The Question
What is your management approach to patients with relapsed/refractory hairy cell leukemia?
My Response
Hairy cell leukemia (HCL) is an indolent B-cell malignancy, originally with a median survival of approximately four years.1 Single-agent purine analog therapy with pentostatin or cladribine achieves complete remission (CR) rates of 70 to 90 percent and median relapse-free survivals in excess of 15 years.2-4 However, minimal residual disease (MRD) often remains, and may be associated with relapse, particularly in young patients with longer follow-up.5 Thus, in the past 25 to 30 years, many patients have neither died nor been cured, leading to the increasingly common question of how to treat relapsed and refractory patients. The answer requires accurate diagnosis, understanding the history of response and toxicity to prior agents, and a strategy for prioritizing options including exciting new targeted therapies.
Diagnosis of HCL and Recognition of Variant Disease
HCL diagnosis today is most accurately made by flow cytometry showing strong expression of B-cell antigens CD20 and CD22; strong CD11c; and positive CD25, CD103, and CD123.6 Bone marrow immunohistochemistry is positive for CD20, tartrate-resistant acid phosphatase (TRAP), annexin 1A (Anxa1), and for the V600E mutated form of BRAF.7 Variant HCL (HCLv) has a similar immunophenotype but lacks CD25, Anxa1, CD123, and TRAP.6 Additionally, BRAF is wild type. HCLv is more aggressive, with worse splenomegaly, malignant lymphocytosis rather than cytopenias, and exhibits a poor response to single-agent purine analog; it also may require adding rituximab. Although classified in 2008 by the World Health Organization as a separate disorder, HCLv is also discussed here. Another aggressive variant is characterized by an immunophenotype of HCLv or classic HCL, but with the IGHV4-34 type of immunoglobulin rearrangement that is more than 98 percent homologous (unmutated) to its germline sequence.8 Even with the classic immunophenotype, these patients resemble those with HCLv with malignant lymphocytosis, purine analog resistance, and wild-type BRAF.8,9 By shipping blood samples and unstained bone marrow slides to an expert center, accurate diagnosis is also possible without travel. Treatment should only be initiated after accurate diagnosis is established.
Treatment of Patients in First Relapse
With some exceptions, remission rates are lower after second-line versus after first-line purine analog therapy3,4 ; however, if the length of the first remission is at least two to four years, many will recommend repeating the initial purine analog. The shorter the treatment-free interval, the greater the risk of overlapping toxicity. Patients relapsing after shorter intervals often receive the anti-CD20 MAb rituximab alone in four to eight weekly doses. While single-agent rituximab can achieve response without overlapping chemotherapy toxicity, response rates are relatively low in patients with prior purine analog therapy who have cytopenias requiring treatment. A more effective strategy is to combine a purine analog with rituximab. Elimination of MRD, detectable by immunohistochemistry of the bone marrow biopsy, as well as by flow cytometry of the blood or bone marrow aspirate, is possible with rituximab one month after cladribine.10 We treat once-relapsed HCL/HCLv with rituximab, starting immediately with cladribine to effect synergy,11 or delayed at least six months so MRD status can be determined.
Therapy of Multiply Relapsed HCL
Several options are being tested for multiply relapsed HCL. In order to avoid chemotherapy toxicities, but to retain cytotoxic power, we use recombinant immunotoxins containing an Fv fragment of a Mab and a truncated form of Pseudomonas exotoxin. High CR rates in multiply relapsed HCL were reported in 2001 using the anti-CD22 recombinant immunotoxin BL22,12 including patients with HCLv. Mutations were made in the VH domain of BL22 that improve its binding to CD22, and the resulting affinity-matured recombinant immunotoxin moxetumomab pasudotox achieved CRs in approximately 50 percent of patients with multiply relapsed HCL, many without MRD.13 Another strategy to achieve MRD-negative CR is to use six cycles of either pentostatin plus rituximab,4 or bendamustine plus rituximab.14 Our protocol at the National Institutes of Health (NIH) prospectively evaluates and randomizes patients between both regimens and allows crossover to the other regimen if needed. Most can achieve MRDnegative CR, but with more toxicity than patients receiving non-chemotherapy approaches such as moxetumomab pasudotox.
Targeted Approaches in HCL
In the BRAF pathway of normal cells, BRAF phosphorylates MEK, phospho-MEK phosphorylates ERK, and phospho-ERK leads to cell proliferation. BRAF containing the V600E mutation, present in nearly half of malignant melanomas, in lower percentages of other tumors, but in nearly all cases of classic HCL, exhibits uncontrolled phosphorylation leading to the malignant phenotype. Inhibition of this mutant BRAF with vemurafenib, or with dabrafenib combined with the MEK inhibitor trametinib, is approved for malignant melanoma and is currently being tested in HCL. Oral BRAF inhibitors in HCL can rapidly reverse severe cytopenias but have not yet been reported to clear MRD and prevent relapse. The oral agent ibrutinib, which targets the Bruton’s tyrosine kinase pathway, is also being tested in patients with HCL and HCLv. Though most patients achieve stable disease, this can have palliative benefit, particularly for patients with HCLv.
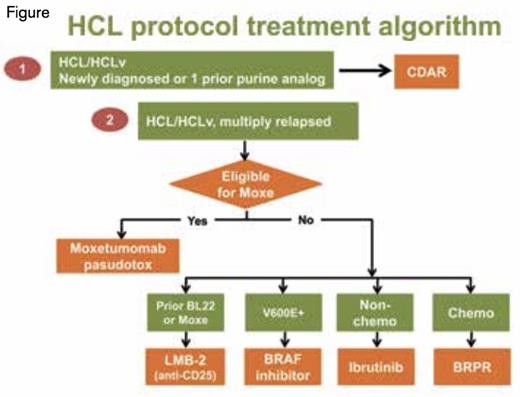
HCL Protocol Treatment Algorithm. Newly diagnosed HCL patients typically receive a purine analog, most commonly cladribine in five daily doses, less commonly pentostatin in six to 12 biweekly doses. Newly diagnosed or once-relapsed patients may receive repeat courses of single-agent purine analog, but at MD Anderson and the NIH, are also eligible for protocol treatment with rituximab and cladribine (CDAR). At the NIH, multiply relapsed HCL/HCLv patients eligible for moxetumomab pasudotox receive that option (also available in other centers), while ineligible patients may receive LMB-2, BRAF inhibition, ibrutinib, and BRPR (bendamustine-rituximab vs pentostatinrituximab) in that order of priority. Common off-protocol options for multiply-relapsed HCL include repeat courses of single-agent purine analog, single-agent rituximab, and pentostatin-rituximab. Splenectomy and even splenic radiation can have palliative benefit at least for a limited period of time.
HCL Protocol Treatment Algorithm. Newly diagnosed HCL patients typically receive a purine analog, most commonly cladribine in five daily doses, less commonly pentostatin in six to 12 biweekly doses. Newly diagnosed or once-relapsed patients may receive repeat courses of single-agent purine analog, but at MD Anderson and the NIH, are also eligible for protocol treatment with rituximab and cladribine (CDAR). At the NIH, multiply relapsed HCL/HCLv patients eligible for moxetumomab pasudotox receive that option (also available in other centers), while ineligible patients may receive LMB-2, BRAF inhibition, ibrutinib, and BRPR (bendamustine-rituximab vs pentostatinrituximab) in that order of priority. Common off-protocol options for multiply-relapsed HCL include repeat courses of single-agent purine analog, single-agent rituximab, and pentostatin-rituximab. Splenectomy and even splenic radiation can have palliative benefit at least for a limited period of time.
Summary and Algorithm for Treatment Approach to Patients with Relapsed/Refractory HCL
Figure 1 shows our algorithm for prioritization of clinical trials for relapsed HCL; additional options can be used off-protocol. Patients with once-relapsed HCL after purine analog can be retreated with the same or different purine analog as a single-agent, particularly if the first remission duration was long, but we prefer combining cladribine with rituximab on study to eliminate MRD. For patients with multiply relapsed HCL, moxetumomab pasudotox is our preferred option due to its unique ability to achieve MRD-negative CR without chemotherapy toxicities. For patients who responded to anti-CD22 recombinant immunotoxins (BL22 or moxetumomab pasudotox) but need additional therapy, we target CD25 with recombinant immunotoxin LMB-2. For patients ineligible for moxetumomab pasudotox or LMB-2, we use BRAF or BRAF/MEK inhibition, provided patients are able to handle toxicities and understand that remission durations may be limited if MRD persists. For patients with HCL/HCLv ineligible for these approaches, we prefer either the ibrutinib protocol or our protocol using rituximab combined with either bendamustine or pentostatin. Ibrutinib offers oral therapy without chemotherapy toxicity, while the rituximab-chemotherapy combination offers a likely path to high MRD-negative CR rates and the potential (as in patients after moxetumomab pasudotox) of not needing therapy for many years. Further follow-up and testing will be needed to determine the long-term benefit of these approaches and whether MRD-negative CR can translate into cure.
References
Competing Interests
Dr. Kreitman is the co-inventor of the NIH patent for Moxetumomab pasudotox.