patients with immune thrombocytopenia (ITP)5 and are higher than expected in patients with essential thrombocythemia (ET).6 Interestingly, some patients with myeloproliferative neoplasms (MPNs) with increased platelet numbers exhibit decreased Mpl expression on the surface of their platelets, which results in decreased TPO uptake and therefore increased plasma TPO levels.7 Mplfl/flPf4-Cre mice that specifically lack Mpl on megakaryocytes and platelets but that normally express Mpl on stem/progenitor cells, display profound megakaryocytosis and thrombocytosis with a remarkable expansion of megakaryocyte-committed and multipotential progenitor cells.8 In this mouse model, where circulating platelet numbers are high and megakaryocyte counts are significantly increased, the blood TPO levels are not as low as would be expected if Mpl expression on platelets and megakaryocytes were the only mechanism regulating TPO levels. It appears therefore that platelet numbers and TPO levels are regulated in a complicated fashion.
A number of inflammatory states (e.g., ulcerative colitis, rheumatoid arthritis, ovarian cancer) are associated with increased blood TPO levels and thrombocytosis.9,10 The inflammation-induced increase in TPO expression is mediated by interleukin (IL)-6, which stimulates TPO mRNA expression both in hepatocytes in vivo and in HepG2 and Hep3B cells in vitro.11 Hepatic TPO regulation by IL-6 is well characterized, but the ligand-receptor pair regulating hepatic TPO production at steady state has remained elusive.
A new model that helps to explain the regulation of blood TPO levels and thrombopoiesis is the clearance of senescent, desialylated platelets via the hepatic Ashwell-Morrell receptor (AMR), which enhances hepatic TPO production.12 Studies have shown that platelet surface glycans mediate platelet clearance.13 Sialic acid is normally considered as a “do not eat me” signal. Most cell surface glycans are presumably fully capped by sialic acid residues (i.e., fully sialylated). A recent study identified loss of sialic acid as a determinant of senescent platelet removal.12 This study shows that platelets lose sialic acid during circulation, likely due to upregulation of platelet intrinsic lysosomal sialidases, Neu1 and Neu3.14 Desialylated platelets are cleared via the hepatic AMR, a transmembrane heteroligomeric glycoprotein complex composed of ASGPR1 (CLEC4H1, HL-1) and ASGPR2 (CLEC4H2, HL-2) subunits. This highly conserved receptor has been largely regarded as an endocytic receptor, and its regulatory role has remained unclear since its discovery four decades ago.15 Specifically, mice lacking either the ASGPR1 or ASGPR2 subunit do not accumulate plasma proteins or lipids lacking sialic acid, which has been the predicted outcome.15 However, it was a surprising discovery that platelets with reduced α2,3-linked sialic acid due to sepsis, cold storage (in vitro aging), or in mice lacking the sialyltransferase ST3GalIV, are cleared by the hepatic AMR.15
The notion that loss of sialic acid determines platelet life span is not entirely novel.13,16 However, our recent study elucidates the specific mechanisms by which senescent, desialylated platelets regulate hepatic TPO mRNA production in vivo via the AMR.12 This feedback mechanism presents the desialylated platelet–AMR pair as the critical control point for TPO homeostasis and shows that hepatic TPO production is regulated and not constitutive. In support of this notion, injection of desialylated platelets into rabbits stimulates platelet production,17 presumably by stimulating liver TPO secretion.
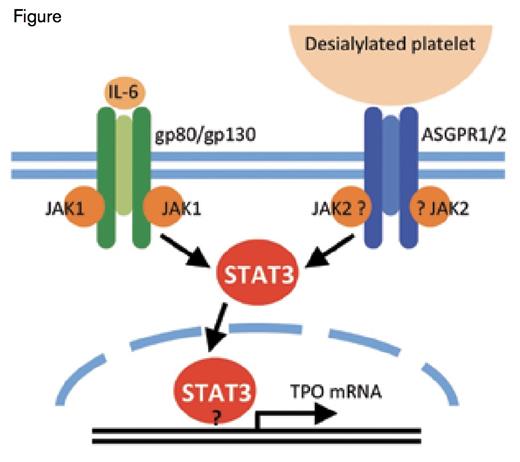
Comparison Between the AMR and IL-6R Signaling Pathways Leading to TPO mRNA Expression. Binding of desialylated platelets to the hepatic AMR composed of 1 ASGPR2 and 2 ASGPR1 subunits activates JAK2. IL-6 binding to its hepatic receptor composed of 1 gp80 and 2 gp130 subunits activates gp130-associated JAK1. Both JAK1 and JAK2 phosphorylate STAT3, resulting in its translocation to the nucleus where it stimulates mRNA expression of TPO and acute phase response proteins. It is unclear whether JAK2 directly associates with ASGPR1 and whether STAT3 directly binds to the TPO promoter.
Comparison Between the AMR and IL-6R Signaling Pathways Leading to TPO mRNA Expression. Binding of desialylated platelets to the hepatic AMR composed of 1 ASGPR2 and 2 ASGPR1 subunits activates JAK2. IL-6 binding to its hepatic receptor composed of 1 gp80 and 2 gp130 subunits activates gp130-associated JAK1. Both JAK1 and JAK2 phosphorylate STAT3, resulting in its translocation to the nucleus where it stimulates mRNA expression of TPO and acute phase response proteins. It is unclear whether JAK2 directly associates with ASGPR1 and whether STAT3 directly binds to the TPO promoter.
These findings led to the discovery that removal of senescent, desialylated platelets drives hepatic TPO mRNA expression via JAK2 and STAT3 phosphorylation, and translocation of the latter to the nucleus.12 Sequence analysis shows that the TPO promoter contains STAT3 binding sites; however, their function remains to be investigated. Interestingly, the AMR signaling cascade shares similarities with that of the IL-6 receptor (IL-6R). Binding of IL-6 to its hepatic receptor engages the signal transducing subunit gp130, leading to STAT3 tyrosine phosphorylation and activation by gp130-associated JAK1. Thus, both desialylated platelets and IL-6 lead to STAT3-mediated hepatic TPO mRNA expression downstream of the AMR-JAK2 and IL-6R-JAK1 signaling cascades, respectively. It remains to be determined whether JAK2 and STAT3 directly associate with the AMR or require gp130.
Hepatic STAT3 controls the transcription of mRNA for acute phase plasma proteins. It is therefore tempting to speculate that acute phase proteins are produced in response to AMR ligation, which would establish clearance of desialylated platelets as a component of the acute phase response. Consistent with this hypothesis, the AMR-mediated removal of desialylated platelets improves the probability of host survival during sepsis.15 Separate studies have shown that liver regeneration following injury is promoted by platelets18 and requires AMR and hepatic STAT3 function. Thus, the platelet-AMR-JAK2-STAT3 signaling cascade may connect desialylated platelets to inflammatory responses.
The most compelling evidence showing that TPO expression is promoted via the AMR-STAT3-JAK2 pathway is based on the experiments using JAK1/2 inhibitors. Disruption of the AMR-JAK2 signaling cascade by JAK1/2 inhibitors AZD1480, TG101348, and BMS911543 adversely affects hepatic TPO mRNA expression and secretion in hepatocytes in vivo, and in HepG2 cells in vitro.12 JAK1/2 inhibitors target wild-type and V617F-mutant JAK2, and are clinically used in MPNs.19 Common on-target JAK-inhibitor-associated toxicities include anemia and thrombocytopenia, which relate to JAK2’s essential role in red blood cell and platelet production. Our data indicate that JAK inhibitors could additionally cause thrombocytopenia by inhibiting TPO production downstream of the hepatic AMR-JAK2 signaling cascade. Clinical studies are necessary to investigate this notion, particularly regarding plasma TPO levels in patients with MPNs following JAK1/2 inhibitor treatment.
In conclusion, recent evidence has shown that the hepatic AMR recognizes circulating desialylated platelets under steady-state conditions. Senescent, desialylated platelets and the AMR are the elusive physiological ligand-receptor pair regulating hepatic TPO mRNA production, resolving the longstanding mystery of steady state hepatic TPO regulation. This feedback mechanism, which recruits hepatic JAK2 and STAT3, contributes to our understanding of the mechanisms of thrombocytopenia observed in patients with MPNs treated with JAK1/2 inhibitors.
Acknowledgment
This work was supported by National Institutes of Health grants HL107146 and HL089224 (K.M.H.); and the Brigham Research Institute Fund to Sustain Research Excellence (H.F.).
References
Competing Interests
Dr. Grozovsky, Dr. Falet, and Dr. Hoffmeister indicated no relevant conflicts of interest.