Red blood cell (RBC) transfusion is a frequently used therapy worldwide. In many countries, including the United States, regulations permit storage of RBC units for up to 42 days; however, prolonged RBC unit storage has been associated with shape and rheologic changes as well as changes in metabolism, and oxygen affinity/delivery, commonly referred to as the RBC “storage lesion.”1 Multiple observational and retrospective analyses have been performed examining the effects of RBC storage age on patient outcomes. The results of these studies have been mixed and complicated with bias.1 Two recently published randomized controlled trials, by Dr. Jacques Lacroix and colleagues (ABLE) and by Dr. Marie Steiner and colleagues (RECESS), examined the impact of RBC storage age on different patient populations and have helped shed light on this critical issue.
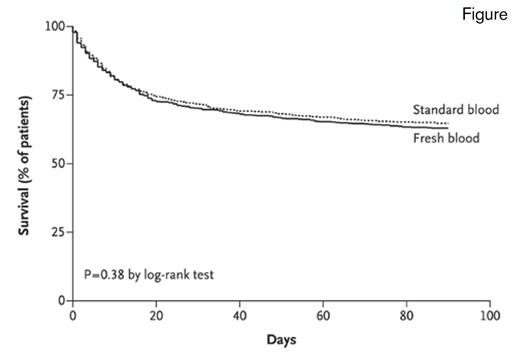
Kaplan–Meier Survival Analysis of Time to Death in the Intention-to-Treat Population. The intention-to-treat population included 2,430 patients. The hazard ratio in the fresh-blood group, as compared with the standard-blood group, was 1.1 (95% CI, 0.9-1.2).From The New England Journal of Medicine, Lacroix J et al, Age of Transfused Blood in Critically Ill Adults, vol. 372, page 1416, Copyright © 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Kaplan–Meier Survival Analysis of Time to Death in the Intention-to-Treat Population. The intention-to-treat population included 2,430 patients. The hazard ratio in the fresh-blood group, as compared with the standard-blood group, was 1.1 (95% CI, 0.9-1.2).From The New England Journal of Medicine, Lacroix J et al, Age of Transfused Blood in Critically Ill Adults, vol. 372, page 1416, Copyright © 2015 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
The ABLE trial examined the storage age of transfused RBCs in critically ill adult patients across 64 centers in Canada and Europe, with 1,211 patients assigned to receive fresh RBC units stored for less than eight days, and 1,219 patients assigned to receive standard issue RBC units (mean age of 22 days). No difference was observed between the two groups in the primary outcome of 90-day all-cause mortality (p=0.38), and there were no significant differences between the two groups with respect to major illnesses; duration of respiratory, hemodynamic, or renal support; length of hospital stay; and transfusion reactions. Importantly, the ABLE trial used leukocyte-reduced RBC units, and thus, could not address whether leukocytes contribute to the degradation of RBCs or have other deleterious effects associated with long-term storage. Additionally, this trial did not address the use of RBCs that are stored for longer periods of time, such as 35 to 42 days. It is noteworthy that the ABLE subjects were transfused at a mean pre-transfusion hemoglobin level of 7.7 g/dL and likely received less exposure to RBC transfusions than patients at centers with more liberal transfusion policies.
The RECESS trial examined the storage age of RBC transfusion in 1,098 patients 12 years or older undergoing complex cardiac surgery at 33 U.S. hospitals. These patients received either RBCs stored for less than 10 days versus older RBCs stored for greater than 21 days. The fresh RBC storage median age was 7 days and the older RBC storage median age was 28 days. The number of RBC units transfused per patient and the percentage of patients who received eight or more units were similar between the two groups. No significant difference was observed between the two groups in the primary outcome of organ dysfunction at seven days, as assessed by the Multiple Organ Dysfunction Score (MODS; p=0.44). Similarly, no significant difference was observed in secondary outcomes of MODS changes at 28 days, all-cause mortality at seven and 28 days, or length of stay in intensive care or the hospital. Adverse events were similar between the groups, with the exception of a significant increase in hyperbilirubinemia in those subjects receiving older blood (p<0.01). Similar to the ABLE trial, patients in the RECESS trial received leukoreduced RBCs, and the study did not address the clinical impact of RBC units toward the end of their storage life.
In Brief
The results of the ABLE and RECESS trials argue that restricting RBC transfusion to units stored for a shorter period is not necessary for critically ill adults or patients 12 years of age or older undergoing complex cardiac surgery. However, these results may not be generalizable to non–leukocyte-reduced RBC units. Additionally, these studies did not address RBC units transfused close to the end of the maximum-allowed storage period. The ABLE and RECESS results echo the findings of the Age of Red Blood Cells in Premature Infants (ARIPI) trial, which also showed no significant differences in outcomes among premature and low-birth-weight infants.2 Collectively, these results have important implications for the blood supply, where the management of special requests (freshest blood, irradiated blood, cytomegalovirus seronegative, etc.) is complex, time consuming, and expensive. Whether there are other patient populations that may be impacted from RBC storage duration has yet to be determined.
References
Competing Interests
Dr. George and Dr. Lockhart indicated no relevant conflicts of interest.