Development of cancer is usually thought to arise after gradual accumulation of mutations over time, but there is a more profound event termed “chromothripsis” that represents a catastrophic change of genome structure, with widespread damage and simultaneous acquisition of multiple mutations. This latter process involves a cluster of chromosomal rearrangements. The mechanism for the occurrence of the widespread damage was not clear for a long time, but current models have shed new insight.
The phenomenon of chromothripsis was observed after genome sequencing of a sample from a case of chronic lymphocytic leukemia that exhibited 42 genomic rearrangements involving the long arm of chromosome 4, with concomitant rearrangements of portions of chromosomes 1, 12 and 15.1 Chromothripsis was observed in about 3 percent of other cancers as well.
Dr. Cheng-Zhong Zhang and colleagues at the Dana-Farber Cancer Institute identified that the damage occurred after partitioning of intact chromosomes into micronuclei by combining live cell imaging with single-cell genomic analysis they termed “Look-Seq.” This group had previously identified the central role of the micronucleus in this process.2 These “lagging” chromosomes that fail to properly attach to the mitotic spindle during cell division are physically separated in micronuclei that have a separate nuclear envelope and chromatin. The micronuclei have a predilection to irreversibly rupture, and the damaged micronuclei accumulate as the cells progress through the S and G2 phases of the cell cycle, with DNA damage arising during this timeframe of DNA replication.
The investigators created an experimental system by treating synchronized cells with nocodazole, a chemical that destabilizes the mitotic spindle. They observed DNA damage in S and G2, but not G0, after rupture of the micronuclear envelope by fluorescent marking for DNA strand breaks with agents such as γH2AX. Labeling cells with EdU (5-ethinyl-2'-deoxyuridine) demonstrated that incorporation was lower in the micronuclei than in the primary cell nucleus, indicating that the chromosomes in the micronuclei are under-replicated. Look-Seq involved separation of single cells into 384 well plates after treatment with nocodazole. Live cell imaging showed cells with micronuclear envelope rupture during the beginning of S phase. They also used siRNA to knock down expression of the p53 tumor suppressor gene, preventing cell cycle arrest in G1 that would otherwise proceed. The micronuclear chromosome was then reincorporated into the primary nucleus of the daughter cell, allowing DNA damage repair. The under-replicated chromosome was unevenly distributed to the daughter cells, leading to copy number alteration in either a 2:1 or 3:2 ratio, with the first number of the ratio representing the daughter cell that assimilated the mis-segregated chromosome.
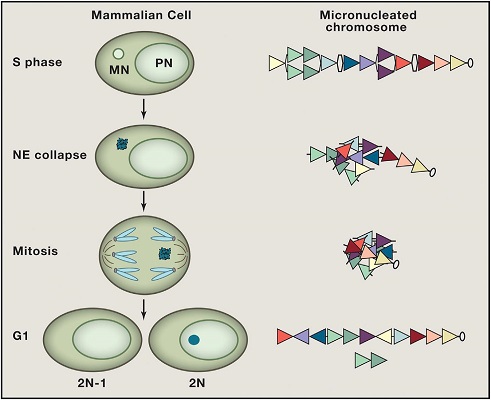
Chromothripsis from Ruptured Micronuclei. When a chromosome mis-segregates during mitosis, it can result in a daughter cell with two nuclei, the primary nucleus (PN) containing most of the genome and the micronucleus (MN), containing the missegregated chromatin (upper-left). After the cell enters S phase, DNA replication can occur on the micronucleated chromatin (upper-right). Rupture of the nuclear envelope during replication (middle-left) causes DNA damage, including double-stranded DNA breaks (middle-right). When the damaged chromatin is re-enclosed in a nuclear envelope after mitosis, DNA damage repair pathways can recognize the shattered chromatin and randomly reassemble the pieces to form a new chromosome (lower-right).Reprinted from Cell, Vol 161, Hatch EM and Hetzer MW, Linking Micronuclei to Chromosome Fragmentation, Pages No. 1502-1504, Copyright 2015, with permission from Elsevier.
Chromothripsis from Ruptured Micronuclei. When a chromosome mis-segregates during mitosis, it can result in a daughter cell with two nuclei, the primary nucleus (PN) containing most of the genome and the micronucleus (MN), containing the missegregated chromatin (upper-left). After the cell enters S phase, DNA replication can occur on the micronucleated chromatin (upper-right). Rupture of the nuclear envelope during replication (middle-left) causes DNA damage, including double-stranded DNA breaks (middle-right). When the damaged chromatin is re-enclosed in a nuclear envelope after mitosis, DNA damage repair pathways can recognize the shattered chromatin and randomly reassemble the pieces to form a new chromosome (lower-right).Reprinted from Cell, Vol 161, Hatch EM and Hetzer MW, Linking Micronuclei to Chromosome Fragmentation, Pages No. 1502-1504, Copyright 2015, with permission from Elsevier.
Whole genome sequencing was performed on 10 parent cells and nine daughter pairs derived from the micronucleated cells. Each of the daughter cells had at least one chromosome with copy number asymmetry representing the chromosome that was formerly localized in the micronucleus. Rearrangements were enhanced (a median 12.5-fold increase) in the mis-segregated chromosome (p<0.0001) and long-range rearrangements greater than 150 kb were more frequently observed. The researchers concluded that the long-range rearrangements arose from breakage of the chromatids in the ruptured micronucleus. Fragments of DNA were re-joined in random order and orientation. For one daughter cell, 14 of 16 breakpoints formed an uninterrupted chain. For another daughter cell, both chromosomes 4 and 11 appeared to have been in the micronucleus, as both intra- and eight inter-chromosomal rearrangements were observed. Lastly, in one daughter cell, four 1 to 3 Mb circular chromosomes were observed that might correspond to the double minute chromosomes that can be present at high copy number and carry oncogenes.
In Brief
This experimental model and technique of “Look-seq” provide insight into the mechanism for the “pulverization” of chromosomes that can be observed in cancer cells, with widespread chromosome fragmentation and reassembly. Loss of p53 permits survival in this setting to bypass cell-cycle arrest.3 The role of micronuclei that have been observed in many cancer types and normal cells in localizing individual chromosomes and mediating this damage is also highlighted. Perhaps this extensive damage can be prevented in the future by methods that interfere with formation of the micronucleus.
Figure. Chromothripsis from ruptured micronuclei. When a chromosome mis-segregates during mitosis, it can result in a daughter cell with two nuclei, the primary nucleus (PN) containing most of the genome and the micronucleus (MN), containing the missegregated chromatin (upper-left). After the cell enters S phase, DNA replication can occur on the micronucleated chromatin (upper-right). Rupture of the nuclear envelope during replication (middle-left) causes DNA damage, including double-stranded DNA breaks (middle-right). When the damaged chromatin is re-enclosed in a nuclear envelope after mitosis, DNA damage repair pathways can recognize the shattered chromatin and randomly reassemble the pieces to form a new chromosome (lower-right).
Reprinted from Cell, Vol 161, Hatch EM and Hetzer MW, Linking Micronuclei to Chromosome Fragmentation, Pages No. 1502-1504, Copyright 2015, with permission from Elsevier.
References
Competing Interests
Dr. Becker indicated no relevant conflicts of interest.